Aberrant Expression of Immunohistochemical Markers in Malignant Melanoma: A Review
Abstract
:1. Introduction
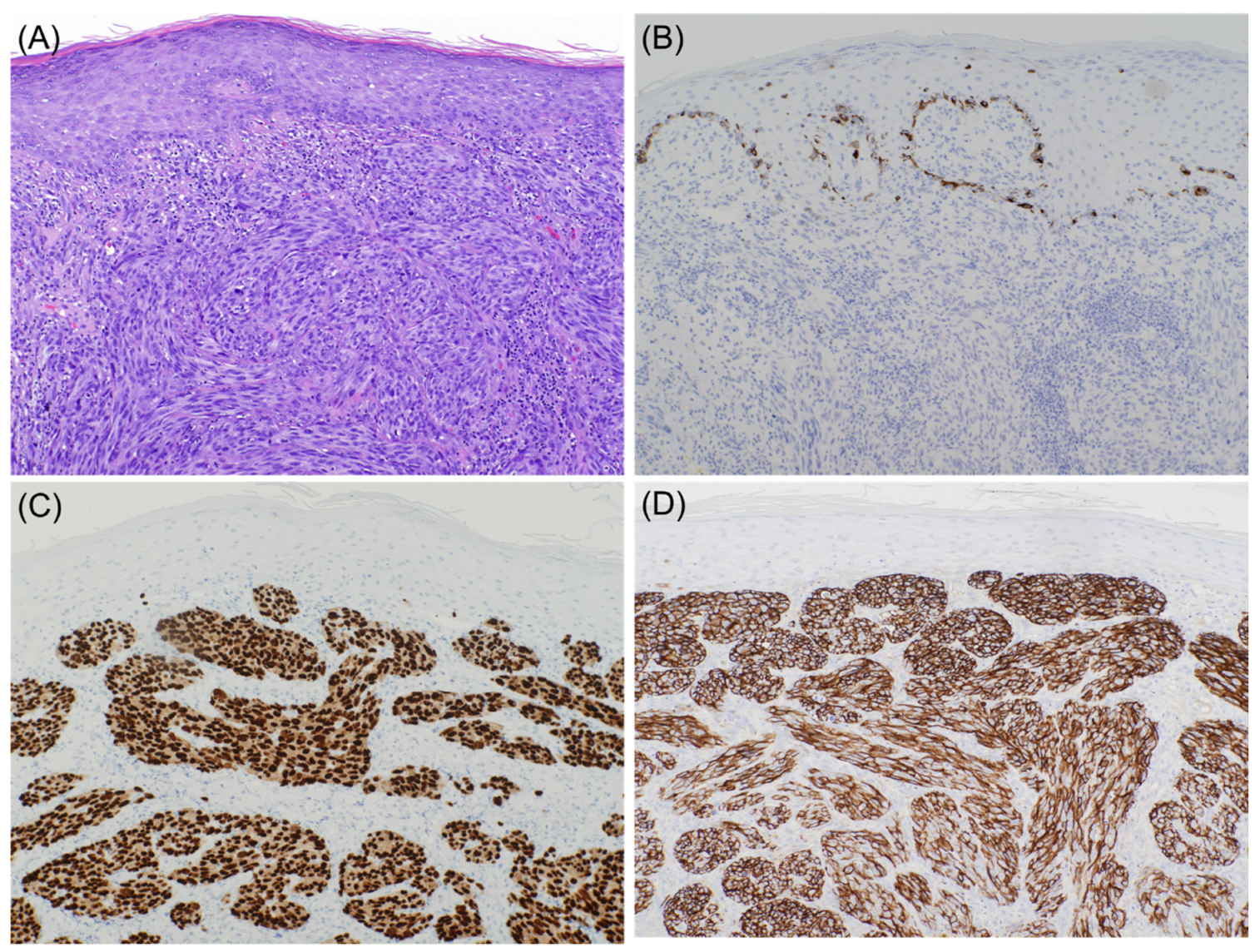
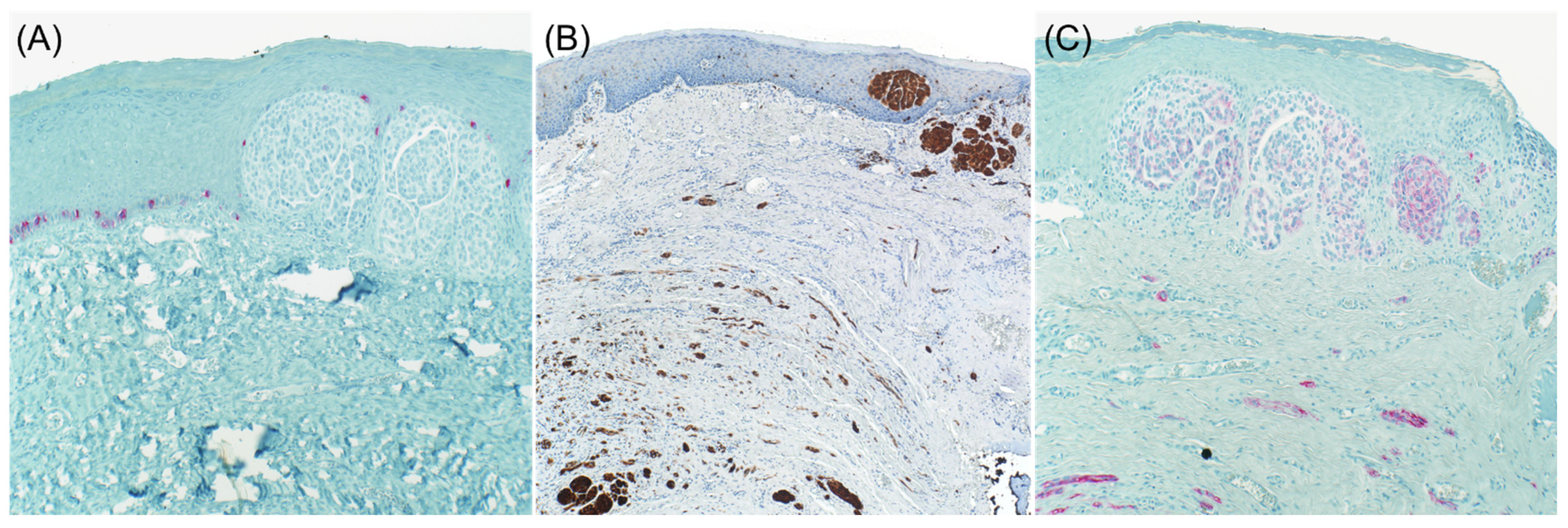
2. Loss of Melanocytic Markers in Malignant Melanoma
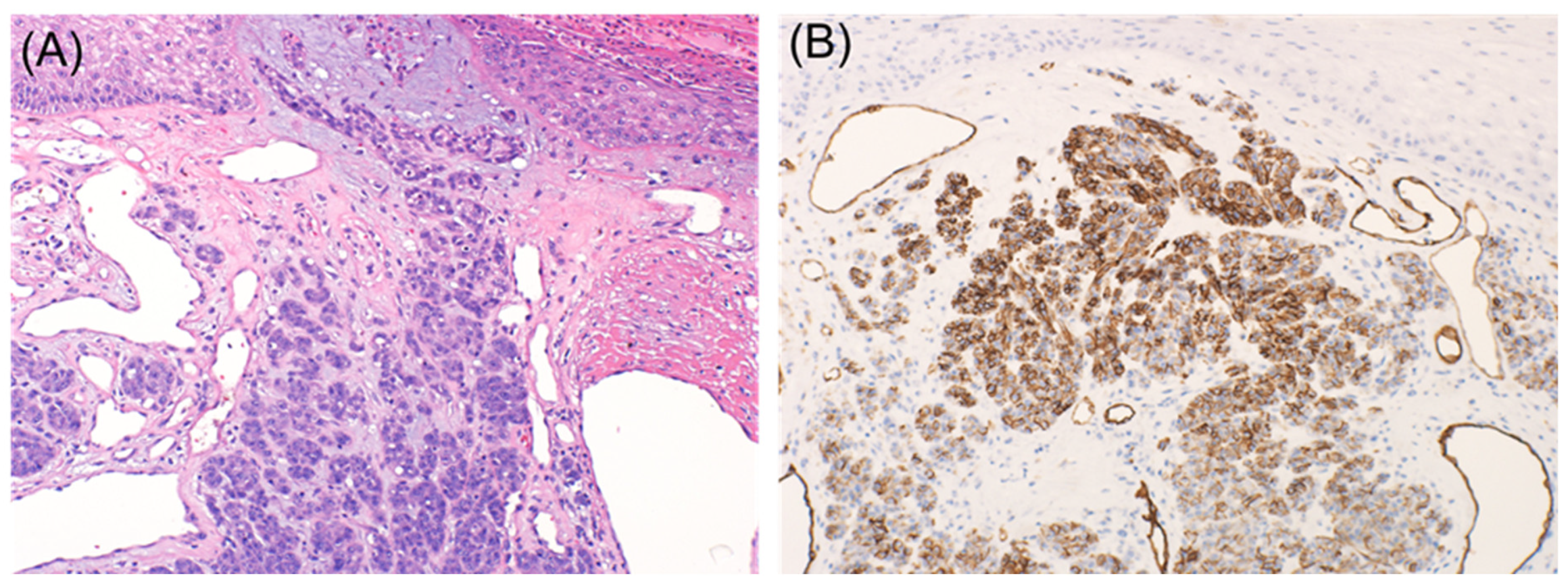
2.1. Metastatic Malignant Melanoma
2.2. Desmoplastic Melanoma
2.3. Primary Melanoma
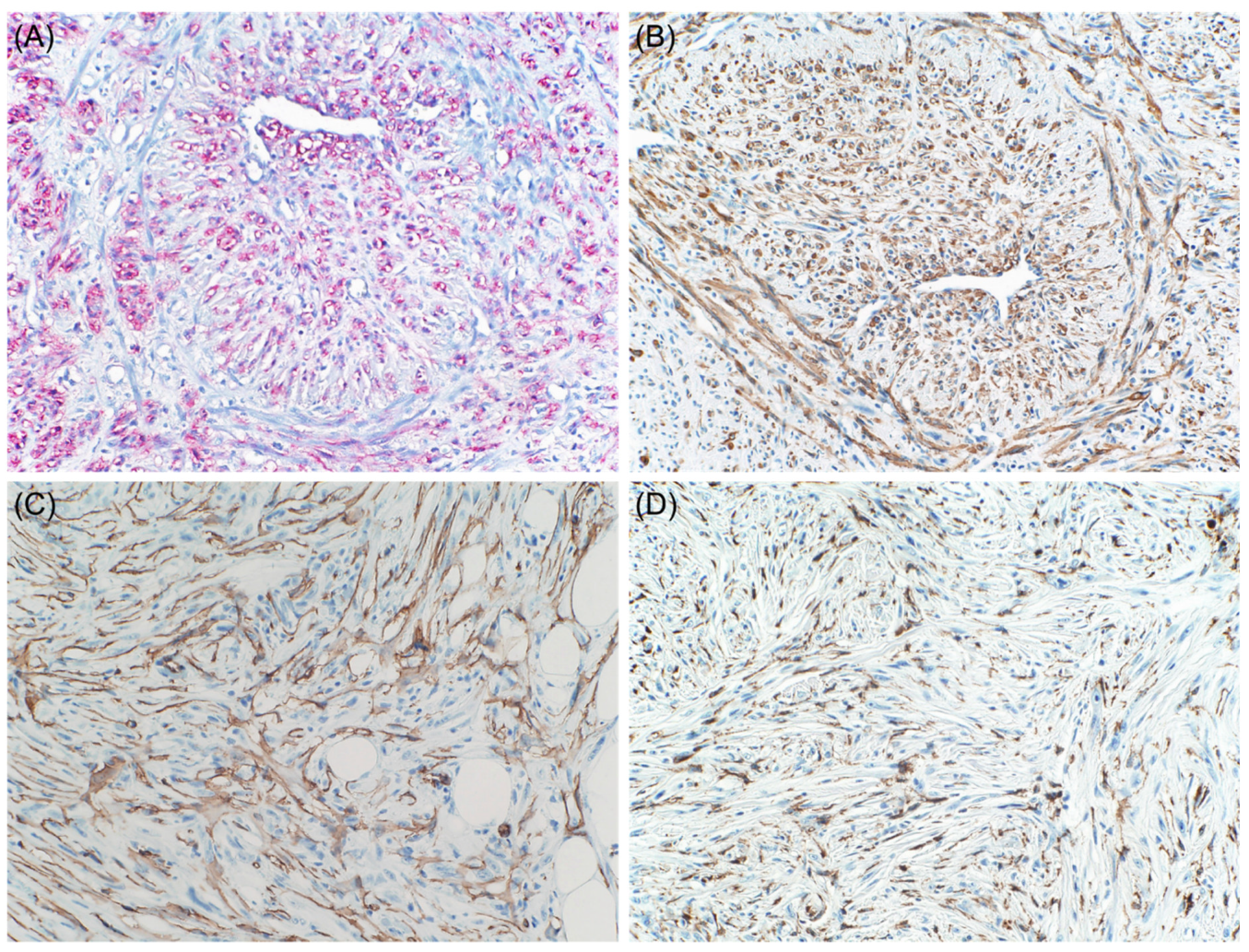
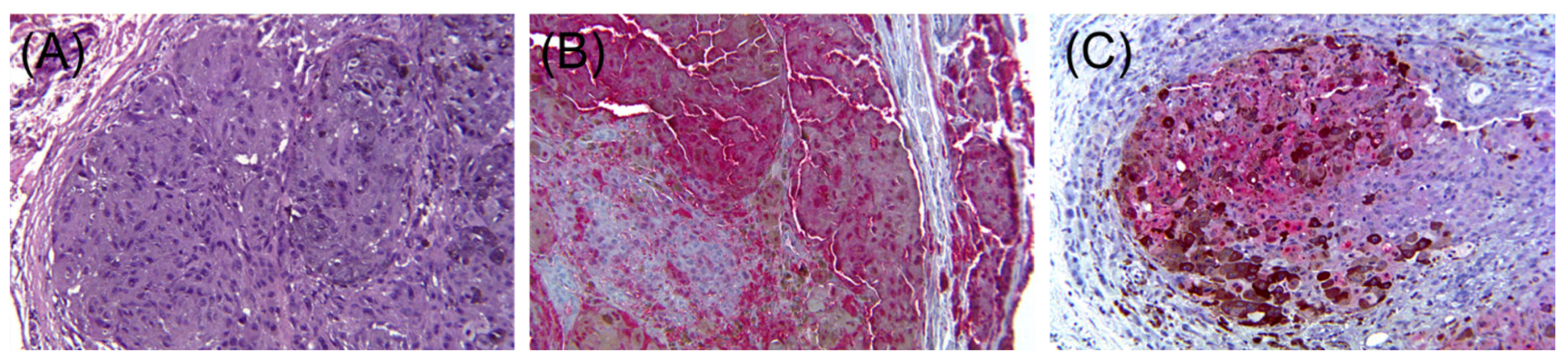
3. Aberrant Expression of Non-Melanocytic Markers
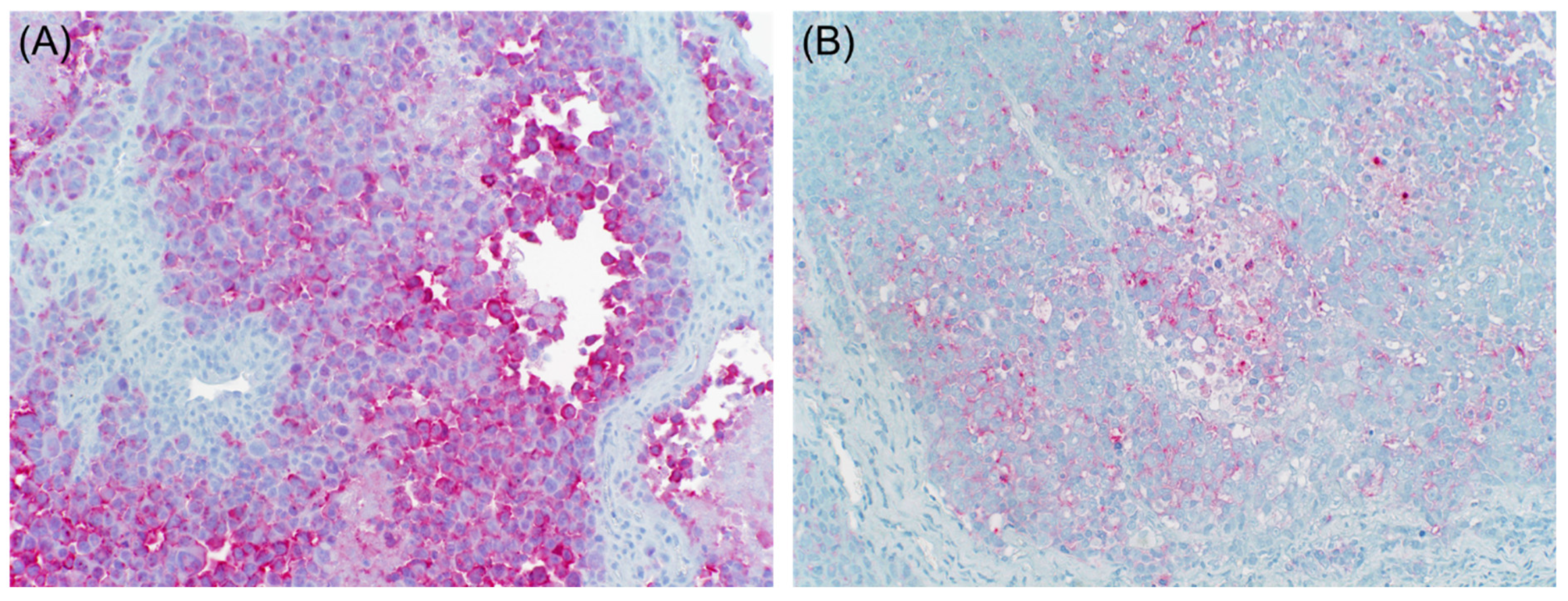
3.1. Muscle-Specific Markers
3.2. Neuroendocrine Markers
3.3. Keratin
3.4. Macrophage Markers
3.5. Vascular Markers
3.6. Hematopoietic Markers
3.7. FLI-1
3.8. GATA3
3.9. CEA
3.10. Calretinin
3.11. PAX8 and PAX2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dinehart, M.S.; Dinehart, S.M.; Sukpraprut-Braaten, S.; High, W.A. Immunohistochemistry utilization in the diagnosis of melanoma. J. Cutan. Pathol. 2020, 47, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez, N.G. Value of melanocytic-associated immunohistochemical markers in the diagnosis of malignant melanoma: A review and update. Hum. Pathol. 2014, 45, 191–205. [Google Scholar] [CrossRef]
- Kim, R.H.; Meehan, S.A. Immunostain use in the diagnosis of melanomas referred to a tertiary medical center: A 15-year retrospective review (2001–2015). J. Cutan. Pathol. 2016, 44, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Aisner, D.L.; Maker, A.; Rosenberg, S.A.; Berman, D.M. Loss of S100 antigenicity in metastatic melanoma. Hum. Pathol. 2005, 36, 1016–1019. [Google Scholar] [CrossRef] [Green Version]
- Agaimy, A.; Specht, K.; Stoehr, R.; Lorey, T.; Märkl, B.; Niedobitek, G.; Straub, M.; Hager, T.; Reis, A.-C.; Schilling, B.; et al. Metastatic Malignant Melanoma with Complete Loss of Differentiation Markers (Undifferentiated/Dedifferentiated Melanoma). Am. J. Surg. Pathol. 2016, 40, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Busam, K.J. Cutaneous Desmoplastic Melanoma. Adv. Anat. Pathol. 2005, 12, 92–102. [Google Scholar] [CrossRef]
- Ohsie, S.J.; Sarantopoulos, G.P.; Cochran, A.J.; Binder, S.W. Immunohistochemical characteristics of melanoma. J. Cutan. Pathol. 2008, 35, 433–444. [Google Scholar] [CrossRef]
- Kanik, A.B.; Yaar, M.; Bhawan, J. p75 nerve growth factor receptor staining helps identify desmoplastic and neurotropic melanoma. J. Cutan. Pathol. 1996, 23, 205–210. [Google Scholar] [CrossRef]
- Radfar, A.; Stefanato, C.M.; Ghosn, S.; Bhawan, J. NGFR-Positive Desmoplastic Melanomas with Focal or Absent S-100 Staining. Am. J. Dermatopathol. 2006, 28, 162–167. [Google Scholar] [CrossRef]
- Palla, B.; Su, A.; Binder, S.; Dry, S. SOX10 Expression Distinguishes Desmoplastic Melanoma FROM Its Histologic Mimics. Am. J. Dermatopathol. 2013, 35, 576–581. [Google Scholar] [CrossRef]
- Kooper-Johnson, S.; Mahalingam, M.; Loo, D.S. SOX-10 and S100 Negative Desmoplastic Melanoma. Am. J. Dermatopathol. 2020, 42. [Google Scholar] [CrossRef]
- Shinohara, M.M.; Deubner, H.; Argenyi, Z.B. S100, HMB-45, and Melan-A negative primary melanoma. Dermatol. Online J. 2009, 15, 7. [Google Scholar] [CrossRef]
- Chang, O.; Argenyi, Z. Loss of Conventional Melanocytic Markers in Malignant Melanoma and Lymph Node Metastasis; an Uncommon but Dangerous Pitfall. Am. J. Dermatopathol. 2017, 39, 760–763. [Google Scholar] [CrossRef]
- Truong, L.D.; Rangdaeng, S.; Cagle, P.; Ro, J.Y.; Hawkins, H.; Font, R.L. The Diagnostic Utility of Desmin: A Study of 584 Cases and Review of the Literature. Am. J. Clin. Pathol. 1990, 93, 305–314. [Google Scholar] [CrossRef]
- Prieto-Torres, L.; Alegría-Landa, V.; Llanos, C.; Córdoba, A.; Kutzner, H.; Requena, L. Cutaneous Malignant Melanoma with Rhabdoid Morphology and Smooth Muscle Differentiation: A Challenging Histopathologic Diagnosis. Am. J. Dermatopathol. 2017, 39, 397–403. [Google Scholar] [CrossRef]
- Smith, S.M.; Schmitt, A.C.; Carrau, R.L.; Iwenofu, O.H. Primary Sinonasal Mucosal Melanoma with Aberrant Diffuse and Strong Desmin Reactivity: A Potential Diagnostic Pitfall! Head Neck Pathol. 2014, 9, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Trevisan, F.; Tregnago, A.C.; Pinto, C.A.L.; Urvanegia, A.C.M.; Morbeck, D.L.; Bertolli, E.; Neto, F.R.R.; Neto, J.P.D.; de Macedo, M.P. Osteogenic Melanoma with Desmin Expression. Am. J. Dermatopathol. 2017, 39, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.C.; Carter, J.M.; Folpe, A.L. Aberrant intermediate filament and synaptophysin expression is a frequent event in malignant melanoma: An immunohistochemical study of 73 cases. Mod. Pathol. 2015, 28, 1033–1042. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.S.; Harris, M. Morphological and immunophenotypic variations in malignant melanoma. Histopathology 2000, 36, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Riccioni, L.; Di Tommaso, L.; Collina, G. Actin-Rich Desmoplastic Malignant Melanoma. Am. J. Dermatopathol. 1999, 21, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Okamoto-Inoue, M.; Nakayama, J.; Hori, Y.; Taniguchi, S. Human malignant melanoma cells release a factor that inhibits the expression of smooth muscle α-actin. J. Dermatol. Sci. 2000, 23, 170–177. [Google Scholar] [CrossRef]
- Lee, H.; Torres, F.X.; McLean, S.A.; Chen, R.; Lee, M.W. Immunophenotypic Heterogeneity of Primary Sinonasal Melanoma with Aberrant Expression of Neuroendocrine Markers and Calponin. Appl. Immunohistochem. Mol. Morphol. 2011, 19, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Eyden, B.; Pandit, D.; Banerjee, S.S. Malignant melanoma with neuroendocrine differentiation: Clinical, histological, immuno-histochemical and ultrastructural features of three cases. Histopathology 2005, 47, 402–409. [Google Scholar] [CrossRef]
- Katerji, H.; Childs, J.M.; Bratton, L.E.; Peyre, C.G.; Huber, A.R. Primary Esophageal Melanoma with Aberrant CD56 Expression: A Potential Diagnostic Pitfall. Case Rep. Phatol. 2017, 2017, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Lewin, K.J. Miscellaneous tumors of the esophagus. Tumors of the esophagus and stomach. In Atlas of Tumor Pathology, 3rd ed.; Armed Forces Institute of Pathology: Washington, DC, USA, 1996; pp. 3–16. [Google Scholar]
- Steppert, C.; Krugmann, J.; Sterlacci, W. Simultaneous endocrine expression and loss of melanoma markers in malignant melanoma metastases, a retrospective analysis. Pathol. Oncol. Res. 2019, 26, 1777–1779. [Google Scholar] [CrossRef] [PubMed]
- Gatter, K.C.; Ralfkiaer, E.; Skinner, J.; Brown, D.; Heryet, A.; A Pulford, K.; Hou-Jensen, K.; Mason, D.Y. An immunocytochemical study of malignant melanoma and its differential diagnosis from other malignant tumours. J. Clin. Pathol. 1985, 38, 1353–1357. [Google Scholar] [CrossRef] [Green Version]
- Miettinen, M.; Franssila, K. Immunohistochemical spectrum of malignant melanoma. The common presence of keratins. Lab. Investig. 1989, 61, 623–628. [Google Scholar]
- Zarbo, R.J.; Gown, A.M.; Nagle, R.B.; Visscher, D.W.; Crissman, J.D. Anomalous cytokeratin expression in malignant melanoma: One- and two-dimensional western blot analysis and immunohistochemical survey of 100 melanomas. Mod. Pathol. 1990, 3, 494–501. [Google Scholar]
- Plotzke, J.M.; Zhao, R.; Hrycaj, S.M.; Harms, P.W.; Mehra, R.; Chan, M.P. Immunohistochemical expression of PAX8, PAX2, and cytokeratin in melanomas. J. Cutan. Pathol. 2021. [Google Scholar] [CrossRef]
- Saggini, A.; Cerroni, L.; Casini, B.; Baciorri, F.; Cota, C. Primary intrafascial desmoplastic melanoma with pseudoglandular differentiation and aberrant cytokeratins expression: An exceptional presentation. Pathol. Res. Pract. 2019, 215, 152668. [Google Scholar] [CrossRef] [PubMed]
- Krugmann, J.; Sterlacci, W.; Veits, L.; Vieth, M.; Rieker, R.J.; Altmann, T.; Sokolowski, T.; Fend, F. Aberrant CD68 expression is a rare pitfall in the diagnosis of primary amelanotic malignant melanoma in ascites fluid. Cytopathology 2015, 27, 295–296. [Google Scholar] [CrossRef] [PubMed]
- Facchetti, F.; Bertalot, G.; Grigolato, P. KP1 (CD 68) staining of malignant melanomas. Histopathology 1991, 19, 141–145. [Google Scholar] [CrossRef]
- Shah, M.I.A.; Gani, M.O.S.; Wheler, R.L. Comparative Immunoreactivity of CO-68 and HMB-45 in Malignant Melanoma, Neural Tumors and Nevi. Pathol. Res. Pract. 1997, 193, 497–502. [Google Scholar] [CrossRef]
- Jensen, T.O.; Schmidt, H.; Møller, H.J.; Høyer, M.; Maniecki, M.B.; Sjoegren, P.; Christensen, I.J.; Steiniche, T. Macrophage Markers in Serum and Tumor Have Prognostic Impact in American Joint Committee on Cancer Stage I/II Melanoma. J. Clin. Oncol. 2009, 27, 3330–3337. [Google Scholar] [CrossRef]
- Pisacane, A.M.; Picciotto, F.; Risio, M. CD31 and CD34 Expression as Immunohistochemical Markers of Endothelial Transdifferentiation in Human Cutaneous Melanoma. Anal. Cell. Pathol. 2007, 29, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Salven, P.; Heikkilä, P.; Joensuu, H. Enhanced expression of vascular endothelial growth factor in metastatic melanoma. Br. J. Cancer 1997, 76, 930–934. [Google Scholar] [CrossRef] [Green Version]
- Hoang, M.P.; Selim, M.A.; Bentley, R.C.; Burchette, J.L.; Shea, C.R. CD34 expression in desmoplastic melanoma. J. Cutan. Pathol. 2001, 28, 508–512. [Google Scholar] [CrossRef]
- Breza, T.S.; Magro, C.M. CD34 expression in primary cutaneous malignant melanoma: Apropos of a case and review of the ab-errant melanoma phenotype. J. Cutan. Pathol. 2005, 32, 685–689. [Google Scholar] [CrossRef]
- Chatzopoulos, K.; O’Brien, D.R.; Sotiriou, S.; Khazaie, K.; Jen, J.; Kocher, J.A.; Markovic, S.N.; Flotte, T.J. Aberrant immunohistochemical expression of CD4 as a rare finding in metastatic melanoma. J. Cutan. Pathol. 2020, 47, 1223–1226. [Google Scholar] [CrossRef]
- Fang, D.; Nguyen, T.K.; Leishear, K.; Finko, R.; Kulp, A.N.; Hotz, S.; Van Belle, P.A.; Xu, X.; Elder, D.E.; Herlyn, M. A Tumorigenic Subpopulation with Stem Cell Properties in Melanomas. Cancer Res. 2005, 65, 9328–9337. [Google Scholar] [CrossRef] [Green Version]
- Ramani, N.; Aung, P.P.; Hwu, W.-J.; Nagarajan, P.; Tetzlaff, M.T.; Curry, J.L.; Ivan, D.; Prieto, V.G.; Torres-Cabala, C.A. Aberrant expression of FLI-1 in melanoma. J. Cutan. Pathol. 2017, 44, 790–793. [Google Scholar] [CrossRef]
- Rossi, S.; Orvieto, E.; Furlanetto, A.; Laurino, L.; Ninfo, V.; Tos, A.D. Utility of the immunohistochemical detection of FLI-1 expression in round cell and vascular neoplasm using a monoclonal antibody. Mod. Pathol. 2004, 17, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Torlakovic, E.E.; Slipicevic, A.; Flørenes, V.A.; Chibbar, R.; DeCoteau, J.F.; Bilalovic, N. Fli-1 expression in malignant melanoma. Histol. Histopathol. 2008, 1309–1314. [Google Scholar] [CrossRef]
- Alkhasawneh, A.; Nassri, A.B.; John, I. Dedifferentiated Melanoma with Expression of Cytokeratin and GATA3 in a Patient with History of Breast Carcinoma. Am. J. Dermatopathol. 2019, 41, 502–504. [Google Scholar] [CrossRef]
- Selby, W.L.; Nance, K.V.; Park, H.K. CEA immunoreactivity in metastatic malignant melanoma. Mod. Pathol. 1992, 5, 415–419. [Google Scholar]
- Sanders, D.S.A.; Evans, A.T.; Allen, C.A.; Bryant, F.J.; Johnson, G.D.; Hopkins, J.; Stocks, S.C.; Marsden, J.R.; Kerr, M.A. Classification of CEA-related positivity in primary and metastatic malignant melanoma. J. Pathol. 1994, 172, 343–348. [Google Scholar] [CrossRef]
- Ihn, H. Clinical Usefulness of Immunostaining with CEA in Distinction between Malignant Melanoma and Spitz Nevus. Investig. Dermatol. Venereol. Res. 2016, 2, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.S.; Ong, S. An interesting case of melanoma with divergent differentiation aberrantly expressing calretinin stain. Proc. Singap. Healthc. 2020, 29, 139–141. [Google Scholar] [CrossRef] [Green Version]
| Loss of Melanocytic Markers |
|---|
| Primary melanoma including desmoplastic |
| Metastatic melanoma |
| Aberrant Expression of Non-Melanocytic Markers |
| Muscle-specific markers |
| Desmin and α-SMA |
| Calponin |
| Neuroendocrine markers |
| Neurofilament protein, glial fibrillary acidic protein, synaptophysin, and chromogranin |
| CD56 |
| Keratin |
| CK AE1/AE3, OSCAR, and CAM 5.2 |
| Macrophage markers |
| CD68 and CD163 |
| Vascular markers |
| CD31 and CD34 |
| Hematopoietic markers |
| CD4 |
| CD20 |
| Miscellaneous |
| FLI-1 |
| GATA3 |
| CEA |
| Calretinin |
| PAX8 and PAX2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saliba, E.; Bhawan, J. Aberrant Expression of Immunohistochemical Markers in Malignant Melanoma: A Review. Dermatopathology 2021, 8, 359-370. https://doi.org/10.3390/dermatopathology8030040
Saliba E, Bhawan J. Aberrant Expression of Immunohistochemical Markers in Malignant Melanoma: A Review. Dermatopathology. 2021; 8(3):359-370. https://doi.org/10.3390/dermatopathology8030040
Chicago/Turabian StyleSaliba, Elie, and Jag Bhawan. 2021. "Aberrant Expression of Immunohistochemical Markers in Malignant Melanoma: A Review" Dermatopathology 8, no. 3: 359-370. https://doi.org/10.3390/dermatopathology8030040
APA StyleSaliba, E., & Bhawan, J. (2021). Aberrant Expression of Immunohistochemical Markers in Malignant Melanoma: A Review. Dermatopathology, 8(3), 359-370. https://doi.org/10.3390/dermatopathology8030040







