“Chasing Rainbows” Beyond Kaposi Sarcoma’s Dermoscopy: A Mini-Review
Abstract
1. Introduction
2. Case Presentations
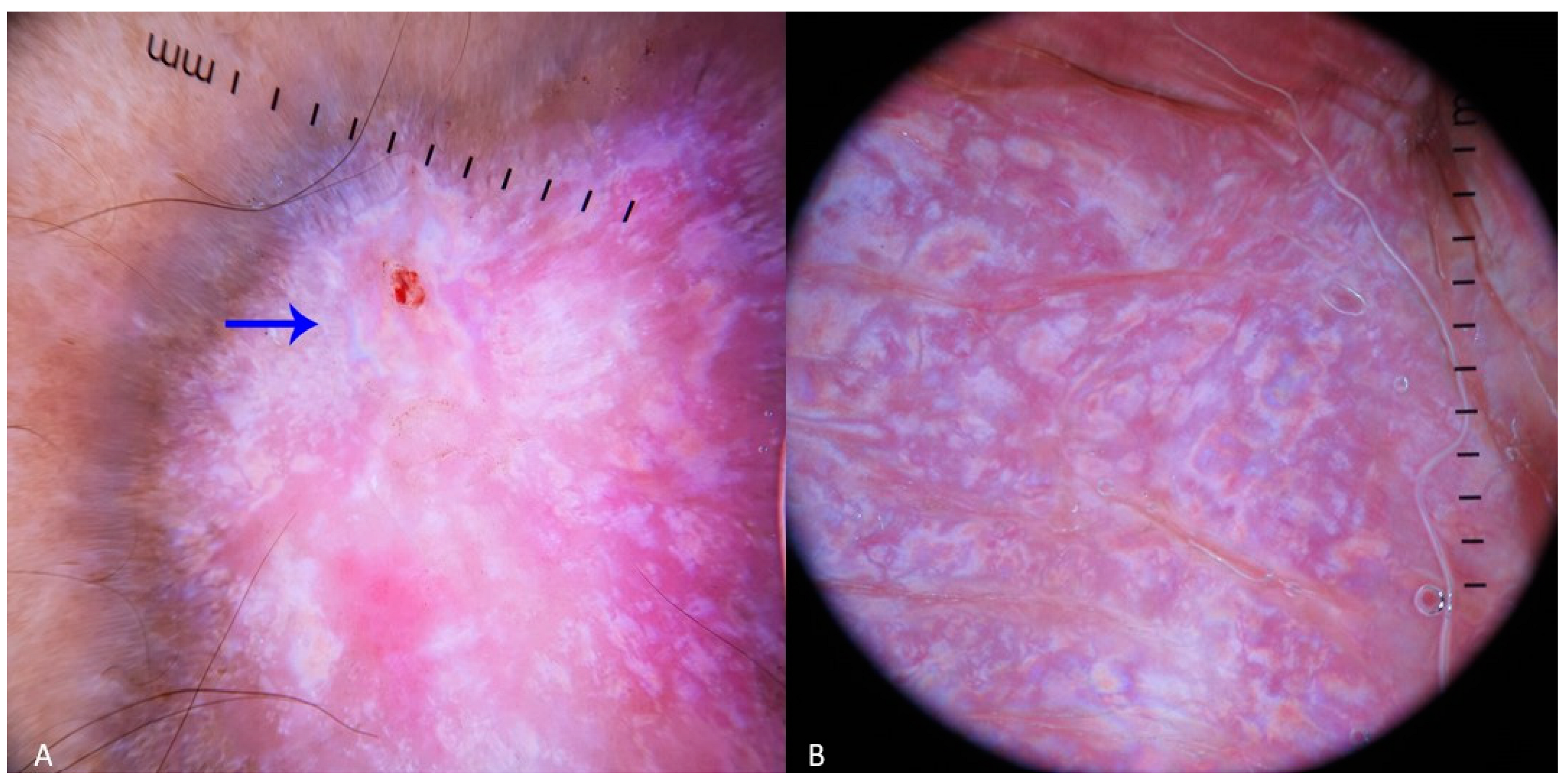
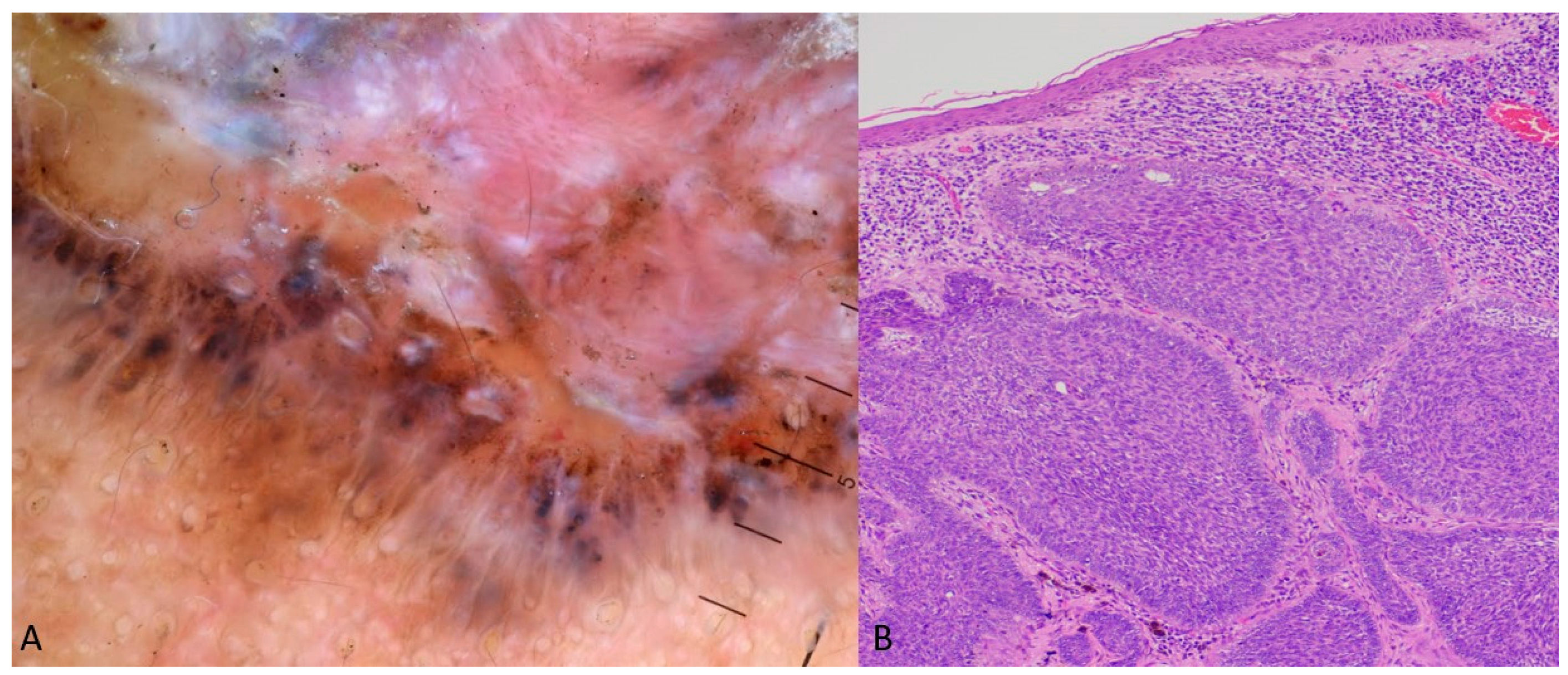
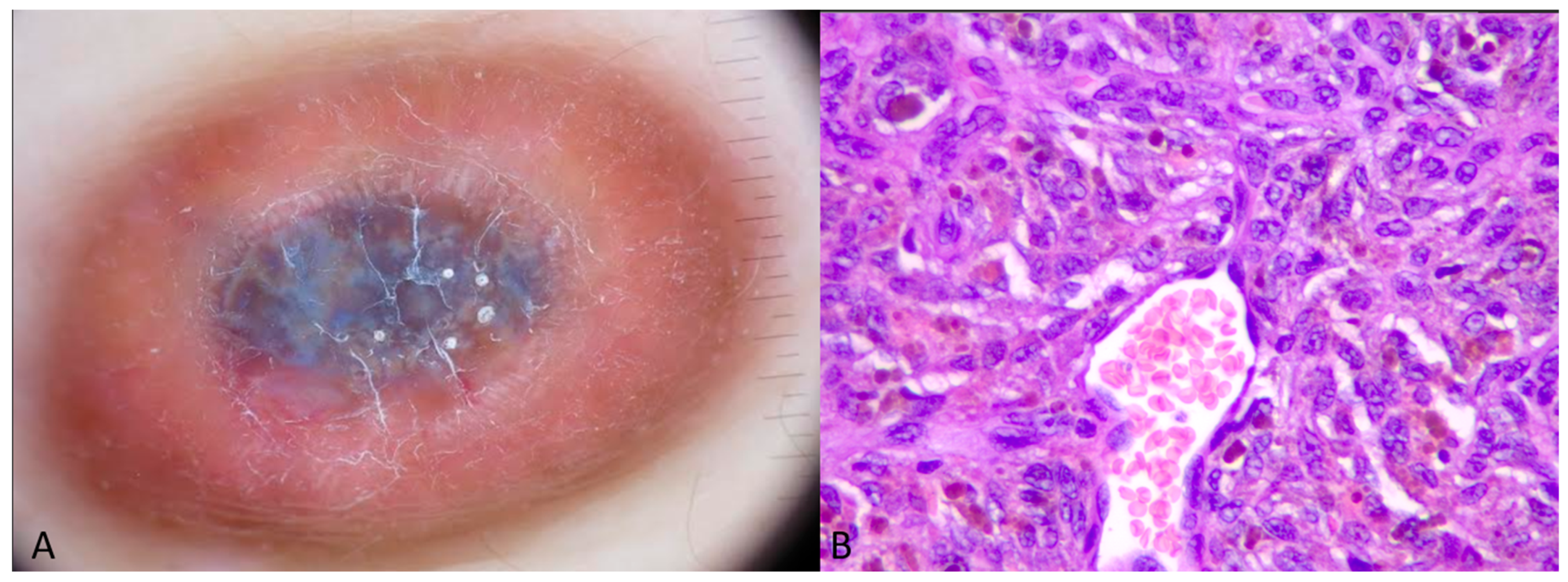
2.1. Case 1
2.2. Case 2
3. Methods
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elmas, Ö.F. Dermoscopic Rainbow Pattern: A Strong Clue to Malignancy or Just a Light Show? North Clin. Istanb. 2020, 7, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Kelati, A.; Mernissi, F.Z. The Rainbow Pattern in Dermoscopy: A Zoom on Nonkaposi Sarcoma Skin Diseases. Biomed. J. 2018, 41, 209–210. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.; Palit, A.; Sethy, M.; Nayak, A.K.; Dash, S.; Ayyanar, P. Multicoloured Rainbow Pattern in a Case of Aneurysmal Dermatofibroma. Australas. J. Dermatol. 2020, 61, e432–e434. [Google Scholar] [CrossRef]
- Al-Sukhni, L.; Mui, U.N.; Tarbox, M. A Spectrum of Diseases with the Dermatoscopic Rainbow Pattern. JAAD Case Rep. 2022, 21, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Fernandez, I.; Duran-Vian, C.; Gonzalez-Vela, M.C.; Yange-Zambrano, G.; Gonzalez-Lopez, M.A. Is Dermoscopy a Useful Tool in Pseudo-Kaposi Sarcoma? Indian J. Dermatol. Venereol. Leprol. 2021, 87, 709. [Google Scholar] [CrossRef]
- Vázquez-López, F.; García-García, B.; Rajadhyaksha, M.; Marghoob, A.A. Dermoscopic Rainbow Pattern in Non-Kaposi Sarcoma Lesions. Br. J. Dermatol. 2009, 161, 474–475. [Google Scholar] [CrossRef]
- Garcia-Garcia, B.; Perez-Oliva, N. Dermoscopic Rainbow Pattern in Basal Cell Carcinoma. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 499–500. [Google Scholar] [CrossRef]
- Vinay, K.; Ankad, B.S.; Narayan R., V.; Chatterjee, D.; Bhat, Y.J.; Neema, S.; Shah, S.; Chauhan, P.; Khare, S.; Rajput, C.; et al. A Multicentric Study on Dermoscopic Patterns and Clinical–Dermoscopic–Histological Correlates of Basal Cell Carcinoma in Indian Skin. Clin. Exp. Dermatol. 2022, 47, 1982–1990. [Google Scholar] [CrossRef]
- Suppa, M.; Micantonio, T.; Di Stefani, A.; Soyer, H.P.; Chimenti, S.; Fargnoli, M.C.; Peris, K. Dermoscopic Variability of Basal Cell Carcinoma According to Clinical Type and Anatomic Location. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1732–1741. [Google Scholar] [CrossRef]
- Rodríguez-Lomba, E.; Lozano-Masdemont, B.; Nieto-Benito, L.M.; Hernández de la Torre, E.; Suárez-Fernández, R.; Avilés-Izquierdo, J.A. Dermoscopic Predictors of Tumor Thickness in Cutaneous Melanoma: A Retrospective Analysis of 245 Melanomas. Dermatol. Pract. Concept. 2021, 11, e2021059. [Google Scholar] [CrossRef]
- Rodríguez-Lomba, E.; García-Piqueras, P.; Lozano-Masdemont, B.; Nieto-Benito, L.M.; Hernández de la Torre, E.; Parra-Blanco, V.; Suárez-Fernández, R.; Lázaro-Ochaita, P.; Avilés-Izquierdo, J.A. ‘Rainbow Pattern’: A Dermoscopic Sign of Invasive Melanoma. Clin. Exp. Dermatol. 2022, 47, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Pinos León, V.H.; Granizo Rubio, J.D. Acral Pseudolymphomatous Angiokeratoma of Children with Rainbow Pattern: A Mimicker of Kaposi Sarcoma. J. Am. Acad. Dermatol. 2017, 76, S25–S27. [Google Scholar] [CrossRef] [PubMed]
- Kayıran, M.A.; Sahin, A.S.; Simsek, B.C. A Lesion Surrounded by the Rainbow: Merkel Cell Carcinoma. Dermatol. Pract. Concept. 2024, 14, e2024064. [Google Scholar] [CrossRef] [PubMed]
- di Meo, N.; Vernoni, S.; Longone, M.; Trevisan, G. Image Gallery: Merkel Cell Carcinoma under the Rainbow. Br. J. Dermatol. 2017, 177, e166. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pitarch, G. Patrón Dermatoscópico En Arcoíris En Fibroxantoma Atípico. Actas Dermosifiliogr. 2014, 105, 97–99. [Google Scholar] [CrossRef]
- Savoia, F.; Medri, M.; Raulli, G.D.; Menna, C.; Melandri, D.; Stanganelli, I. Subungual Metastasis from Ovarian Cancer: Case Report and Brief Review of the Literature. Skin Appendage Disord. 2023, 9, 54–57. [Google Scholar] [CrossRef]
- Uzuncakmak, T.; Ozkanli, S.; Karadag, A. Dermoscopic Rainbow Pattern in Blue Nevus. Dermatol. Pract. Concept. 2017, 7, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Robles-Tenorio, A.; Preciado-Aguiar, M.S.; Quiñones-Venegas, R.; Salazar-Torres, F.J. Dermoscopic Rainbow Pattern in a Deep Penetrating Nevus. Dermatol. Pract. Concept. 2022, 12, e2022046. [Google Scholar] [CrossRef]
- Martinez-Ortega, J.I.; Perez Hernandez, F.d.J.; Flores-Reyes, I.A.; Quiñones-Venega, R.; Fernández-Reyna, I.; Valdivieso-Jimenez, J.A. Sebaceous Adenoma: A Dermoscopic Case Perspective. Cureus 2023, 15, e49126. [Google Scholar] [CrossRef]
- Pérez-Pérez, L.; García-Gavín, J.; Allegue, F.; Zulaica, A. Patrón Dermoscópico En Arcoíris y Rosetas En Cicatrices Cutáneas. Actas Dermosifiliogr. 2014, 105, 96–97. [Google Scholar] [CrossRef]
- Navarrete, J.; Cabrera, R.; Bunker, C.B.; Agorio, C. Dermoscopy of Penile Sclerosing Granuloma. BMJ Case Rep. 2021, 14, e239846. [Google Scholar] [CrossRef] [PubMed]
- Roster, K.; Tarawneh, O.H.; Zufall, A.; Farabi, B.; Russo, M.; Shulman, K.; Safai, B. Dermatoscopic Rainbow Pattern in Lichen Planus Pigmentosus Inversus in a Middle-Aged African American Man. JAAD Case Rep. 2023, 35, 118–121. [Google Scholar] [CrossRef] [PubMed]
- España, A.G.; Pimentel, M.I.F.; Lyra, J.P.d.M.; Valete-Rosalino, C.M.; Lyra, M.R. Description of the Dermatoscopic Features Observed in Sporotrichosis and American Cutaneous Leishmaniasis in a Reference Center in Rio de Janeiro, Brazil. An. Bras. Dermatol. 2023, 98, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-T.; Ke, C.-L.K.; Lee, C.-H.; Wu, C.-S.; Chen, G.-S.; Hu, S.C.-S. Dermoscopic Rainbow Pattern in Non-Kaposi Sarcoma Lesions—Reply. Br. J. Dermatol. 2010, 162, 458–459. [Google Scholar] [CrossRef] [PubMed]
- Karampinis, E.; Georgopoulou, K.-E.; Kampra, E.; Zafiriou, E.; Lallas, A.; Lazaridou, E.; Apalla, Z.; Behera, B.; Errichetti, E. Clinical and Dermoscopic Patterns of Basal Cell Carcinoma and Its Mimickers in Skin of Color: A Practical Summary. Medicina 2024, 60, 1386. [Google Scholar] [CrossRef]
- Karampinis, E.; Lallas, A.; Lazaridou, E.; Errichetti, E.; Apalla, Z. Race-Specific and Skin of Color Dermatoscopic Characteristics of Skin Cancer: A Literature Review. Dermatol. Pract. Concept. 2023, 13, e2023311S. [Google Scholar] [CrossRef]
- Batool, S.; Nisar, M.; Mangini, F.; Frezza, F.; Fazio, E. Scattering of Light from the Systemic Circulatory System. Diagnostics 2020, 10, 1026. [Google Scholar] [CrossRef]
| Non-Kaposi Lesions Exhibiting RP |
|---|
Angiokeratoma [1,2] Aneurysmatic dermatofibroma [3,4] Pyogenic granuloma [1] Masson tumor [4] Pseudo-Kaposi [5,6]
Melanoma [1,2,6,10,11] Pseudolymphoma [12] Merkel cell carcinoma [13,14] Atypical fibroxanthoma [15] Subungual metastasis [16]
Deep penetrating nevi [18]
Granuloma annular [1] Penile sclerosing granuloma [21]
Lichen planus [6,22] Dissecting cellulitis [1]
Sporotrichosis [23] |
| Characteristics of the Lesion-Predisposing Factors for RP Appearance | Non-Kaposi Skin Lesion Examples |
|---|---|
| Nodular subtype—raised lesions | Nodular BCCs, Merkel cell carcinomas, bleeding nodules, pyogenic granola, hypertrophic scars |
| Lesions with abundant vascularization | -With vascular laminas: vascular lesions such as angiokeratomas, strawberry angiomas, aneurismatic dermatofibroma, pseudo-Kaposi -With network-type abundant vascular pattern: BCCs and scars |
| Skin lesion compositions | Instances: BCC: basaloid cell islands with abundant vascularization Dermatofibroma: abundance of fibers and collagen |
| Hematomas | Bleeding nodules or subungual hematoma |
| Pigment skin—dark-skinned patients | Granulomatous reactions, BCCs, aneurismatic dermatofibroma, pseudo-Kaposi |
| Depth of the lesion | Deep penetrating nevi, blue nevus |
| Thickness of lesions | Thick melanomas |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the European Society of Dermatopathology. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karampinis, E.; Toli, O.; Pappa, G.; Vardiampasi, A.; Theofili, M.; Zafiriou, E.; Bobos, M.; Lallas, A.; Lazaridou, E.; Behera, B.; et al. “Chasing Rainbows” Beyond Kaposi Sarcoma’s Dermoscopy: A Mini-Review. Dermatopathology 2024, 11, 333-341. https://doi.org/10.3390/dermatopathology11040035
Karampinis E, Toli O, Pappa G, Vardiampasi A, Theofili M, Zafiriou E, Bobos M, Lallas A, Lazaridou E, Behera B, et al. “Chasing Rainbows” Beyond Kaposi Sarcoma’s Dermoscopy: A Mini-Review. Dermatopathology. 2024; 11(4):333-341. https://doi.org/10.3390/dermatopathology11040035
Chicago/Turabian StyleKarampinis, Emmanouil, Olga Toli, Georgia Pappa, Anna Vardiampasi, Melpomeni Theofili, Efterpi Zafiriou, Mattheos Bobos, Aimilios Lallas, Elizabeth Lazaridou, Biswanath Behera, and et al. 2024. "“Chasing Rainbows” Beyond Kaposi Sarcoma’s Dermoscopy: A Mini-Review" Dermatopathology 11, no. 4: 333-341. https://doi.org/10.3390/dermatopathology11040035
APA StyleKarampinis, E., Toli, O., Pappa, G., Vardiampasi, A., Theofili, M., Zafiriou, E., Bobos, M., Lallas, A., Lazaridou, E., Behera, B., & Apalla, Z. (2024). “Chasing Rainbows” Beyond Kaposi Sarcoma’s Dermoscopy: A Mini-Review. Dermatopathology, 11(4), 333-341. https://doi.org/10.3390/dermatopathology11040035








