Bortezomib-Induced Reticular Eruption in Patient with Multiple Myeloma
Abstract
1. Introduction
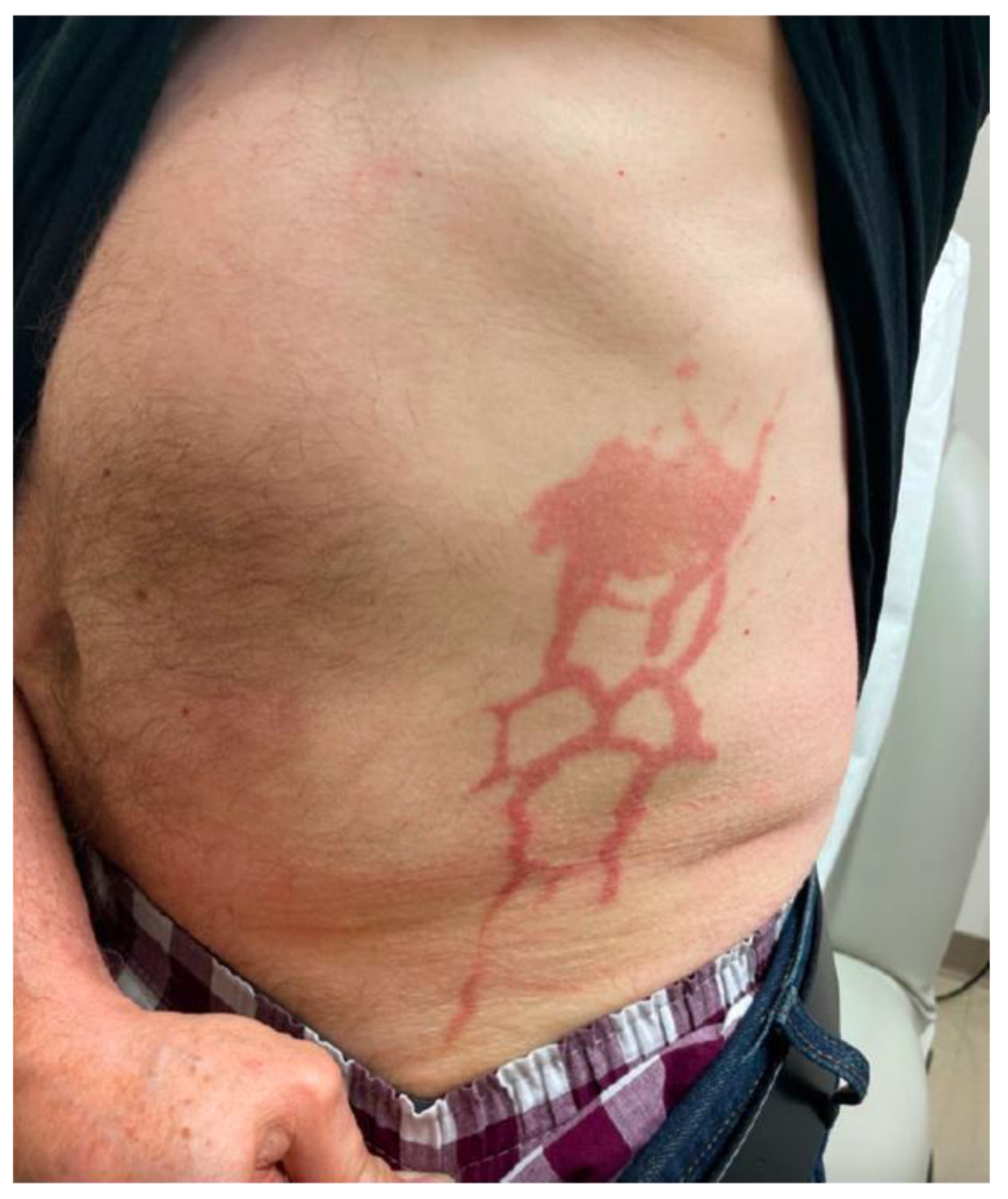
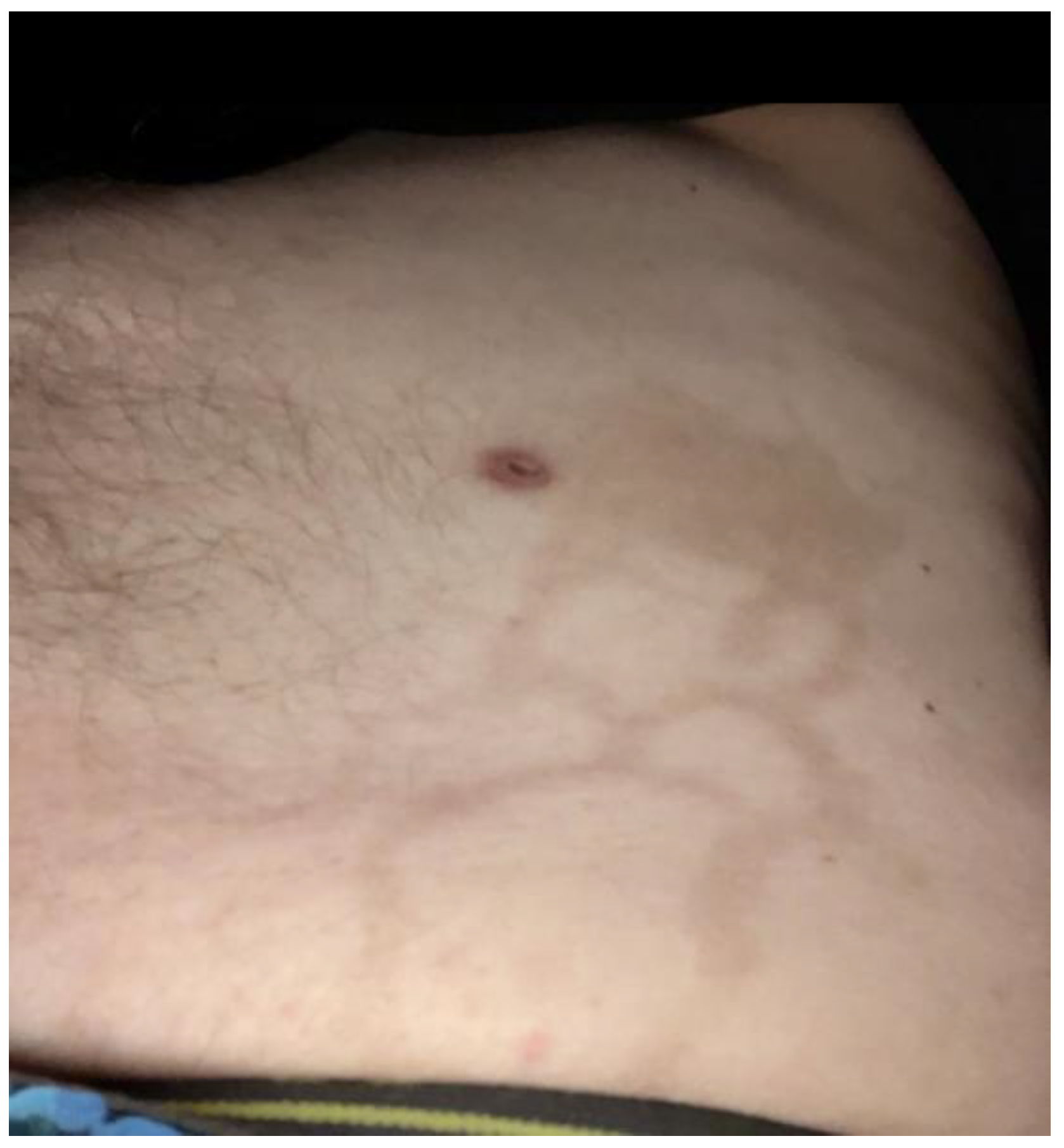
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.K.; Jacobus, S.J.; Cohen, A.D.; Weiss, M.; Callander, N.; Singh, A.K.; Parker, T.L.; Menter, A.; Yang, X.; Parsons, B.; et al. Carfilzomib or bortezomib in combination with lenalidomide and dexamethasone for patients with newly diagnosed multiple myeloma without intention for immediate autologous stem-cell transplantation (ENDURANCE): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020, 21, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Villarrubia, B.; Betlloch, I.; Mataix, J.; Lucas, A.; Botella, C. Bortezomib-associated rash: A new recognizable and avoidable side-effect. Br. J. Dermatol. 2007, 156, 784–785. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.L.; Heule, F.; Lam, K.; Sonneveld, P. Pleomorphic presentation of cutaneous lesions associated with the proteasome inhibitor bortezomib in patients with multiple myeloma. J. Am. Acad. Dermatol. 2006, 55, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Navarro, X.; Puig, L.; Fernandez-Figueras, M.T.; Dalmau, J.; Roe, E.; Alomar, A. Bortezomib-associated cutaneous vasculitis. Br. J. Dermatol. 2007, 157, 799–801. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Cavelier Balloy, B.; Andreoli, A.; Briere, J.; Petit, A. Bortezomib-induced neutrophilic dermatosis with CD30+ lymphocytic infiltration. Ann. Dermatol Venereol. 2009, 136, 438–442. [Google Scholar] [CrossRef]
- Reyes-Habito, C.M.; Roh, E.K. Cutaneous reactions to chemotherapeutic drugs and targeted therapy for cancer. J. Am. Acad. Dermatol. 2014, 71, 217.e1–217.e11. [Google Scholar] [CrossRef] [PubMed]
- Patrizi, A.; Venturi, M.; Dika, E.; Maibach, H.; Tacchetti, P.; Brandi, G. Cutaneous adverse reactions linked to targeted anticancer therapies bortezomib and lenalidomide for multiple myeloma: New drugs, old side effects. Cutan. Ocul. Toxicol. 2013, 33, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Plume, M.A.; Sibaud, V.; Bobin, A.; Hainaut, E.; Frouin, E.; Masson Regnault, M. Spider-like injection site reaction after subcutaneous administration of haematological treatments. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e142–e144. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martin, M.; Saez-Rodriguez, M.; Garcia-Bustinduy, M.; Martin-Herrera, A.; Noda-Cabrera, A. Bortezomib-induced cutaneous lesions in multiple myeloma patients: A case report. Dermatol. Online J. 2008, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Alsaad, K.O.; Ghazarian, D. My approach to superficial inflammatory dermatoses. J. Clin. Pathol. 2005, 58, 1233–1241. [Google Scholar] [CrossRef]
- Akyurek, F.T.; Sari, N.; Ugurluoglu, C.; Kurtipek, G.S. Serpentine supravenous hyperpigmentation related to carboplatin and vinorelbine chemotherapy: A case report. Dermatol. Ther. 2019, 32, e12981. [Google Scholar] [CrossRef]
- Aydogan, I.; Kavak, A.; Parlak, A.H.; Alper, M.; Annakkaya, A.N.; Erbas, M. Persistent serpentine supravenous hyperpigmented eruption associated with docetaxel. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 345–347. [Google Scholar] [CrossRef]
- Pujol, R.M.; Rocamora, V.; Lopez-Pousa, A.; Taberner, R.; Alomar, A. Persistent supravenous erythematous eruption: A rare local complication of intravenous 5-fluorouracil therapy. J. Am. Acad. Dermatol. 1998, 39, 839–842. [Google Scholar] [CrossRef]
- McClain, R.W.; Yentzer, B.A.; Feldman, S.R. Comparison of skin concentrations following topical versus oral corticosteroid treatment: Reconsidering the treatment of common inflammatory dermatoses. J. Drugs Dermatol. 2009, 8, 1076–1079. [Google Scholar]
- Betlloch, I.; Mataix, J.; Lucas, A.; Villarrubia, B. Cutaneous reaction to bortezomib therapy: A new recognizable and avoidable side effect. J. Am. Acad. Dermatol. 2007, 56, AB133. [Google Scholar] [CrossRef]
- Kurtin, S.; Knop, C.S.; Milliron, T. Subcutaneous administration of bortezomib: Strategies to reduce injection site reactions. J. Adv. Pr. Oncol. 2012, 3, 406–410. [Google Scholar] [CrossRef]
- Minarik, J.; Pavlicek, P.; Pour, L.; Pika, T.; Maisnar, V.; Špička, I.; Jarkovsky, J.; Krejčí, M.; Bacovský, J.; Radocha, J.; et al. Subcutaneous Bortezomib in Multiple Myeloma Patients Induces Similar Therapeutic Response Rates as Intravenous Application But It Does Not Reduce the Incidence of Peripheral Neuropathy. PLoS ONE 2015, 10, e0123866. [Google Scholar] [CrossRef]
- Moreau, P.; Pylypenko, H.; Grosicki, S.; Karamanesht, I.; Leleu, X.; Grishunina, M.; Rekhtman, G.; Masliak, Z.; Robak, T.; Shubina, A.; et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: A randomised, phase 3, non-inferiority study. Lancet Oncol. 2011, 12, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; De Jesus, O. Medication Routes of Administration; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Loke, C.; Mollee, P.; McPherson, I.; Walpole, E.; Yue, M.; Mutsando, H.; Wong, P.; Weston, H.; Tomlinson, R.; Hollingworth, S. Bortezomib use and outcomes for the treatment of multiple myeloma. Intern. Med. J. 2020, 50, 1059–1066. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Owji, S.; Agarwal, A.; Kamat, S.; Luu, Y.; Mubasher, A.; Niedt, G.; Ray, C.; Cho, H.J.; Gulati, N.; et al. Bortezomib-Induced Reticular Eruption in Patient with Multiple Myeloma. Dermatopathology 2023, 10, 226-230. https://doi.org/10.3390/dermatopathology10030031
Han J, Owji S, Agarwal A, Kamat S, Luu Y, Mubasher A, Niedt G, Ray C, Cho HJ, Gulati N, et al. Bortezomib-Induced Reticular Eruption in Patient with Multiple Myeloma. Dermatopathology. 2023; 10(3):226-230. https://doi.org/10.3390/dermatopathology10030031
Chicago/Turabian StyleHan, Joseph, Shayan Owji, Aneesh Agarwal, Samir Kamat, Yen Luu, Adnan Mubasher, George Niedt, Chloe Ray, Hearn Jay Cho, Nicholas Gulati, and et al. 2023. "Bortezomib-Induced Reticular Eruption in Patient with Multiple Myeloma" Dermatopathology 10, no. 3: 226-230. https://doi.org/10.3390/dermatopathology10030031
APA StyleHan, J., Owji, S., Agarwal, A., Kamat, S., Luu, Y., Mubasher, A., Niedt, G., Ray, C., Cho, H. J., Gulati, N., & Lamb, A. (2023). Bortezomib-Induced Reticular Eruption in Patient with Multiple Myeloma. Dermatopathology, 10(3), 226-230. https://doi.org/10.3390/dermatopathology10030031





