Bilateral Nipple Enlargement as a Secondary Effect of Anabolic Drugs: A Histopathological Mimicker of Smooth Muscle Hamartoma
Abstract
1. Introduction
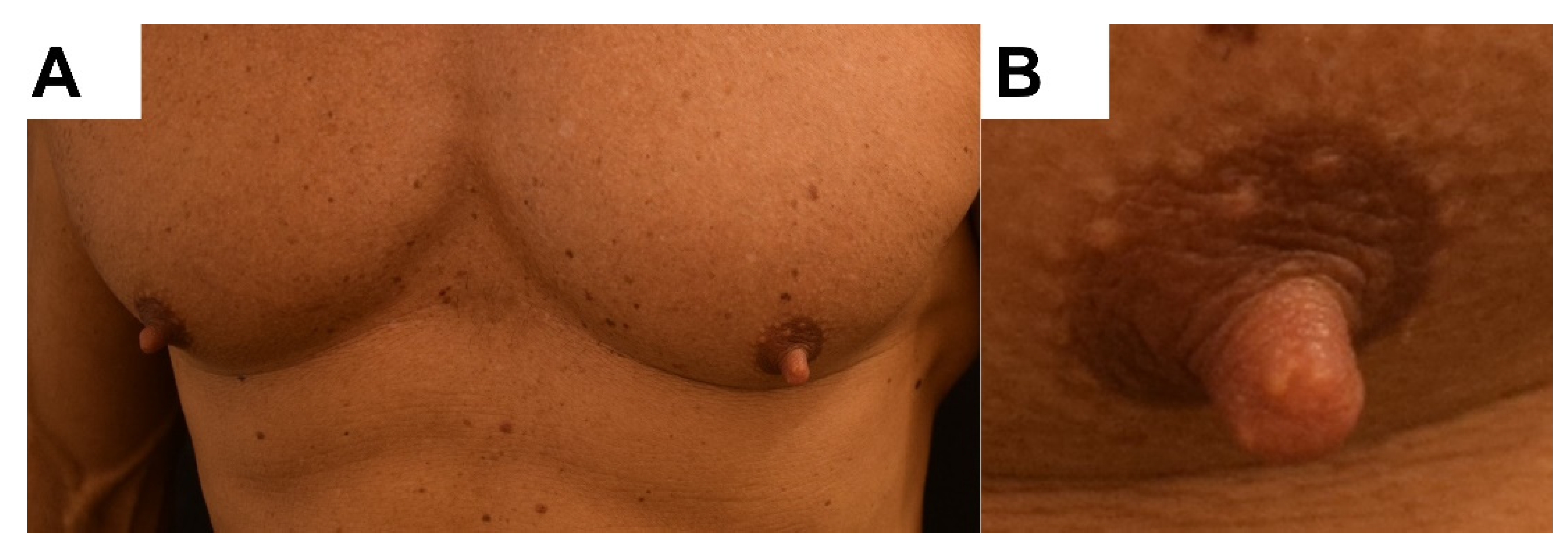
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Guillot, B.; Huet, P.; Joujoux, J.; Lorette, G. Multiple congenital smooth-muscle hamartomas. Ann. Dermatol. Venereol. 1998, 125, 118–120. [Google Scholar]
- Fernandez-Flores, A.; Saeb-Lima, M. Combined cutaneous smooth muscle hamartoma and nevus flammeus. J. Cutan. Pathol. 2014, 41, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Wallach, E. Naevus musclarie generalise avec aspect clinique de “bebe Michelin”. Ann. Dermatol. Venereol. 1980, 107, 923–927. [Google Scholar] [PubMed]
- Gualandri, L.; Cambiaghi, S.; Ermacora, E.; Tadini, G.; Gianotti, R.; Caputo, R. Multiple familial smooth muscle hamartomas. Pediatr. Dermatol. 2001, 18, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Ramos Rodriguez, A.J.; Guo, R.; Bridges, A.G. Estrogen and progesterone receptor-positive bilateral nipple leiomyoma in a man. Int. J. Dermatol. 2017, 56, 1512–1513. [Google Scholar] [CrossRef] [PubMed]
- Deveci, U.; Kapakli, M.S.; Altintoprak, F.; Cayirci, M.; Manukyan, M.N.; Kebudi, A. Bilateral nipple leiomyoma. Case Rep. Surg. 2013, 2013, 475215. [Google Scholar] [CrossRef] [PubMed]
- Newman, P.L.; Fletcher, C.D. Smooth muscle tumours of the external genitalia: Clinicopathological analysis of a series. Histopathology 1991, 18, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Carpintero, I.; Mihm, M.C.; Mizeracki, A.; Waner, M.; North, P.E. Epithelial and mesenchymal hamartomatous changes in a mature port-wine stain: Morphologic evidence for a multiple germ layer field defect. J. Am. Acad. Dermatol. 2004, 50, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Quinn, T.R.; Young, R.H. Smooth-muscle hamartoma of the tunica dartos of the scrotum: Report of a case. J. Cutan. Pathol. 1997, 24, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Dal Vechio, A.; Nakajima, E.; Pinto, D.; Azevedo, L.H.; Migliari, D.A. Rhabdomyomatous (mesenchymal) hamartoma presenting as haemangioma on the upper lip: A case report with immunohistochemical analysis and treatment with high-power lasers. Case Rep. Dent. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Tièche, M. Über benigne Melanome (“Chromatophorome”) der Haut—“blaue Naevi”. Virchows Arch. Pathol. Anat. Und Physiol. Und Klin. Med. 1906, 186, 212–229. [Google Scholar] [CrossRef]
- Tzu, J.; Goldman, C.; Perry, A.E.; Meehan, S.A. Combined blue nevus-smooth muscle hamartoma: A series of 12 cases. J. Cutan. Pathol. 2013, 40, 879–883. [Google Scholar] [CrossRef]
- Townsend, M.; Wald, J.; Murphy, M.; Kristjansson, A. Blue Nevus-Smooth Muscle Hamartoma: A Rarely Reported Entity. Am. J. Dermatol. 2015, 37, 662–663. [Google Scholar] [CrossRef]
- Ferran, M.; Tribó, M.J.; González-Rivero, M.A.; Alameda, F.; Pujol, R.M. Congenital hamartoma of the scalp with meningothelial, sebaceus, muscular, and immature glandular components. Am. J. Dermatol. 2007, 29, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Sevim, Y.; Kocaay, A.F.; Eker, T.; Celasin, H.; Karabork, A.; Erden, E.; Genc, V. Breast hamartoma: A clinicopathologic analysis of 27 cases and a literature review. Clinics 2014, 69, 515–523. [Google Scholar] [CrossRef]
- Huang, W.-C.; Yu, C.-M.; Chang, Y.-Y. Geometric incision design for reduction nippleplasty. Aesthetic Plast. Surg. 2012, 36, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Agostini, T.; Perello, R.; Famiglietti, M.; Li, A. Reconstructive, Surgery A. Six factors justify the pathologic analysis of subcutaneous mastectomy specimens in patients with gynaecomastia. J. Plast Reconstr. Aesthet. Surg. 2014, 67, 1760–1761. [Google Scholar] [CrossRef] [PubMed]
- Christou, M.A.; Christou, P.A.; Markozannes, G.; Tsatsoulis, A.; Mastorakos, G.; Tigas, S. Effects of anabolic androgenic steroids on the reproductive system of athletes and recreational users: A systematic review and meta-analysis. Sports Med. 2017, 47, 1869–1883. [Google Scholar] [CrossRef] [PubMed]
- Nieschlag, E.; Vorona, E. Mechanisms in Endocrinology: Medical consequences of doping with anabolic androgenic steroids (AAS): Effects on reproductive functions. Eur. J. Endocrinol. 2015, 173, R47–R58. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llamas-Velasco, M.; Bianciardi Valassina, M.F.; Ovejero-Merino, E.; Massi, G.; Mentzel, T. Bilateral Nipple Enlargement as a Secondary Effect of Anabolic Drugs: A Histopathological Mimicker of Smooth Muscle Hamartoma. Dermatopathology 2021, 8, 103-106. https://doi.org/10.3390/dermatopathology8020016
Llamas-Velasco M, Bianciardi Valassina MF, Ovejero-Merino E, Massi G, Mentzel T. Bilateral Nipple Enlargement as a Secondary Effect of Anabolic Drugs: A Histopathological Mimicker of Smooth Muscle Hamartoma. Dermatopathology. 2021; 8(2):103-106. https://doi.org/10.3390/dermatopathology8020016
Chicago/Turabian StyleLlamas-Velasco, Mar, Maria Francesca Bianciardi Valassina, Enrique Ovejero-Merino, Guido Massi, and Thomas Mentzel. 2021. "Bilateral Nipple Enlargement as a Secondary Effect of Anabolic Drugs: A Histopathological Mimicker of Smooth Muscle Hamartoma" Dermatopathology 8, no. 2: 103-106. https://doi.org/10.3390/dermatopathology8020016
APA StyleLlamas-Velasco, M., Bianciardi Valassina, M. F., Ovejero-Merino, E., Massi, G., & Mentzel, T. (2021). Bilateral Nipple Enlargement as a Secondary Effect of Anabolic Drugs: A Histopathological Mimicker of Smooth Muscle Hamartoma. Dermatopathology, 8(2), 103-106. https://doi.org/10.3390/dermatopathology8020016





