Granular Cell Dermatofibroma: When Morphology Still Matters
Abstract
:1. Introduction
2. Materials and Methods
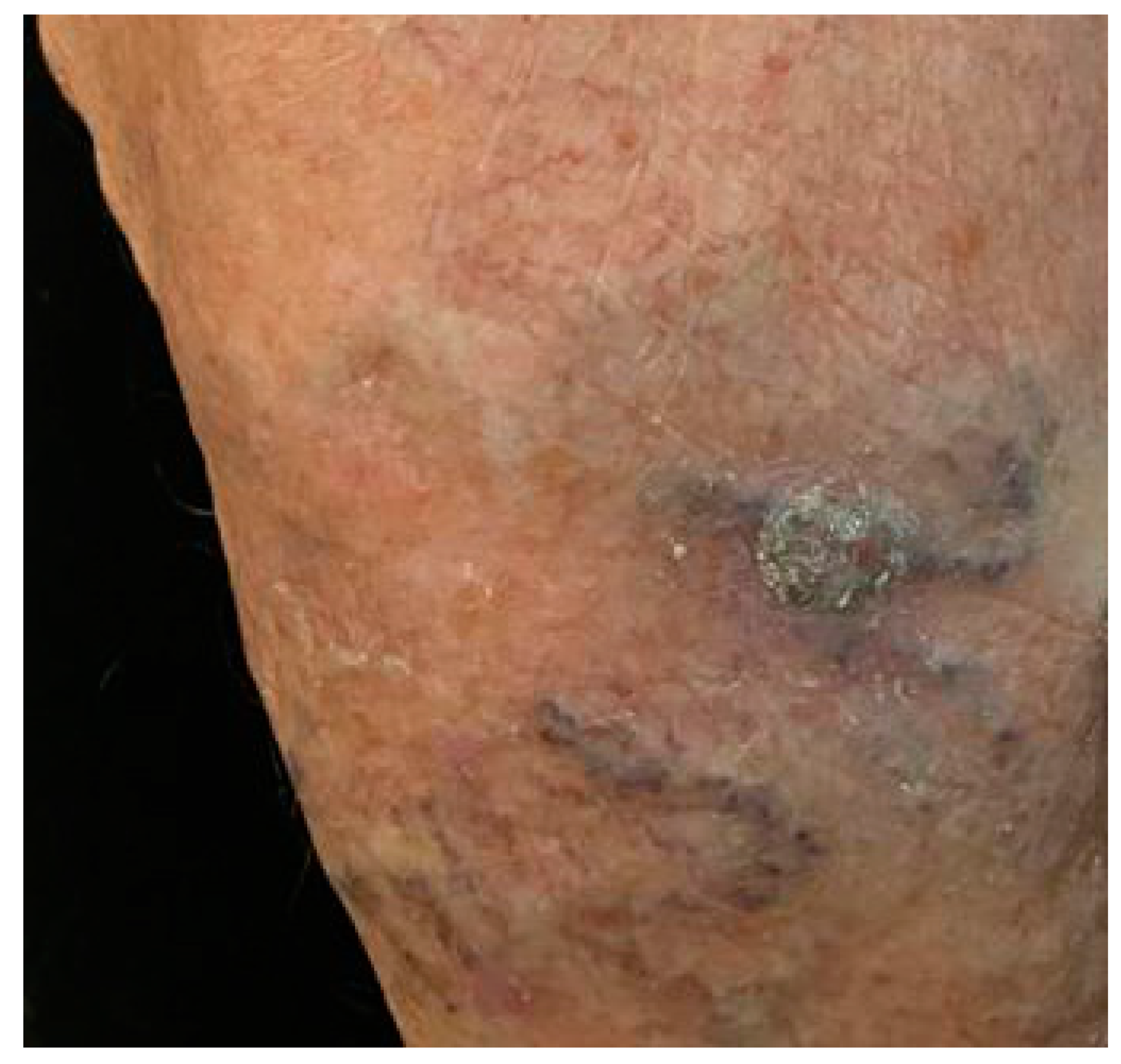
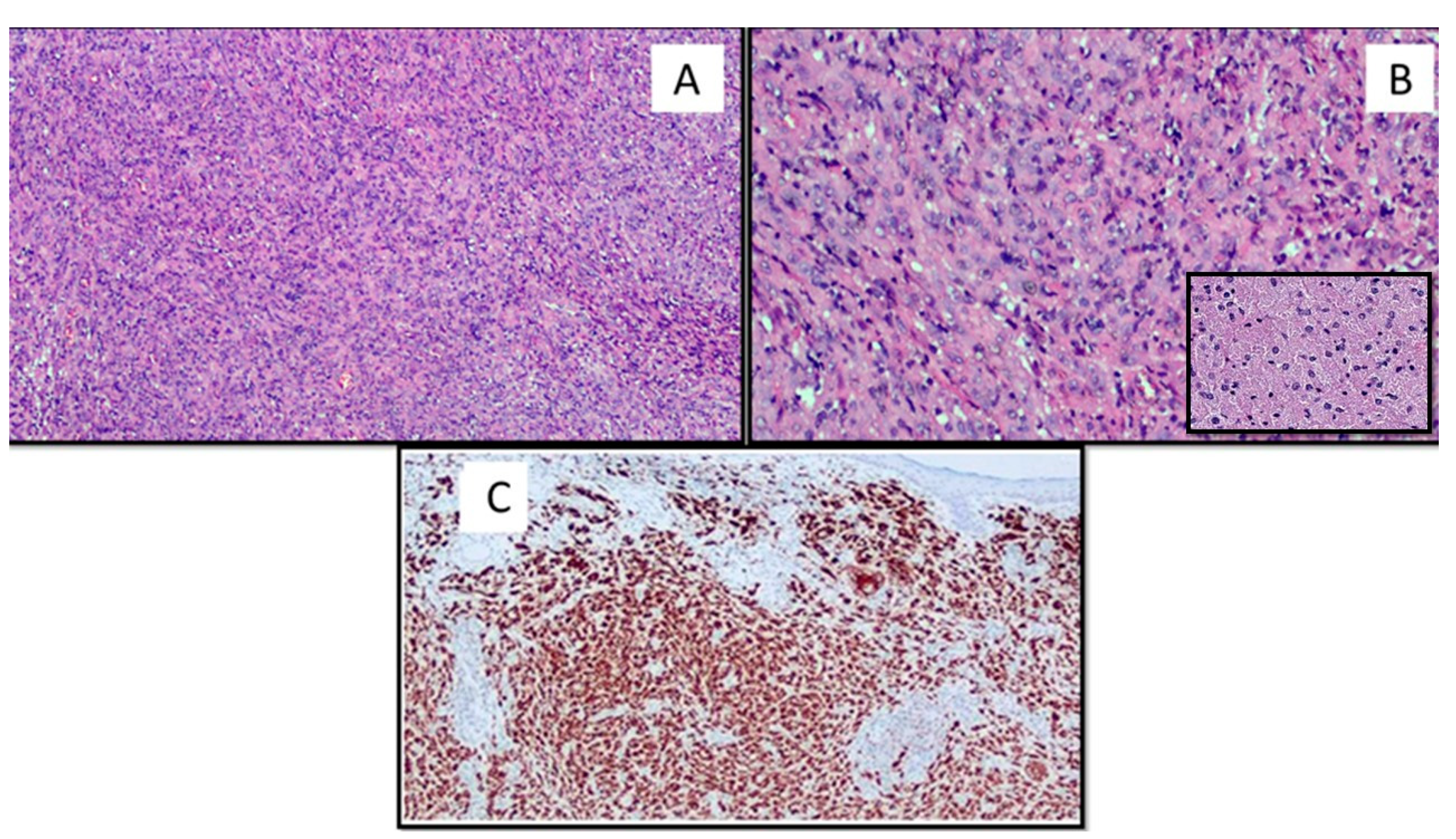
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Myers, D.J.; Fillman, E.P. Dermatofibroma; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Han, T.Y.; Chang, H.S.; Lee, J.H.; Lee, W.M.; Son, S.J. A clinical and histopathological study of 122 cases of dermatofibroma (benign fibrous histiocytoma). Ann. Dermatol. 2011, 23, 185–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeBoit, P.E.; Barr, R.J.; Bural, S.; Metcalf, J.S.; Yen, T.S.B.; Wick, M.R. Primitive polypoid granular-cell tumor and other granular-cell neoplasms of apparent nonneural origin. Am. J. Surg. Pathol. 1991, 15, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Aloi, D.F.; Albertazzi, M.P. Dermatofibroma with Granular Cells: A Report of Two Cases. Dermatology 1999, 199, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, J. Epithelioid Cell Histiocytoma with Granular Cells. (Another Nonneural Granular Cell Neoplasm). Am. J. Dermatopathol. 2007, 29, 475–476. [Google Scholar] [CrossRef] [PubMed]
- Martin, Y.; Sathyakumar, M.; Premkumar, J.; Magesh, K.T. Granular Cell Ameloblastoma. Case Rep. J. Oral Maxillofac. Pathol. 2017, 21, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perret, R.E.; Jullie, M.L.; Vergier, B.; Coindre, J.M.; Le Loarer, F. A subset of so-called dermal non-neural granular cell tumours are underlined by ALK fusions, further supporting the idea that they represent a variant of epithelioid fibrous histiocytoma. Histopathology 2018, 73, 532–534. [Google Scholar] [CrossRef] [PubMed]
- Soukup, J.; Hadzi-Nikolov, D.; Ryska, A. Dermatofibroma-like granular cell tumour: A potential diagnostic pitfall. J. Pathol. 2016, 67, 291–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazzato, G.; Colagrande, A.; Cimmino, A.; Liguori, G.; Lettini, T.; Serio, G.; Ingravallo, G.; Marzullo, A. Atypical Fibroxanthoma-Like Amelanotic Melanoma: A Diagnostic Challenge. Dermatopathology 2021, 8, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Cives, M.; Mannavola, F.; Lospalluti, L.; Sergi, M.C.; Cazzato, G.; Filoni, E.; Cavallo, F.; Giudice, G.; Stucci, L.S.; Porta, C.; et al. Non-Melanoma Skin Cancers: Biological and Clinical Features. Int. J. Mol. Sci. 2020, 21, 5394. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.V.; Matos, D.M.; Barreiros, H.F.; Bártolo, E.A. Variants of dermatofibroma—A histopathological study. An. Bras. Dermatol. 2014, 89, 472–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.D.; Usmani, A.S.; DeYoung, B.R.; Ly, M.; Pellegrini, A.E. Dermatofibroma-like Granular Cell Tumor: Case Reports. J. Cutan. Pathol. 2001, 28, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Rawal, Y.B.; Dodson, T.B. S-100 Negative Granular Cell Tumor (So-called Primitive Polypoid Non-neural Granular Cell Tumor) of the Oral Cavity; Case Reports. Head Neck Pathol. 2017, 11, 404–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luzar, B.; Calonje, E. Morphological and immunohistochemical characteristics of atypical fibroxanthoma with a special emphasis on potential diagnostic pitfalls: A review. J. Cutan. Pathol. 2010, 37, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Cardis, M.A.; Ni, J.; Bhawan, J. Granular cell differentiation: A review of the published work. J. Dermatol. 2017, 44, 251–258. [Google Scholar] [CrossRef] [PubMed]
| Benign granular cell tumor | Granular cell basal cell carcinoma |
| Malignant granular cell tumor | Granular cell schwannoma |
| Primitive polypoid granular cell tumor | Granular cell leiomyoma |
| Granular cell ameloblastoma | Granular cell leiomyosarcoma and angiosarcoma |
| Granular cell fibrous papule of the nose | Granular cell dermatofibrosarcoma protuberans |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazzato, G.; Colagrande, A.; Cimmino, A.; Marrone, M.; Stellacci, A.; Arezzo, F.; Lettini, T.; Resta, L.; Ingravallo, G. Granular Cell Dermatofibroma: When Morphology Still Matters. Dermatopathology 2021, 8, 371-375. https://doi.org/10.3390/dermatopathology8030041
Cazzato G, Colagrande A, Cimmino A, Marrone M, Stellacci A, Arezzo F, Lettini T, Resta L, Ingravallo G. Granular Cell Dermatofibroma: When Morphology Still Matters. Dermatopathology. 2021; 8(3):371-375. https://doi.org/10.3390/dermatopathology8030041
Chicago/Turabian StyleCazzato, Gerardo, Anna Colagrande, Antonietta Cimmino, Maricla Marrone, Alessandra Stellacci, Francesca Arezzo, Teresa Lettini, Leonardo Resta, and Giuseppe Ingravallo. 2021. "Granular Cell Dermatofibroma: When Morphology Still Matters" Dermatopathology 8, no. 3: 371-375. https://doi.org/10.3390/dermatopathology8030041
APA StyleCazzato, G., Colagrande, A., Cimmino, A., Marrone, M., Stellacci, A., Arezzo, F., Lettini, T., Resta, L., & Ingravallo, G. (2021). Granular Cell Dermatofibroma: When Morphology Still Matters. Dermatopathology, 8(3), 371-375. https://doi.org/10.3390/dermatopathology8030041










