Multiply Recurrent Composite Hemangioendothelioma of Penis with Histologic Progression to High-Grade Features
Abstract
1. Introduction
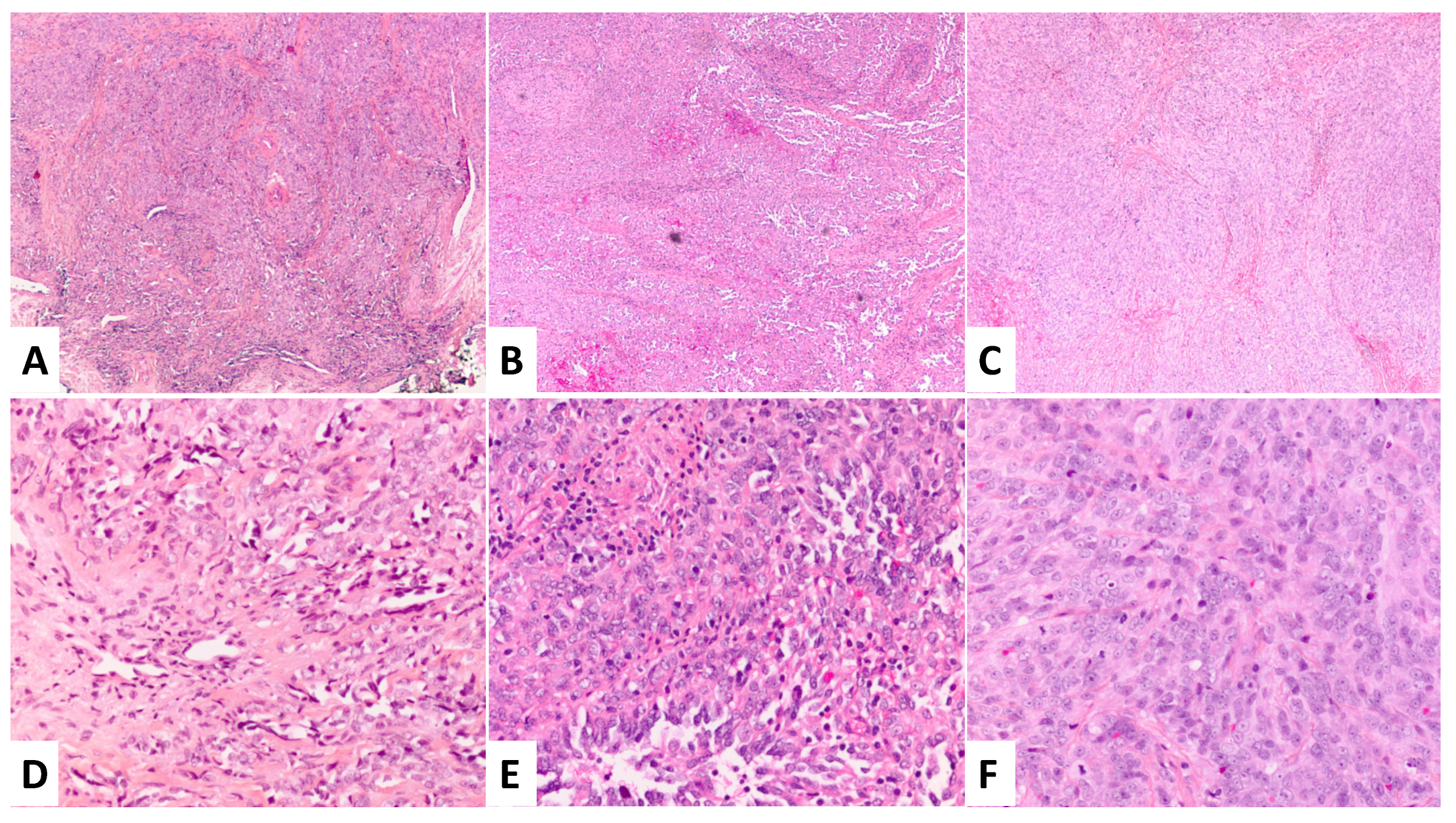
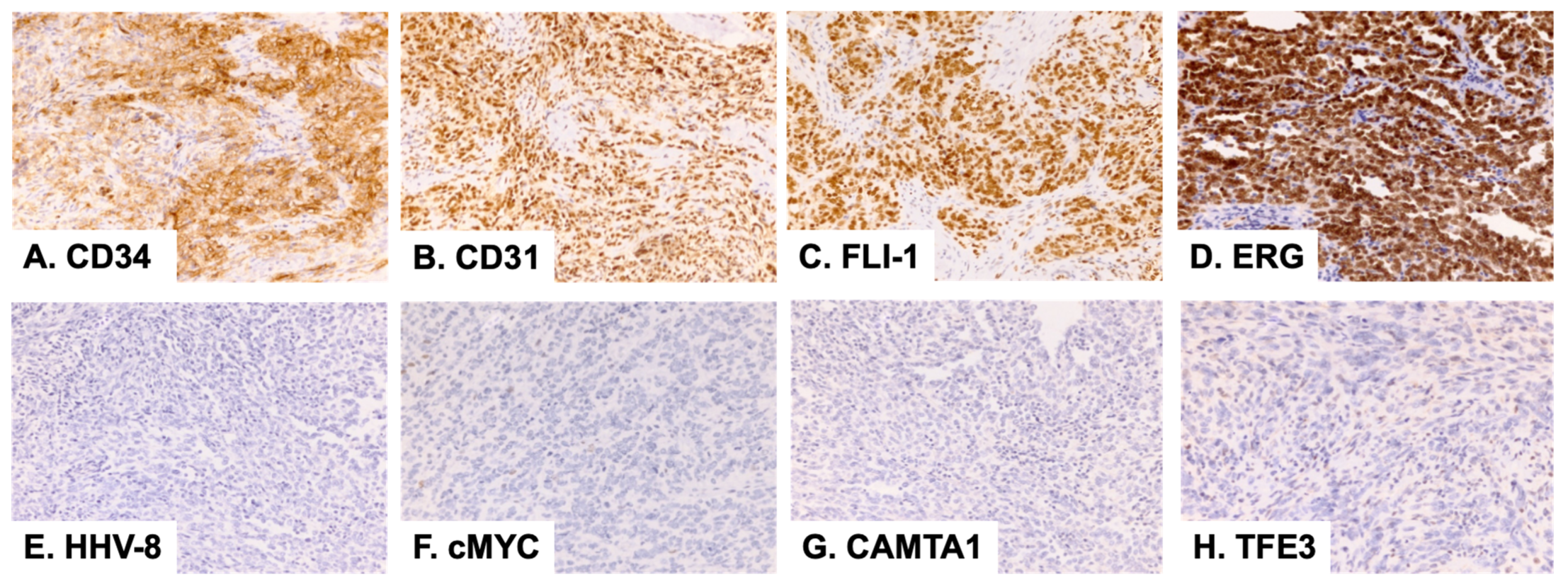
2. Case Presentation
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nayler, S.J.; Rubin, B.P.; Calonje, E.; Chan, J.K.; Fletcher, C.D. Composite hemangioendothelioma: A complex, low-grade vascular lesion mimicking angiosarcoma. Am. J. Surg. Pathol. 2000, 24, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Rokni, G.R.; Montazer, F.; Sharifian, M.; Goldust, M. Composite hemangioendothelioma of the forehead and right eye; a case report. BMC Dermatol. 2017, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Goldblum, J.R.; Lamps, L.W.; McKenney, J.K.; Myers, J.L. Rosai and Ackerman’s Surgical Pathology, 11th ed.; Mosby Inc.: Philadelphia, PA, USA, 2018; pp. 1862–1863. [Google Scholar]
- Zhang, J.; Wu, B.; Zhou, G.Q.; Zhang, R.S.; Wei, X.; Yu, B.; Lu, Z.F.; Ma, H.H.; Shi, Q.L.; Zhou, X.J. Composite hemangioendothelioma arising from the kidney: A case report with review of the literature. Int. J. Clin. Exp. Pathol. 2013, 6, 1935–1941. [Google Scholar] [PubMed]
- Chen, Y.L.; Chen, W.X.; Wang, J.; Jiang, Y. Composite hemangioendothelioma on the neck. Kaohsiung J. Med. Sci. 2012, 28, 564–565. [Google Scholar] [CrossRef] [PubMed]
- Fasolis, M.; Iaquinta, C.; Montesco, M.C.; Garzino-Demo, P.; Tosco, P.; Tanteri, G.; Bonandini, E.; Ninfo, V.; Berrone, S. Composite hemangioendothelioma of the oral cavity: Case report and review of the literature. Head Neck 2008, 30, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Cakir, E.; Demirag, F.; Gulhan, E.; Oz, G.; Tastepez, I. Mediastinal composite hemangioendothelioma. A rare tumor at an unusual location. Tumori J. 2009, 95, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Gok, S.; Berkman, M.Z.; Baykara, E. Composite Hemangioendothelioma Settled in the Paraspinal Region: A Rare Case Report. Turk. Neurosurg. 2020, 30, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Stojsic, Z.; Brasanac, D.; Stojanovic, M.; Boricic, M. Cutaneous composite hemangioendothelioma: Case report and review of published reports. Ann. Saudi. Med. 2014, 34, 182–188. [Google Scholar] [CrossRef] [PubMed]
- McCollum, K.J.; Al-Rohil, R.N. Cutaneous Vascular Neoplasms of Uncertain Biological Behavior. Biology 2021, 10, 1160. [Google Scholar] [CrossRef] [PubMed]
- Shon, W.; Sukov, W.R.; Jenkins, S.M.; Folpe, A.L. MYC amplification and overexpression in primary cutaneous angiosarcoma: A fluorescence in-situ hybridization and immunohistochemical study. Mod. Pathol. 2014, 27, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Tejera-Vaquerizo, A.; Herrera-ceballos, E.; Bosch-garcia, R.; Fernandez-orland, A.; Matilla, A. Composite cutaneous hemangioendothelioma on the back. Am. J. Dermatopathol. 2008, 30, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.C.; Choi, S.J.; Kim, L.; Han, J.Y.; Kim, J.M. Composite hemangioendothelioma. A case report. Korean J. Pathol. 2006, 40, 142–147. [Google Scholar]
- Leen, S.L.S.; Fisher, C.; Thway, K. Composite Hemangioendothelioma: Clinical and Histologic Features of an Enigmatic Entity. Adv. Anat. Pathol. 2015, 22, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Deyrup, A.T.; Tighiouart, M.; Montag, A.G.; Weiss, S.W. Epithelioid hemangioendothelioma of soft tissue: A proposal for risk stratification based on 49 cases. Am. J. Surg. Pathol. 2008, 32, 924–927. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.A.; Fletcher, C.D.; Hornick, J.L. Nuclear Expression of CAMTA1 Distinguishes Epithelioid Hemangioendothelioma From Histologic Mimics. Am. J. Surg. Pathol. 2016, 40, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Antonescu, C.R.; Dickson, B.C.; Sung, Y.S.; Zhang, L.; Suurmeijer, A.J.H.; Stenzinger, A.; Mechtersheimer, G.; Fletcher, C.D.M. Recurrent YAP1 and MAML2 Gene Rearrangements in Retiform and Composite Hemangioendothelioma. Am. J. Surg. Pathol. 2020, 44, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Dermawan, J.K.; Westra, W.H.; Antonescu, C.R. Recurrent PTBP1::MAML2 fusions in composite hemangioendothelioma with neuroendocrine differentiation: A report of two cases involving neck lymph nodes. Genes Chromosomes Cancer 2022, 61, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Perry, K.D.; Al-Lbraheemi, A.; Rubin, B.P.; Jen, J.; Ren, H.; Jang, J.S.; Nair, A.; Davila, J.; Pambuccian, S.; Horvai, A.; et al. Composite hemangioendothelioma with neuroendocrine marker expression: An aggressive variant. Mod. Pathol. 2017, 30, 1589–1602. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui, C.M.; Balzer, B. Multiply Recurrent Composite Hemangioendothelioma of Penis with Histologic Progression to High-Grade Features. Dermatopathology 2023, 10, 41-45. https://doi.org/10.3390/dermatopathology10010005
Bui CM, Balzer B. Multiply Recurrent Composite Hemangioendothelioma of Penis with Histologic Progression to High-Grade Features. Dermatopathology. 2023; 10(1):41-45. https://doi.org/10.3390/dermatopathology10010005
Chicago/Turabian StyleBui, Chau M., and Bonnie Balzer. 2023. "Multiply Recurrent Composite Hemangioendothelioma of Penis with Histologic Progression to High-Grade Features" Dermatopathology 10, no. 1: 41-45. https://doi.org/10.3390/dermatopathology10010005
APA StyleBui, C. M., & Balzer, B. (2023). Multiply Recurrent Composite Hemangioendothelioma of Penis with Histologic Progression to High-Grade Features. Dermatopathology, 10(1), 41-45. https://doi.org/10.3390/dermatopathology10010005




