Risk Assessment Profiles for Caregiver Burden in Family Caregivers of Persons Living with Alzheimer’s Disease: An Exploratory Study with Machine Learning
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics, Procedures, and Sampling
2.2. Instruments
2.3. Data Analysis
2.4. Pre-Processing
2.5. Training
3. Results
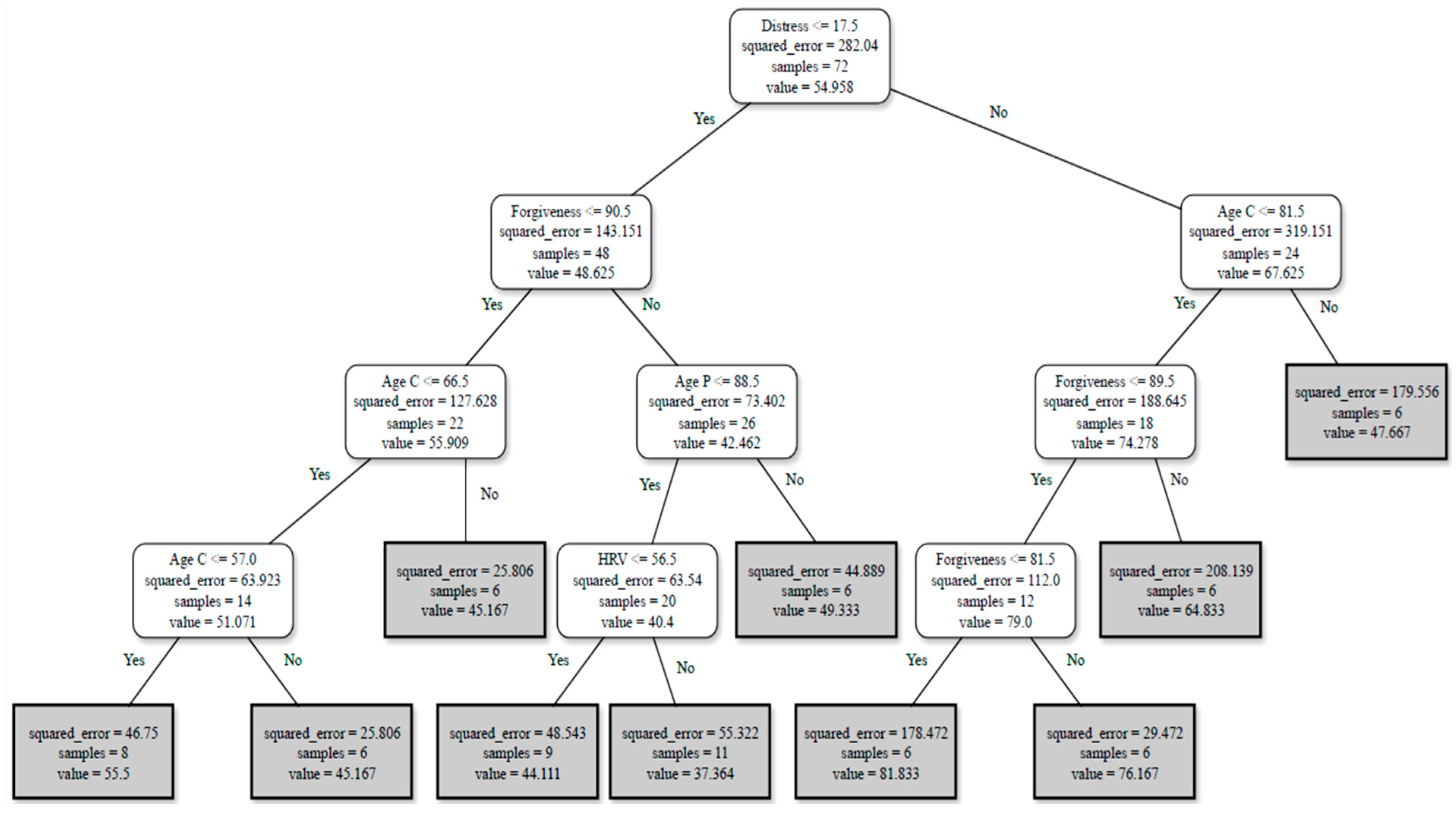
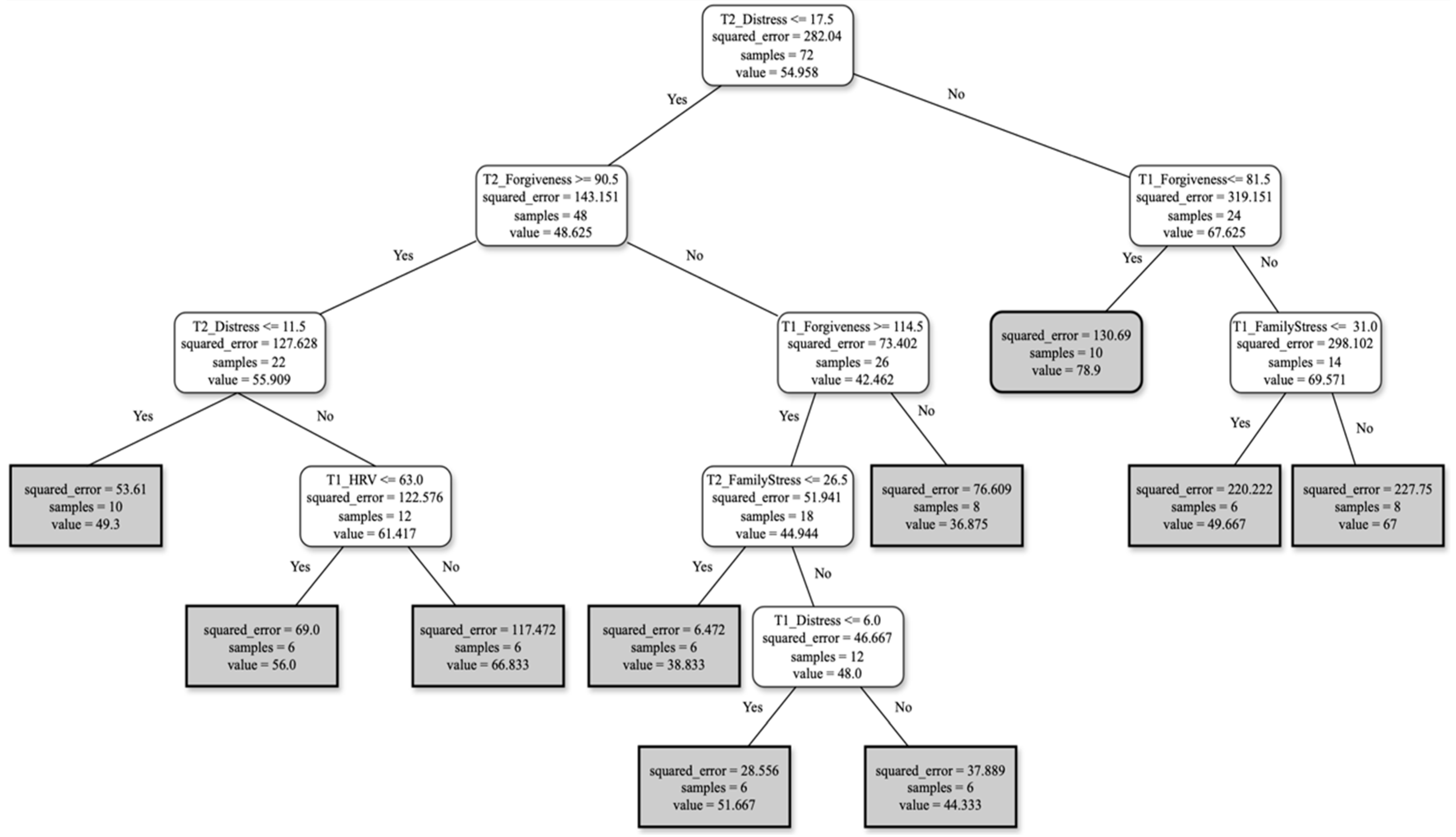
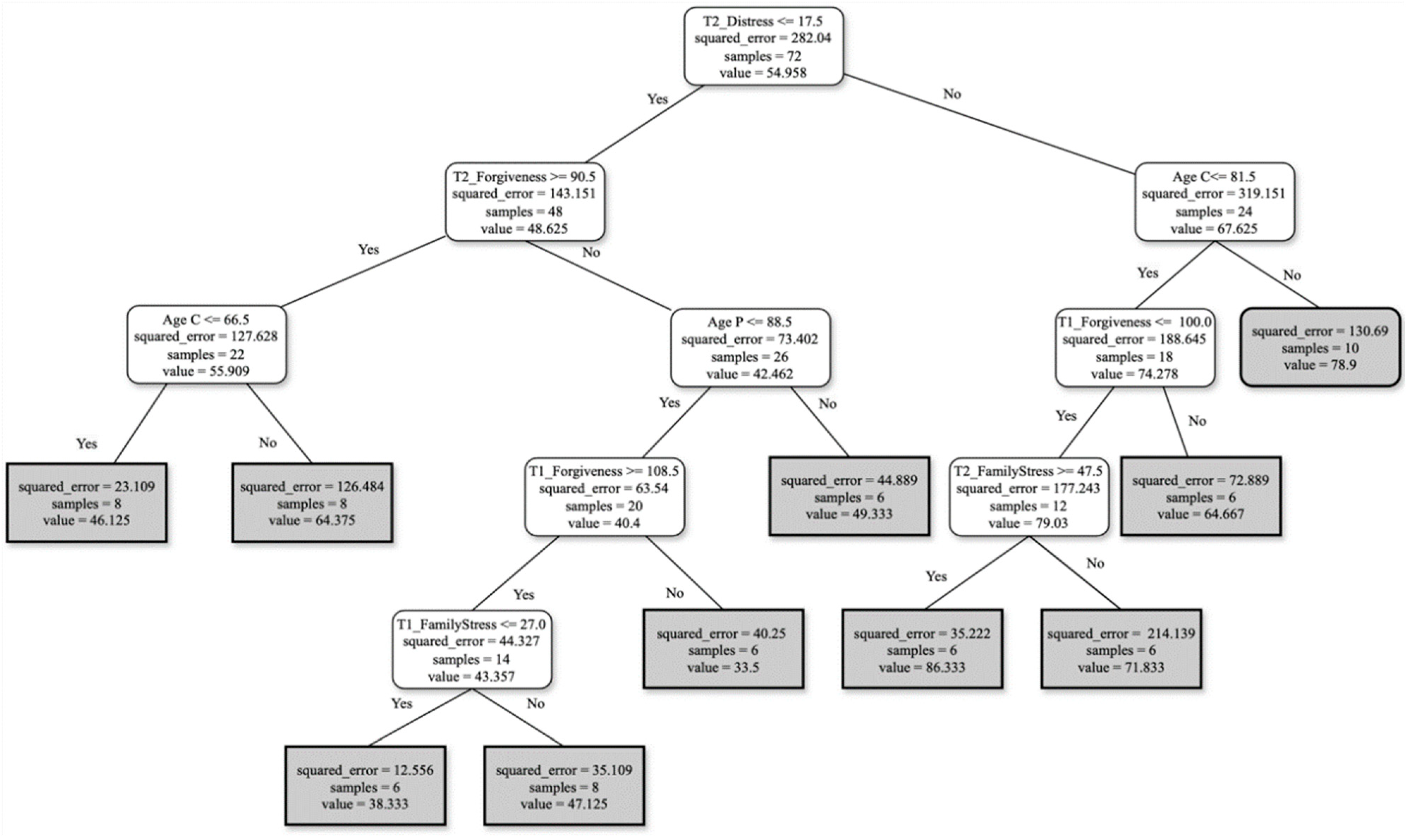
Burden Profiles
- For FCs reporting ≤ 17.5 on distress scores and ≥90.5 in forgiveness at T2, with an FCs’ age ≤ 66.5 or ≤57, the burden score perception will be around 51.
- For FCs reporting ≤ 17.5 on distress scores and ≥90.5 in forgiveness at T2, with the person living with AD’s age ≤ 88.5 and HRV ≥ 56.5, the burden score perception will be around 40.
- For FCs reporting >17.5 on distress scores, with age ≤ 81.5 and forgiveness at T2 ≤ 89.5 or ≤81.5, the burden score perception will be around 79.
- For FCs reporting distress ≤ 17.5 and forgiveness ≥ 90.5 at T2, with distress ≤ 11.5 and HRV ≤ 63 at T1, the burden score perception will be around 61.
- For FCs reporting distress ≤ 17.5 and forgiveness ≥ 90.5 at T2, with forgiveness at T1 ≥ 114.5, family stress at T2 ≤ 26.5 and distress ≤ 6 at T1, the burden score perception will be around 48.
- FCs reporting distress ≥ 17.5 at T2, with forgiveness ≤ 81.5 at T1 and family stress at T1 ≥ 31, will show a burden score perception of around 69.
- For FCs reporting distress ≤ 17.5 and forgiveness ≥ 90.5 at T2, with the FC’s age ≤ 66.5, the burden score perception will be around 56.
- For FCs reporting distress ≤ 17.5 and forgiveness ≥ 90.5 at T2, with person living with AD’s age ≤ 88.5, and finally, forgiveness ≥ 108.5 and family stress ≤ 27 at T1, the burden score perception will be around 43.
- For FCs reporting distress ≥ 17.5, with FC’s age ≤ 81.5, forgiveness at T1 ≤ 100, and family stress, at T2 ≥ 47.5, the burden score perception will be around 79.
4. Discussion
5. Limitations
6. Clinical Implications
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abulaiti, B., Zhang, X., Guan, T., Wang, M., Jia, S., & Wang, A. (2022). The dyadic care experiences of elderly individuals with disabilities and caregivers in the home setting from the perspective of family resilience: A qualitative study. Frontiers in Psychiatry, 13, 963101. [Google Scholar] [CrossRef] [PubMed]
- Allen, A. P., Buckley, M. M., Cryan, J. F., Chorcoráin, A. N., Dinan, T. G., Kearney, P. M., O’Caoimh, R., Calnan, M., Clarke, G., & Molloy, D. W. (2020). Informal caregiving for dementia patients: The contribution of patient characteristics and behaviours to caregiver burden. Age and Ageing, 49(1), 52–56. [Google Scholar] [CrossRef]
- Bessey, L. J., & Walaszek, A. (2019). Management of behavioral and psychological symptoms of dementia. Current Psychiatry Reports, 21(8), 66. [Google Scholar] [CrossRef] [PubMed]
- Bintamur, D. F. (2020, May 25–26). The association between forgiveness and life satisfaction. 1st International Conference on Psychology (pp. 174–180), Banda Aceh, Indonesia. [Google Scholar] [CrossRef]
- Brown, E. E., Kumar, S., Rajji, T. K., Pollock, B. G., & Mulsant, B. H. (2020). Anticipating and mitigating the impact of the COVID-19 pandemic on Alzheimer’s disease and related dementias. The American Journal of Geriatric Psychiatry, 28(7), 712–721. [Google Scholar] [CrossRef]
- Canevelli, M., Valletta, M., Toccaceli Blasi, M., Remoli, G., Sarti, G., Nuti, F., Sciancalepore, F., Ruberti, E., Cesari, M., & Bruno, G. (2020). Facing dementia during the COVID-19 outbreak. Journal of the American Geriatrics Society, 68(8), 1673–1676. [Google Scholar] [CrossRef] [PubMed]
- Costa, E. M. D. D. M. C., De Lucena, M. M., Estrela, Y. da C. A., Neto, H. T. D. O., Neto, T. M., Brito, É. P. R., Rezende, A. C. C., De Souza, J. H., Estrela, Y. M. da C. A., & Brustein, V. P. (2021). Impactos na qualidade de vida de cuidadores de idosos portadores de Alzheimer/Impacts on the quality of life of caregivers of elderly people with Alzheimer’s. Brazilian Journal of Health Review, 4(2), 7726–7741. [Google Scholar] [CrossRef]
- Damian, A. C., Mihăilescu, A. I., Anghele, C., Ciobanu, C. A., Petrescu, C., Riga, S., Dionisie, V., & Ciobanu, A. M. (2023). Quality of life predictors in a group of informal caregivers during the COVID-19 pandemic. Medicina, 59(8), 1486. [Google Scholar] [CrossRef]
- Elite HRV. (2019). CorSense heart rate variability finger sensor by Elite HRV. Available online: https://elitehrv.com/corsense (accessed on 12 January 2024).
- Fagerström, C., Elmståhl, S., & Wranker, L. S. (2020). Analyzing the situation of older family caregivers with a focus on health-related quality of life and pain: A cross-sectional cohort study. Health and Quality of Life Outcomes, 18(1), 1–10. [Google Scholar] [CrossRef]
- Gaspar, T., Raimundo, M., Borges, S., Barata, M., & Cabrita, T. (2023). Relationship between burden, quality of life and difficulties of informal primary caregivers in the context of the COVID-19 pandemic: Analysis of the contributions of public policies. International Journal of Environmental Research and Public Health, 20(6), 5205. [Google Scholar] [CrossRef]
- Grupo de Investigação em Saúde & Família [Health & Family Research Group]. (2023). Health behaviors questionnaire for informal caregivers of Alzheimer’s disease patients: Validation of portuguese research version. School of Psychology, University of Minho. [Google Scholar]
- Gräler, L., Bremmers, L., Bakx, P., van Exel, J., & van Bochove, M. (2022). Informal care in times of a public health crisis: Objective burden, subjective burden and quality of life of caregivers in the Netherlands during the COVID-19 pandemic. Health & Social Care in the Community, 30(6), 5515–5526. [Google Scholar] [CrossRef]
- Greenberg, N. E., Wallick, A., & Brown, L. M. (2020). Impact of COVID-19 pandemic restrictions on community-dwelling caregivers and persons with dementia. Psychological Trauma: Theory, Research, Practice, and Policy, 12(1), 220–221. [Google Scholar] [CrossRef]
- Häikiö, K., Cloutier, D., & Rugkåsa, J. (2020). Is health literacy of family carers associated with carer burden, quality of life, and time spent on informal care for older persons living with dementia? PLoS ONE, 15(11), e0241982. [Google Scholar] [CrossRef]
- Hellis, E., & Mukaetova-Ladinska, E. B. (2023). Informal caregiving and Alzheimer’s disease: The psychological effect. Medicina, 59(1), 48. [Google Scholar] [CrossRef] [PubMed]
- Henry, J. D., & Crawford, J. R. (2005). The short-form version of the depression anxiety stress scales (DASS-21): Construct validity and normative data in a large non-clinical sample. British Journal of Clinical Psychology, 44(2), 227–239. [Google Scholar] [CrossRef] [PubMed]
- Hudson, W. (1993). The walmyr assessment scales scoring manual: Index of family relations (1st ed.). Walmyr Publishing Company. [Google Scholar]
- James, T. A., James, D., & Larkey, L. K. (2021). Heart-focused breathing and perceptions of burden in Alzheimer’s caregivers: An online randomized controlled pilot study. Geriatric Nursing, 42(2), 397–404. [Google Scholar] [CrossRef]
- Kaleta, K., & Mróz, J. (2020). The relationship between basic hope and depression: Forgiveness as a mediator. Psychiatric Quarterly, 91(3), 877–886. [Google Scholar] [CrossRef] [PubMed]
- Kim, H. G., Cheon, E. J., Bai, D. S., Lee, Y. H., & Koo, B.-H. (2018). Stress and heart rate variability: A meta-analysis and review of the literature. Psychiatry Investigation, 15(3), 235–245. [Google Scholar] [CrossRef]
- Kılıç, D., Taşar, P. T., & Cengiz, M. (2024). The effect of mindfulness-based compassionate living training for informal caregivers of palliative inpatients on burnout and caregiving burden: A randomized controlled trial. Health Education Research, 39(4), 313–322. [Google Scholar] [CrossRef]
- Kong, Y.-L., Anis-Syakira, J., Jawahir, S., Tan, Y. R., Rahman, N. H. A., & Tan, E. H. (2021). Factors associated with informal caregiving and its effects on health, work, and social activities of adult informal caregivers in Malaysia: Findings from the national health and morbidity survey 2019. BMC Public Health, 21(1), 1–13. [Google Scholar] [CrossRef]
- Levy, K., Grant, P. C., Clem, K., Eadie, D. S., & Rossi, J. L. (2021). Holding onto hurt: The prevalence of interpersonal hurt and need for forgiveness-focused solutions for hospice family caregivers. Journal of Palliative Medicine, 24(8), 1139–1146. [Google Scholar] [CrossRef]
- Lindeza, P., Rodrigues, M., Costa, J., Guerreiro, M., & Rosa, M. M. (2020). Impact of dementia on informal care: A systematic review of family caregivers’ perceptions. BMJ Supportive & Palliative Care, 14(e1), e38–e49. [Google Scholar] [CrossRef]
- Loo, Y. X., Yan, S., & Low, L. L. (2022). Caregiver burden and its prevalence, measurement scales, predictive factors and impact: A review with an Asian perspective. Singapore Medical Journal, 63(10), 593–603. [Google Scholar] [CrossRef]
- López, J., Serrano, M. I., Giménez, I., & Noriega, C. (2021). Forgiveness interventions for older adults: A review. Journal of Clinical Medicine, 10(9), 1866. [Google Scholar] [CrossRef] [PubMed]
- Lovibond, P., & Lovibond, S. (1995). The structure of negative emotional states: Comparison of the depression anxiety stress scales (DASS) with the beck depression and anxiety inventories. Behaviour Research and Therapy, 33(3), 335–343. [Google Scholar] [CrossRef]
- Manevich, A., Rubin, S. S., Katz, M., Ben-Hayun, R., & Aharon-Peretz, J. (2023). Risk, resilience, and the two-track model of dementia grief among spouses of people living with cognitive decline. Gerontology & Geriatric Medicine, 9, 1–12. [Google Scholar] [CrossRef]
- McGee, J. S., Myers, D. R., Meraz, R., & Davie, M. (2021). Caring for a family member with early stage Alzheimer’s disease: Caregiver perceptions, connections, and relational dynamics with the sacred. Journal of Religion, Spirituality & Aging, 34(3), 196–207. [Google Scholar] [CrossRef]
- Meza-Cely, N., Sabella-Jiménez, V., Acosta-Reyes, J., Otero-Herrera, C., Pérez-Olivo, M. S., Ruiz-Plaza, D., Jiménez-Hernández, D., Silvera-Redondo, C., & Rolón-Martínez, G. (2020). Characterization of patients with early vs. late-onset Alzheimer’s dementia. Acta Médica Colombiana, 45(2), 22–29. [Google Scholar] [CrossRef]
- Morris, J. C. (1993). The clinical dementia rating (CDR): Current version and scoring rules. Neurology, 43(11), 2412. [Google Scholar] [CrossRef]
- Nemcikova, M., Katreniakova, Z., & Nagyova, I. (2023). Social support, positive caregiving experience, and caregiver burden in informal caregivers of older adults with dementia. Frontiers in Public Health, 11, 1104250. [Google Scholar] [CrossRef]
- Organization for Economic Co-Operation and Development [OECD]. (2021). Health at a glance 2021: OECD indicators. OECD Publishing. [Google Scholar] [CrossRef]
- Pearlin, L. I., Mullan, J. T., Semple, S. J., & Skaff, M. M. (1990). Caregiving and the stress process: An overview of concepts and their measures. The gerontologist, 30(5), 583–594. [Google Scholar] [CrossRef]
- Pereira, M. G., & Pedras, S. (2009). Lifestyle questionnaire (research version). Group Studies on Family Health & Illness. School of Psychology, University of Minho. [Google Scholar]
- Quinn, C., Nelis, S. M., Martyr, A., Victor, C., Morris, R. G., & Clare, L. (2019). Influence of positive and negative dimensions of dementia caregiving on caregiver well-being and satisfaction with life: Findings from the IDEAL study. The American Journal of Geriatric Psychiatry, 27(8), 838–848. [Google Scholar] [CrossRef]
- Rasmussen, K. R., Stackhouse, M., Boon, S. D., Comstock, K., & Ross, R. (2019). Meta-analytic connections between forgiveness and health: The moderating effects of forgiveness-related distinctions. Psychology & Health, 34(5), 515–534. [Google Scholar] [CrossRef]
- Rosenbusch, H., Soldner, F., Evans, A. M., & Zeelenberg, M. (2021). Supervised machine learning methods in psychology: A practical introduction with annotated R code. Social and Personality Psychology Compass, 15(2), e12579. [Google Scholar] [CrossRef]
- Ruisoto, P., Contador, I., Fernández-Calvo, B., Serra, L., Jenaro, C., Flores, N., Ramos, F., & Rivera-Navarro, J. (2020). Mediating effect of social support on the relationship between resilience and burden in caregivers of people with dementia. Archives of Gerontology and Geriatrics, 86, 103952. [Google Scholar] [CrossRef] [PubMed]
- So, M. K. P., Yuk, H., Tiwari, A., Cheung, S. T. Y., & Chu, A. M. Y. (2021). Predicting the burden of family caregivers from their individual characteristics. Informatics for Health and Social Care, 47(2), 211–222. [Google Scholar] [CrossRef]
- Sołtys, A., & Tyburski, E. (2020). Predictors of mental health problems in formal and informal caregivers of patients with Alzheimer’s disease. BMC Psychiatry, 20(1), 435. [Google Scholar] [CrossRef]
- Strelan, P. (2020). The stress-and-coping model of forgiveness: Theory, research, and the potential of dyadic coping. In Handbook of forgiveness (pp. 63–73). Routledge. [Google Scholar]
- Thompson, L. Y., Snyder, C. R., Hoffman, L., Michael, S. T., Rasmussen, H. N., & Billings, L. S. (2005). Dispositional forgiveness of self, others, and situations. Journal of Personality, 73(2), 313–359. [Google Scholar] [CrossRef]
- Tsai, C.-F., Hwang, W.-S., Lee, J.-J., Wang, W.-F., Huang, L.-C., Huang, L.-K., Lee, W.-J., Sung, P.-S., Liu, Y.-C., Hsu, C.-C., & Fuh, J.-L. (2021). Predictors of caregiver burden in aged caregivers of demented older patients. BMC Geriatrics, 21(1), 1–9. [Google Scholar] [CrossRef]
- Tulek, Z., Baykal, D., Erturk, S., Bilgic, B., Hanagasi, H., & Gurvit, I. H. (2020). Caregiver burden, quality of life and related factors in family caregivers of dementia patients in Turkey. Issues in Mental Health Nursing, 41(8), 741–749. [Google Scholar] [CrossRef]
- Vrettos, I., Anagnostopoulos, F., Voukelatou, P., Panayiotou, S., Kyvetos, A., Nikas, A., Kollia, D., & Niakas, D. (2023). Factors associated with health-related quality of life of informal caregivers of older patients and the mediating role of subjective caregivers’ burden. Psychogeriatrics, 23(2), 286–297. [Google Scholar] [CrossRef]
- Witten, I. H., Frank, E., & Hall, M. A. (2011). Chapter 11—The explorer. In Data mining: Practical machine learning tools and techniques (3rd ed., pp. 407–494). The Morgan Kaufmann Series in Data Management Systems. Morgan Kaufmann. [Google Scholar] [CrossRef]
- Zarit, S. H., & Zarit, J. M. (1983). The memory and behavior problems checklist and the burden interview. Pennsylvania State University, Gerontology Center. [Google Scholar]
- Zwar, L., König, H. H., & Hajek, A. (2023). Gender differences in mental health, quality of life, and caregiver burden among informal caregivers during the second wave of the COVID-19 pandemic in Germany: A representative, population-based study. Gerontology, 69(2), 149–162. [Google Scholar] [CrossRef] [PubMed]
| Family Caregivers | |||||
|---|---|---|---|---|---|
| Min | Max | Mean | SD | ||
| Age | 42 | 92 | 66.15 | 13.64 | |
| Education (years) | 0 | 20 | 4.53 | 3.58 | |
| Duration of care (years) | 0.5 | 9 | 3.98 | 2.20 | |
| Frequency | % | ||||
| Sex | Male | 29 | 22.3 | ||
| Female | 101 | 77.7 | |||
| Marital status | Married | 109 | 83.8 | ||
| Unmarried | 21 | 16.2 | |||
| Degree of kinship | Daughters | 55 | 42.3 | ||
| Spouses | 58 | 44.6 | |||
| Others (siblings, nieces, or close friends) | 17 | 13.1 | |||
| Co-habitation | Nuclear family | 120 | 90.2 | ||
| Extended family | 10 | 7.7 | |||
| Caregiving hours | 0–12 h | 7 | 5.4 | ||
| 13–24 h | 123 | 94.6 | |||
| First-time caregiver | Yes | 92 | 70.8 | ||
| No | 38 | 29.2 | |||
| Chose to be the primary caregiver | Yes | 103 | 79.2 | ||
| No | 27 | 20.8 | |||
| Presence of a secondary caregiver | Yes | 71 | 54.6 | ||
| No | 59 | 45.4 | |||
| Persons living with Alzheimer’s disease | |||||
| Continuous variables | Min | Max | Mean | SD | |
| Age | 60 | 101 | 85.19 | 5.97 | |
| Education | 0 | 9 | 1.38 | 1.91 | |
| Frequency | % | ||||
| Sex | Male | 44 | 33.8 | ||
| Female | 86 | 66.2 | |||
| Marital status | Married | 66 | 50.8 | ||
| Unmarried | 64 | 49.2 | |||
| Min | Max | Mean | SD | ||
| Duration of memory problems | 6 months | 10 years | 3.93 | 2.29 | |
| Frequency | % | ||||
| Previous treatments for memory problems | Yes | 44 | 33.8 | ||
| No | 86 | 66.2 | |||
| Stages of dementia (CDR) | Mild | 43 | 33.1 | ||
| Moderate | 37 | 28.5 | |||
| Severe | 50 | 38.5 | |||
| T1 | T1_Base | T2 | T2_Base | T1_T2 | T1_T2_Base | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | |
| Decision Trees | 14.89 | 0.49 | 14.71 | 0.50 | 14.21 | 0.47 | 10.52 | 0.71 | 12.21 | 0.61 | 10.78 | 0.69 |
| Random Forests | 16.38 | 0.38 | 17.26 | 0.32 | 14.32 | 0.46 | 11.97 | 0.62 | 10.34 | 0.72 | 11.53 | 0.65 |
| XGBoost | 17.34 | 0.31 | 16.62 | 0.37 | 11.51 | 0.65 | 11.56 | 0.65 | 8.97 | 0.79 | 9.88 | 0.74 |
| LightGBM | 17.97 | 0.26 | 17.9 | 0.27 | 15.06 | 0.40 | 11.57 | 0.65 | 14.18 | 0.47 | 12.19 | 0.61 |
| CatBoost | 13.86 | 0.56 | 14.78 | 0.5 | 13.58 | 0.51 | 14.21 | 0.47 | 12.06 | 0.62 | 11.13 | 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the University Association of Education and Psychology. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brito, L.; Cepa, B.; Brito, C.; Leite, Â.; Pereira, M.G. Risk Assessment Profiles for Caregiver Burden in Family Caregivers of Persons Living with Alzheimer’s Disease: An Exploratory Study with Machine Learning. Eur. J. Investig. Health Psychol. Educ. 2025, 15, 41. https://doi.org/10.3390/ejihpe15030041
Brito L, Cepa B, Brito C, Leite Â, Pereira MG. Risk Assessment Profiles for Caregiver Burden in Family Caregivers of Persons Living with Alzheimer’s Disease: An Exploratory Study with Machine Learning. European Journal of Investigation in Health, Psychology and Education. 2025; 15(3):41. https://doi.org/10.3390/ejihpe15030041
Chicago/Turabian StyleBrito, Laura, Beatriz Cepa, Cláudia Brito, Ângela Leite, and M. Graça Pereira. 2025. "Risk Assessment Profiles for Caregiver Burden in Family Caregivers of Persons Living with Alzheimer’s Disease: An Exploratory Study with Machine Learning" European Journal of Investigation in Health, Psychology and Education 15, no. 3: 41. https://doi.org/10.3390/ejihpe15030041
APA StyleBrito, L., Cepa, B., Brito, C., Leite, Â., & Pereira, M. G. (2025). Risk Assessment Profiles for Caregiver Burden in Family Caregivers of Persons Living with Alzheimer’s Disease: An Exploratory Study with Machine Learning. European Journal of Investigation in Health, Psychology and Education, 15(3), 41. https://doi.org/10.3390/ejihpe15030041







