Mimicking the LOX-Related Autosomal Recessive Congenital Ichthyosis Skin Disease Using a CRISPR-Cas9 System and Unravelling 12S-LOX Function in the Skin
Abstract
1. Introduction
2. Materials and Methods
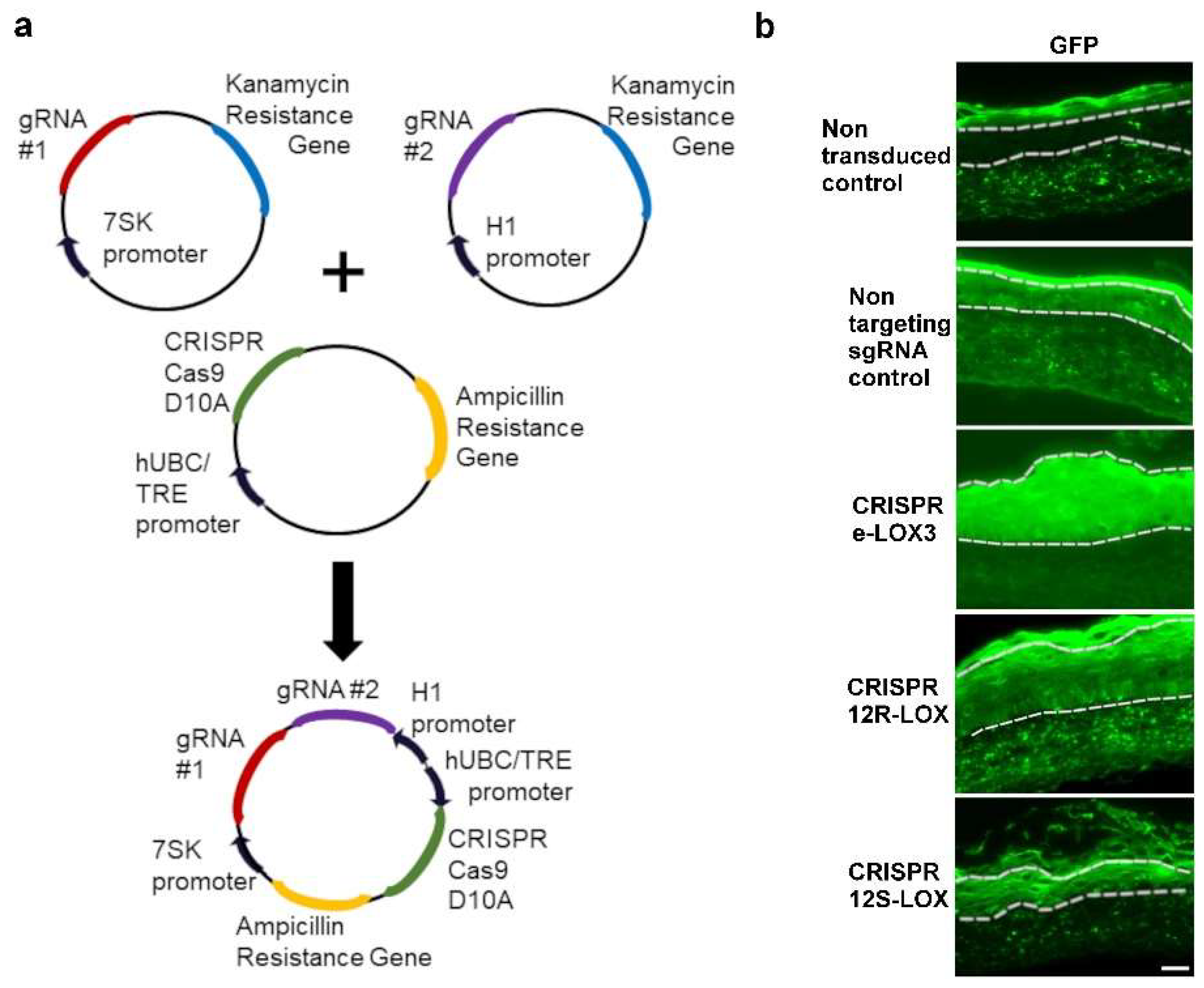
2.1. Production of CRISPR-Cas9(D10A), Lentiviral Vector Production, and Cell Culture
2.2. Skin Biopsies
2.3. Production of CRISPR-LOX Plasmids
2.4. Keratinocyte Transduction, Cell Sorting, and Gene Editing Analysis
2.5. PCR and Mutation Analysis
2.6. Production of TES
2.7. Staining and Immunofluorescence Analysis
2.8. Ultrastructural Analysis
2.9. Statistical Methods
3. Results
3.1. Transduction and Enrichment of Keratinocytes Depleted in the Native Form of Either eLOX3, 12R-, or 12S-LOX Using a CRISPR-Cas9(D10A) Lentiviral System
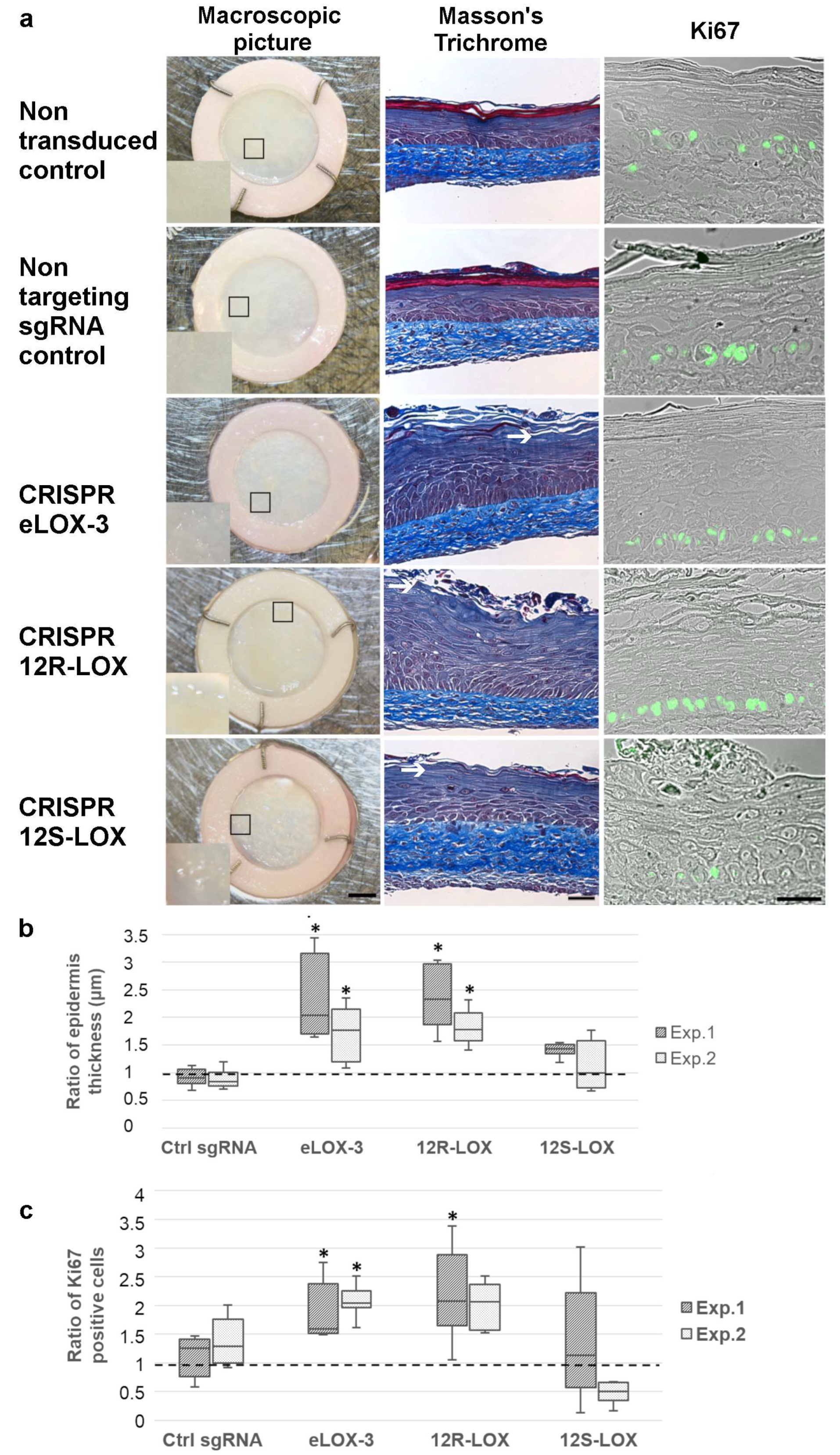
3.2. Impact of the Reduced Expression of the Native Forms of the Selected LOXs on TES Phenotype and Cell Proliferation
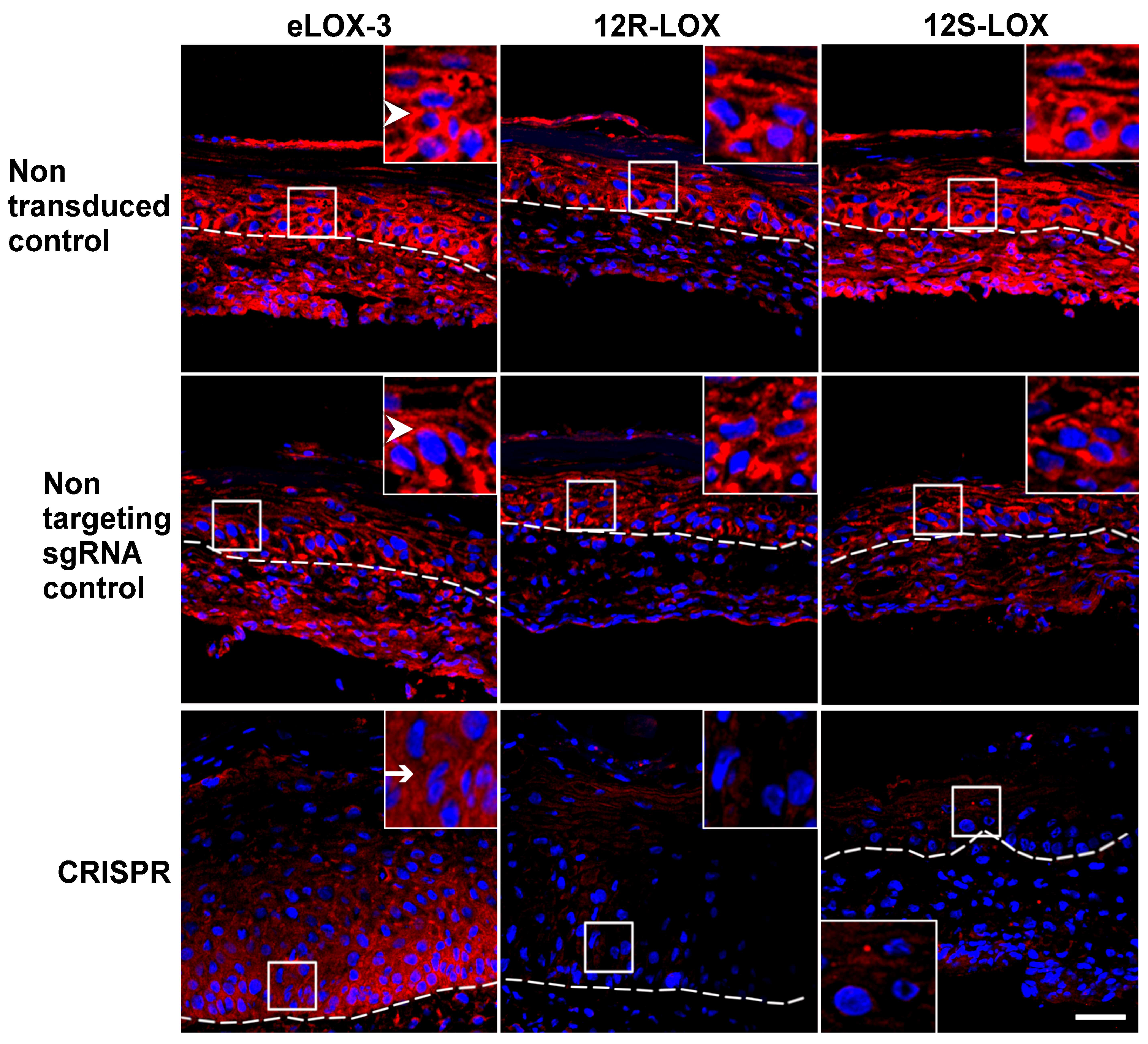
3.3. Impact of the Depletion of the Native Form of the Targeted LOXs on Keratinocyte Differentiation in TES
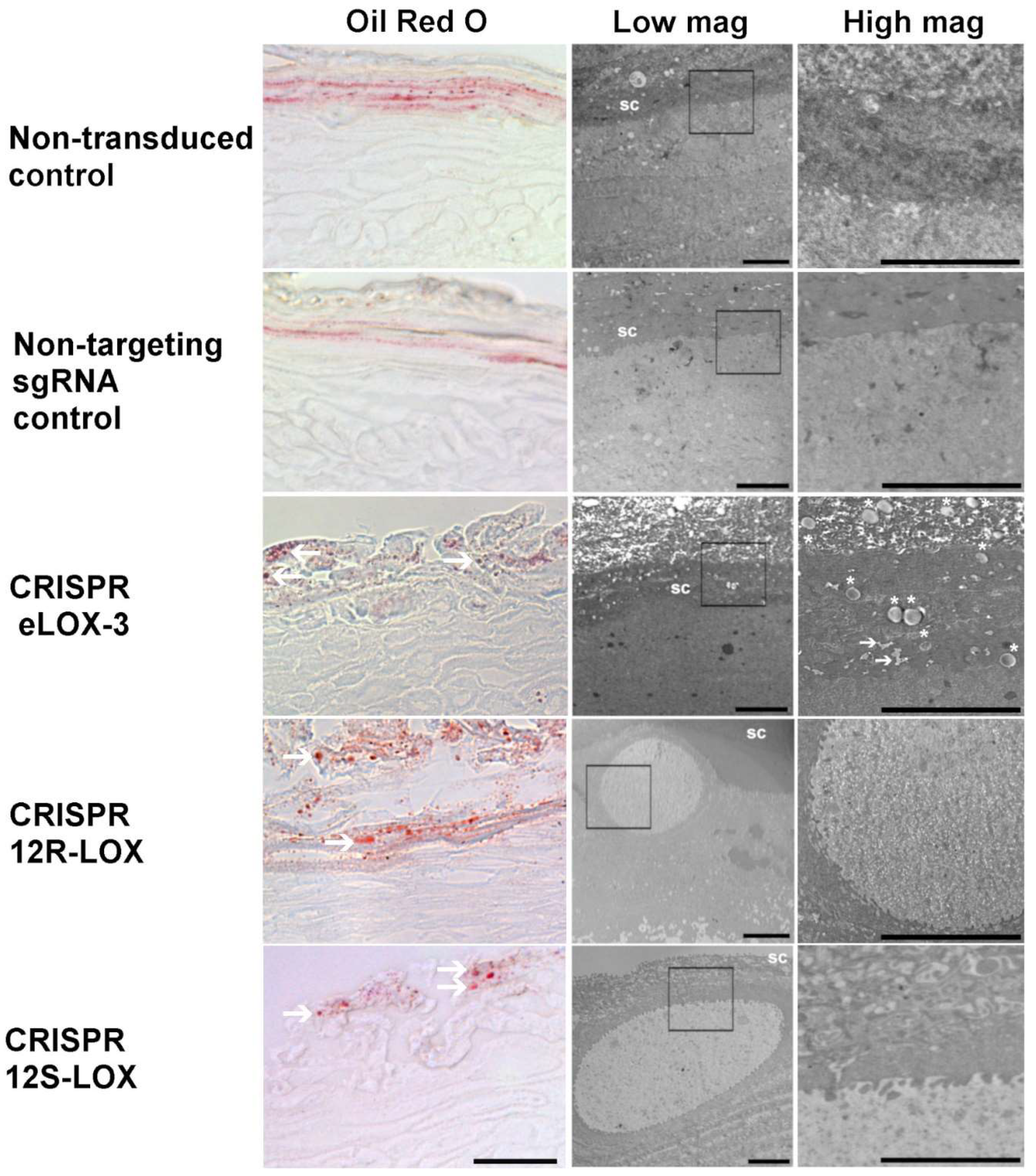
3.4. Lower Levels of the Native Form of the Targeted LOXs in TES Affects Lipid Droplets and Cell Ultrastructure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARCI | Autosomal Recessive Congenital Ichthyosis |
| 12R-LOX | 12R-Lipoxygenase |
| 12S-LOX | 12S-Lipoxygenase |
| CLE | Cornified Lipid Envelope |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| eLOX-3 | Epidermis-type lipoxygenase 3 |
| GFP | Green Fluorescent Protein |
| NCIE | Non-bullous Congenital Ichthyosiform Erythroderma |
| ORO | Oil Red O |
| SC | Stratum Corneum |
| TEM | Transmission Electron Microscopy |
| TES | Tissue-Engineered Skin |
| TEWL | Transepidermal Water Loss |
References
- Takeshi, M.; Masayuki, A. Dissecting the formation, structure and barrier function of the stratum corneum. Int. Immunol. 2015, 27, 269–280. [Google Scholar] [CrossRef]
- Eckhart, L.; Lippens, S.; Tschachler, E.; Declercq, W. Cell death by cornification. Biochim. Biophys. Acta-Mol. Cell. Res. 2013, 1833, 3471–3480. [Google Scholar] [CrossRef]
- Rawlings, A.V.; Lane, M.E.; Voegeli, R. The importance of stratum corneum ω-linoleoyloxyacylceramides in human skin barrier health: Their biochemistry, processing enzymes and metabolites involved in corneocyte lipid envelope maturation. Int. J. Cosmet. Sci. 2024, 46, 623–642. [Google Scholar] [CrossRef] [PubMed]
- Wertz, P.W. Lipid metabolic events underlying the formation of the corneocyte lipid envelope. Skin. Pharmacol. Physiol. 2021, 34, 38–50. [Google Scholar] [CrossRef]
- Akiyama, M. Corneocyte lipid envelope (CLE), the key structure for skin barrier function and ichthyosis pathogenesis. J. Dermatol. Sci. 2017, 88, 3–9. [Google Scholar] [CrossRef]
- Feingold, K.R.; Elias, P.M. Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids. 2014, 1841, 280–294. [Google Scholar] [CrossRef]
- Jobard, F.; Lefèvre, C.; Karaduman, A.; Blanchet-Bardon, C.; Emre, S.; Weissenbach, J.; Özgüc, M.; Lathrop, M.; Prud’homme, J.-F.; Fischer, J. Lipoxygenase-3 (ALOXE3) and 12(R)-lipoxygenase (ALOX12B) are mutated in non-bullous congenital ichthyosiform erythroderma (NCIE) linked to chromosome 17p13.1. Hum. Mol. Genet. 2002, 11, 107–113. [Google Scholar] [CrossRef]
- Akiyama, M.; Sawamura, D.; Shimizu, H. The clinical spectrum of nonbullous congenital ichthyosiform erythroderma and lamellar ichthyosis. Clin. Exp. Dermatol. 2003, 28, 235–240. [Google Scholar] [CrossRef]
- Oji, V.; Tadini, G.; Akiyama, M.; Bardon, C.B.; Bodemer, C.; Bourrat, E.; Coudiere, P.; DiGiovanna, J.J.; Elias, P.; Fischer, J. Revised nomenclature and classification of inherited ichthyoses: Results of the First Ichthyosis Consensus Conference in Sorèze 2009. J. Am. Acad. Dermatol. 2010, 63, 607–641. [Google Scholar] [CrossRef] [PubMed]
- De Juanes, S.; Epp, N.; Latzko, S.; Neumann, M.; Fürstenberger, G.; Hausser, I.; Stark, H.-J.; Krieg, P. Development of an ichthyosiform phenotype in Alox12b-deficient mouse skin transplants. J. Invest. Dermatol. 2009, 129, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Krieg, P.; Dick, A.; Latzko, S.; Rosenberger, S.; Meyer, J.; Crumrine, D.; Hielscher, T.; Elias, P.M.; Rauh, M.; Schneider, H. Conditional Alox12b knockout: Degradation of the corneocyte lipid envelope in a mouse model of autosomal recessive congenital ichthyoses. J. Investig. Dermatol. 2020, 140, 249. [Google Scholar] [CrossRef]
- Krieg, P.; Rosenberger, S.; De Juanes, S.; Latzko, S.; Hou, J.; Dick, A.; Kloz, U.; Van Der Hoeven, F.; Hausser, I.; Esposito, I. Aloxe3 knockout mice reveal a function of epidermal lipoxygenase-3 as hepoxilin synthase and its pivotal role in barrier formation. J. Invest. Dermatol. 2013, 133, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.L.; Qiu, H.; Turbe-Doan, A.; Yun, Y.; Boeglin, W.E.; Brash, A.R.; Beier, D.R. A mouse mutation in the 12R-lipoxygenase, Alox12b, disrupts formation of the epidermal permeability barrier. J. Invest. Dermatol. 2007, 127, 1893–1897. [Google Scholar] [CrossRef]
- Johnson, E.N.; Nanney, L.B.; Virmani, J.; Lawson, J.A.; Funk, C.D. Basal transepidermal water loss is increased in platelet-type 12-lipoxygenase deficient mice. J. Invest. Dermatol. 1999, 112, 861–865. [Google Scholar] [CrossRef]
- Biernacki, M.; Skrzydlewska, E. Metabolic pathways of eicosanoids-derivatives of arachidonic acid and their significance in skin. Cell. Mol. Biol. Letters. 2025, 30, 7. [Google Scholar] [CrossRef]
- Burger, B.; Sagiorato, R.N.; Cavenaghi, I.; Rodrigues, H.G. Abnormalities of sphingolipids metabolic pathways in the pathogenesis of psoriasis. Metabolites 2023, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Shornick, L.P.; Shannon, V.R.; Wilson, J.D.; Funk, C.D.; Pentland, A.P.; Holtzman, M.J. Epidermis contains platelet-type 12-lipoxygenase that is overexpressed in germinal layer keratinocytes in psoriasis. Am. J. Physiol. Cell Physiol. 1994, 266, C243–C253. [Google Scholar] [CrossRef] [PubMed]
- Krieg, P.; Fürstenberger, G. The physiology and pathophysiology of lipoxygenases in the skin. In Lipoxygenases in Inflammation, 1st ed.; Steinhilber, D., Ed.; Progress In Inflammation Research; Springer: Cham, Switzerland, 2016; pp. 159–183. [Google Scholar]
- Takeichi, T.; Kinoshita, F.; Tanaka, H.; Fujita, S.; Kobayashi, Y.; Nakatochi, M.; Sugiura, K.; Akiyama, M. The lipoxygenase-hepoxilin pathway is activated in cutaneous plaque lesions of psoriasis. J. Cutan. Immunol. Allergy 2019, 2, 15–24. [Google Scholar] [CrossRef]
- Voegeli, R.; Monneuse, J.M.; Schoop, R.; Summers, B.; Rawlings, A. The effect of photodamage on the female Caucasian facial stratum corneum corneome using mass spectrometry-based proteomics. Int. J. Cosmet. Sci. 2017, 39, 637–652. [Google Scholar] [CrossRef]
- Blunder, S.; Rühl, R.; Moosbrugger-Martinz, V.; Krimmel, C.; Geisler, A.; Zhu, H.; Crumrine, D.; Elias, P.M.; Gruber, R.; Schmuth, M. Alterations in epidermal eicosanoid metabolism contribute to inflammation and impaired late differentiation in FLG-mutated atopic dermatitis. J. Investig. Dermatol. 2017, 137, 706–715. [Google Scholar] [CrossRef]
- Töröcsik, D.; Weise, C.; Gericke, J.; Szegedi, A.; Lucas, R.; Mihaly, J.; Worm, M.; Rühl, R. Transcriptomic and lipidomic profiling of eicosanoid/docosanoid signalling in affected and non-affected skin of human atopic dermatitis patients. Exp. Dermatol. 2019, 28, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Broughton, G.; Jeffrey, J.E.; Attinger, C.E. The basic science of wound healing. Plast. Reconstr. Surg. 2006, 117, 12S–34S. [Google Scholar] [CrossRef] [PubMed]
- Moch, D.; Schewe, T.; Buntrock, P.; Kühn, H. Anti-inflammatory and antiproliferative actions of FLM 5011, a lipoxygenase inhibitor, in a wound-healing model of the rat. Theor. Surg. 1990, 5, 185–191. [Google Scholar]
- Süss, R.; Arenberger, P.; Gross, E.C.; Ruzicka, T. Regulation of 12 (S)-hydroxyeicosatetraenoic acid (12 (S)-HETE) binding sites on human epidermal cells by interferon-γ. Exp. Cell Res. 1990, 191, 204–208. [Google Scholar]
- Burke, E.; Barik, S. Megaprimer PCR: Application in mutagenesis and gene fusion. In PCR protocols, 2nd ed.; Bartlett, J.M.S., Stirling, D., Eds.; Humana Press: Totowa, NJ, USA, 2003; Volume 226, pp. 525–531. [Google Scholar]
- Germain, L.; Rouabhia, M.; Guignard, R.; Carrier, L.; Bouvard, V.; Auger, F.A. Improvement of human keratinocyte isolation and culture using thermolysin. Burns 1993, 19, 99–104. [Google Scholar] [CrossRef]
- Rompré, P.; Auger, F.A.; Germain, L.; Bouvard, V.; Valle, C.A.L.; Thibault, J.; Duy, A. Influence of initial collagen and cellular concentrations on the final surface area of dermal and skin equivalents: A box-behnken analysis. Vitr. Cell. Dev. Biol. 1990, 26, 983–990. [Google Scholar] [CrossRef]
- Magne, B.; Demers, A.; Savard, É.; Lemire-Rondeau, M.; Veillette, N.; Pruneau, V.; Guignard, R.; Morissette, A.; Larouche, D.; Auger, F.A.; et al. Speeding up the production of clinical-grade skin substitutes using off-the-shelf decellularized self-assembled dermal matrices. Acta Biomater. 2023, 167, 249–259. [Google Scholar] [CrossRef]
- Goyer, B.; Larouche, D.; Kim, D.H.; Veillette, N.; Pruneau, V.; Bernier, V.; Auger, F.A.; Germain, L. Immune tolerance of tissue-engineered skin produced with allogeneic or xenogeneic fibroblasts and syngeneic keratinocytes grafted on mice. Acta Biomater. 2019, 90, 192–204. [Google Scholar] [CrossRef]
- Basic Local Alignment Search Tool. Available online: "https://blast.ncbi.nlm.nih.gov/" (accessed on 7 November 2019).
- Concordet, J.-P.; Haeussler, M. CRISPOR: Intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res. 2018, 46, W242–W245. [Google Scholar] [CrossRef]
- Kabadi, A.M.; Ousterout, D.G.; Hilton, I.B.; Gersbach, C.A. Multiplex CRISPR/Cas9-based genome engineering from a single lentiviral vector. Nucleic Acids Res. 2014, 42, e147. [Google Scholar] [CrossRef] [PubMed]
- Simard-Bisson, C.; Bidoggia, J.; Larouche, D.; Guérin, S.L.; Blouin, R.; Hirai, S.-I.; Germain, L. A Role for DLK in Microtubule Reorganization to the Cell Periphery and in the Maintenance of Desmosomal and Tight Junction Integrity. J. Investig. Dermatol. 2017, 137, 132–141. [Google Scholar] [CrossRef] [PubMed]
- ICE CRISPR Analysis. Available online: https://www.synthego.com/products/bioinformatics/analysis (accessed on 29 August 2025).
- Attiogbe, E.; Larochelle, S.; Chaib, Y.; Mainzer, C.; Mauroux, A.; Bordes, S.; Closs, B.; Gilbert, C.; Moulin, V.J. An in vitro autologous, vascularized, and immunocompetent Tissue Engineered Skin model obtained by the self-assembled approach. Acta Biomater. 2023, 168, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Sumigray, K.D.; Chen, H.; Lechler, T. Lis1 is essential for cortical microtubule organization and desmosome stability in the epidermis. J. Cell Biol. 2011, 194, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Simard-Bisson, C.; Parent, L.A.; Moulin, V.J.; de Laclos, B.F. Characterization of Epidermal Lipoxygenase Expression in Normal Human Skin and Tissue-Engineered Skin Substitutes. J. Histochem. Cytochem. 2018, 66, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Eckl, K.-M.; Krieg, P.; Küster, W.; Traupe, H.; André, F.; Wittstruck, N.; Fürstenberger, G.; Hennies, H.C. Mutation spectrum and functional analysis of epidermis-type lipoxygenases in patients with autosomal recessive congenital ichthyosis. Hum. Mutat. 2005, 26, 351–361. [Google Scholar] [CrossRef]
- Black, A.F.; Berthod, F.; L’Heureux, N.; Germain, L.; Auger, F.A. In vitro reconstruction of a human capillary-like network in a tissue-engineered skin equivalent. FASEB J. 1998, 12, 1331–1340. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Lin, C.-Y.; Gootenberg, J.S.; Konermann, S.; Trevino, A.E.; Scott, D.A.; Inoue, A.; Matoba, S.; Zhang, Y.; et al. Double Nicking by RNA-Guided CRISPR Cas9 for Enhanced Genome Editing Specificity. Cell 2013, 154, 1380–1389. [Google Scholar] [CrossRef]
- Wang, T.; Wei, J.J.; Sabatini, D.M.; Lander, E.S. Genetic Screens in Human Cells Using the CRISPR-Cas9 System. Science 2014, 343, 80–84. [Google Scholar] [CrossRef]
- Alonso, L.; Marquezin, C.A.; Gonçalves, P.J.; Alonso, A. Transmittance and Autofluorescence of Neonatal Rat Stratum Corneum: Nerolidol Increases the Dynamics and Partitioning of Protoporphyrin IX into Intercellular Membranes. J. Fluoresc. 2016, 26, 709–717. [Google Scholar] [CrossRef]
- Makarov, M.; Storozheva, M.; Borovkova, N. Collagen Fiber Autofluorescence Level in Evaluating the Biological Properties of Tissue Grafts. Sovrem. Teh. v Med. 2017, 9, 83. [Google Scholar] [CrossRef]
- A Dale, B.; A Holbrook, K.; Kimball, J.R.; Hoff, M.; Sun, T.T. Expression of epidermal keratins and filaggrin during human fetal skin development. J. Cell Biol. 1985, 101, 1257–1269. [Google Scholar] [CrossRef]
- Wang, T.; Xu, C.; Zhou, X.; Li, C.; Zhang, H.; Lian, B.Q.; Lee, J.J.; Shen, J.; Liu, Y.; Lian, C.G. Homozygous ALOXE3 Nonsense Variant Identified in a Patient with Non-Bullous Congenital Ichthyosiform Erythroderma Complicated by Superimposed Bullous Majocchi’s Granuloma: The Consequences of Skin Barrier Dysfunction. Int. J. Mol. Sci. 2015, 16, 21791–21801. [Google Scholar] [CrossRef]
- Kuri-Harcuch, W.; Ramírez-Zacarías, J.L.; Castro-Muñozledo, F. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with oil red O. Histochem 1992, 97, 493–497. [Google Scholar] [CrossRef]
- Mehlem, A.; E Hagberg, C.; Muhl, L.; Eriksson, U.; Falkevall, A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat. Protoc. 2013, 8, 1149–1154. [Google Scholar] [CrossRef]
- Carriel, V.; Campos, F.; Aneiros-Fernández, J.; Kiernan, J.A. Tissue Fixation and Processing for the Histological Identification of Lipids. Beta-Arrestins 2017, 1560, 197–206. [Google Scholar] [CrossRef]
- Fenini, G.; Grossi, S.; Contassot, E.; Biedermann, T.; Reichmann, E.; French, L.E.; Beer, H.-D. Genome Editing of Human Primary Keratinocytes by CRISPR/Cas9 Reveals an Essential Role of the NLRP1 Inflammasome in UVB Sensing. J. Investig. Dermatol. 2018, 138, 2644–2652. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, V.; Chacón-Solano, E.; Bonafont, J.; Mencía, Á.; Di, W.-L.; Murillas, R.; Llames, S.; Vicente, A.; Del Rio, M.; Carretero, M.; et al. Efficient CRISPR-Cas9-Mediated Gene Ablation in Human Keratinocytes to Recapitulate Genodermatoses: Modeling of Netherton Syndrome. Mol. Ther. -Methods Clin. Dev. 2020, 18, 280–290. [Google Scholar] [CrossRef]
- Robitaille, H.; Simard-Bisson, C.; Larouche, D.; Tanguay, R.M.; Blouin, R.; Germain, L. The Small Heat-Shock Protein Hsp27 Undergoes ERK-Dependent Phosphorylation and Redistribution to the Cytoskeleton in Response to Dual Leucine Zipper-Bearing Kinase Expression. J. Investig. Dermatol. 2010, 130, 74–85. [Google Scholar] [CrossRef]
- Aushev, M.; Koller, U.; Mussolino, C.; Cathomen, T.; Reichelt, J. Traceless Targeting and Isolation of Gene-Edited Immortalized Keratinocytes from Epidermolysis Bullosa Simplex Patients. Mol. Ther. -Methods Clin. Dev. 2017, 6, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.K.; Uppala, R.; Zeng, C.; Billi, A.C.; Tsoi, L.C.; Kidder, A.; Xing, X.; White, B.E.P.; Shao, S.; Plazyo, O.; et al. Keratinocytes sense and eliminate CRISPR DNA through STING/IFN-κ activation and APOBEC3G induction. J. Clin. Investig. 2023, 133. [Google Scholar] [CrossRef] [PubMed]
- Nasrallah, A.; Rezvani, H.-R.; Kobaisi, F.; Hammoud, A.; Rambert, J.; Smits, J.P.H.; Sulpice, E.; Rachidi, W. Generation and characterization of CRISPR-Cas9-mediated XPC gene knockout in human skin cells. Sci. Rep. 2024, 14, 1–21. [Google Scholar] [CrossRef]
- Alipour, F.; Ahmadraji, M.; Yektadoust, E.; Mohammadi, P.; Baharvand, H.; Basiri, M. CRISPR/Cas9-Mediated Generation of COL7A1-Deficient Keratinocyte Model of Recessive Dystrophic Epidermolysis Bullosa. Cell J. 2023, 25, 665–673. [Google Scholar]
- Eckl, K.-M.; Alef, T.; Torres, S.; Hennies, H.C. Full-thickness human skin models for congenital ichthyosis and related keratinization disorders. J. Investig. Dermatol. 2011, 131, 1938–1942. [Google Scholar] [CrossRef]
- Youssef, G.; Ono, M.; Brown, S.J.; Kinsler, V.A.; Sebire, N.J.; Harper, J.I.; O’Shaughnessy, R.F. Identifying a hyperkeratosis signature in autosomal recessive congenital ichthyosis: Mdm2 inhibition prevents hyperkeratosis in a rat ARCI model. J. Investig. Dermatol. 2014, 134, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Cerrajero, C.; Sprecher, E.; Paller, A.S.; Akiyama, M.; Mazereeuw-Hautier, J.; Hernández-Martín, A.; González-Sarmiento, R. Ichthyosis. Nat. Rev. Dis. Primers 2023, 9, 2. [Google Scholar] [CrossRef]
- Dahlqvist, J.; Klar, J.; Hausser, I.; Anton-Lamprecht, I.; Pigg, M.H.; Gedde-Dahl, T.; Gånemo, A.; Vahlquist, A.; Dahl, N. Congenital ichthyosis: Mutations in ichthyin are associated with specific structural abnormalities in the granular layer of epidermis. J. Med. Genet. 2007, 44, 615–620. [Google Scholar] [CrossRef]
- Harting, M.; Brunetti-Pierri, N.; Chan, S.C.; Kirby, J.; Dishop, M.K.; Richard, G.; Scaglia, F.; Yan, A.C.; Levy, M.L. Self-healing collodion membrane and mild nonbullous congenital ichthyosiform erythroderma due to 2 novel mutations in the ALOX12B gene. Arch. Dermatol. 2008, 144, 351–356. [Google Scholar] [CrossRef]
- Epp, N.; Fürstenberger, G.; Müller, K.; de Juanes, S.; Leitges, M.; Hausser, I.; Thieme, F.; Liebisch, G.; Schmitz, G.; Krieg, P. 12R-lipoxygenase deficiency disrupts epidermal barrier function. J. Cell Biol. 2007, 177, 173–182. [Google Scholar] [CrossRef]
- Ponec, M.; Kempenaar, J.; Weerheim, A.; Boonstra, J. Differentiation of human keratinocytes: Changes in lipid synthesis, plasma membrane lipid composition, and 125I-EGF binding upon administration of 25-hydroxycholesterol and mevinolin. J. Cell. Physiol. 1987, 133, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Li, Y.; Jin, G.; Huang, T.; Zou, M.; Duan, S. The biological role of arachidonic acid 12-lipoxygenase (ALOX12) in various human diseases. Biomed. Pharmacother. 2020, 129, 110354. [Google Scholar] [CrossRef] [PubMed]
- Stefanovic, N.; Irvine, A.D. Filaggrin and beyond: New insights into the skin barrier in atopic dermatitis and allergic diseases, from genetics to therapeutic perspectives. Ann. Allergy Asthma Immunol. 2024, 132, 187–195. [Google Scholar] [CrossRef]
- Moosbrugger-Martinz, V.; Leprince, C.; Méchin, M.-C.; Simon, M.; Blunder, S.; Gruber, R.; Dubrac, S. Revisiting the roles of filaggrin in atopic dermatitis. Int. J. Mol. Sci. 2022, 23, 5318. [Google Scholar] [CrossRef]
- Perusquía-Ortiz, A.; Oji, V.; Sauerland, M.; Tarinski, T.; Zaraeva, I.; Seller, N.; Metze, D.; Aufenvenne, K.; Hausser, I.; Traupe, H. Complete filaggrin deficiency in ichthyosis vulgaris is associated with only moderate changes in epidermal permeability barrier function profile. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 1552–1558. [Google Scholar] [CrossRef]
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Gross, E.; Ruzicka, T.; Restorff, B.V.; Stolz, W.; Klotz, K.-N. High-affinity binding and lack of growth-promoting activity of 12 (S)-hydroxyeicosatetraenoic acid (12 (S)-HETE) in a human epidermal cell line. J. Investig. Dermatol. 1990, 94, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Simard, M.; Tremblay, A.; Morin, S.; Martin, C.; Julien, P.; Fradette, J.; Flamand, N.; Pouliot, R. α-Linolenic acid and linoleic acid modulate the lipidome and the skin barrier of a tissue-engineered skin model. Acta Biomater. 2022, 140, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, E. CRISPR-Cas9: How research on a bacterial RNA-guided mechanism opened new perspectives in biotechnology and biomedicine. EMBO Mol. Med. 2015, 7, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Bédard, P.; Gauvin, S.; Ferland, K.; Caneparo, C.; Pellerin, È.; Chabaud, S.; Bolduc, S. Innovative human three-dimensional tissue-engineered models as an alternative to animal testing. Bioengineering 2020, 7, 115. [Google Scholar] [CrossRef]
- Auger, F.A.; Berthod, F.; Moulin, V.; Pouliot, R.; Germain, L. Tissue-engineered skin substitutes: From in vitro constructs to in vivo applications. Biotech. Appl. Biochem. 2004, 39, 263–275. [Google Scholar] [CrossRef]
- Mok, B.R.; Shon, S.-J.; Kim, A.R.; Simard-Bisson, C.; Martel, I.; Germain, L.; Kim, D.H.; Shin, J.U. Structural and functional validation of a full-thickness self-assembled skin equivalent for disease modeling. Pharmaceutics 2022, 14, 1211. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the European Society of Dermatopathology. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simard-Bisson, C.; Larochelle, S.; Moulin, V.J.; Fruteau de Laclos, B. Mimicking the LOX-Related Autosomal Recessive Congenital Ichthyosis Skin Disease Using a CRISPR-Cas9 System and Unravelling 12S-LOX Function in the Skin. Dermatopathology 2025, 12, 30. https://doi.org/10.3390/dermatopathology12030030
Simard-Bisson C, Larochelle S, Moulin VJ, Fruteau de Laclos B. Mimicking the LOX-Related Autosomal Recessive Congenital Ichthyosis Skin Disease Using a CRISPR-Cas9 System and Unravelling 12S-LOX Function in the Skin. Dermatopathology. 2025; 12(3):30. https://doi.org/10.3390/dermatopathology12030030
Chicago/Turabian StyleSimard-Bisson, Carolyne, Sébastien Larochelle, Véronique J. Moulin, and Bernard Fruteau de Laclos. 2025. "Mimicking the LOX-Related Autosomal Recessive Congenital Ichthyosis Skin Disease Using a CRISPR-Cas9 System and Unravelling 12S-LOX Function in the Skin" Dermatopathology 12, no. 3: 30. https://doi.org/10.3390/dermatopathology12030030
APA StyleSimard-Bisson, C., Larochelle, S., Moulin, V. J., & Fruteau de Laclos, B. (2025). Mimicking the LOX-Related Autosomal Recessive Congenital Ichthyosis Skin Disease Using a CRISPR-Cas9 System and Unravelling 12S-LOX Function in the Skin. Dermatopathology, 12(3), 30. https://doi.org/10.3390/dermatopathology12030030





