Cortical Contrast Processing in Retinitis Pigmentosa: Evidence of PVEPs Spatial Functions
Abstract
1. Introduction
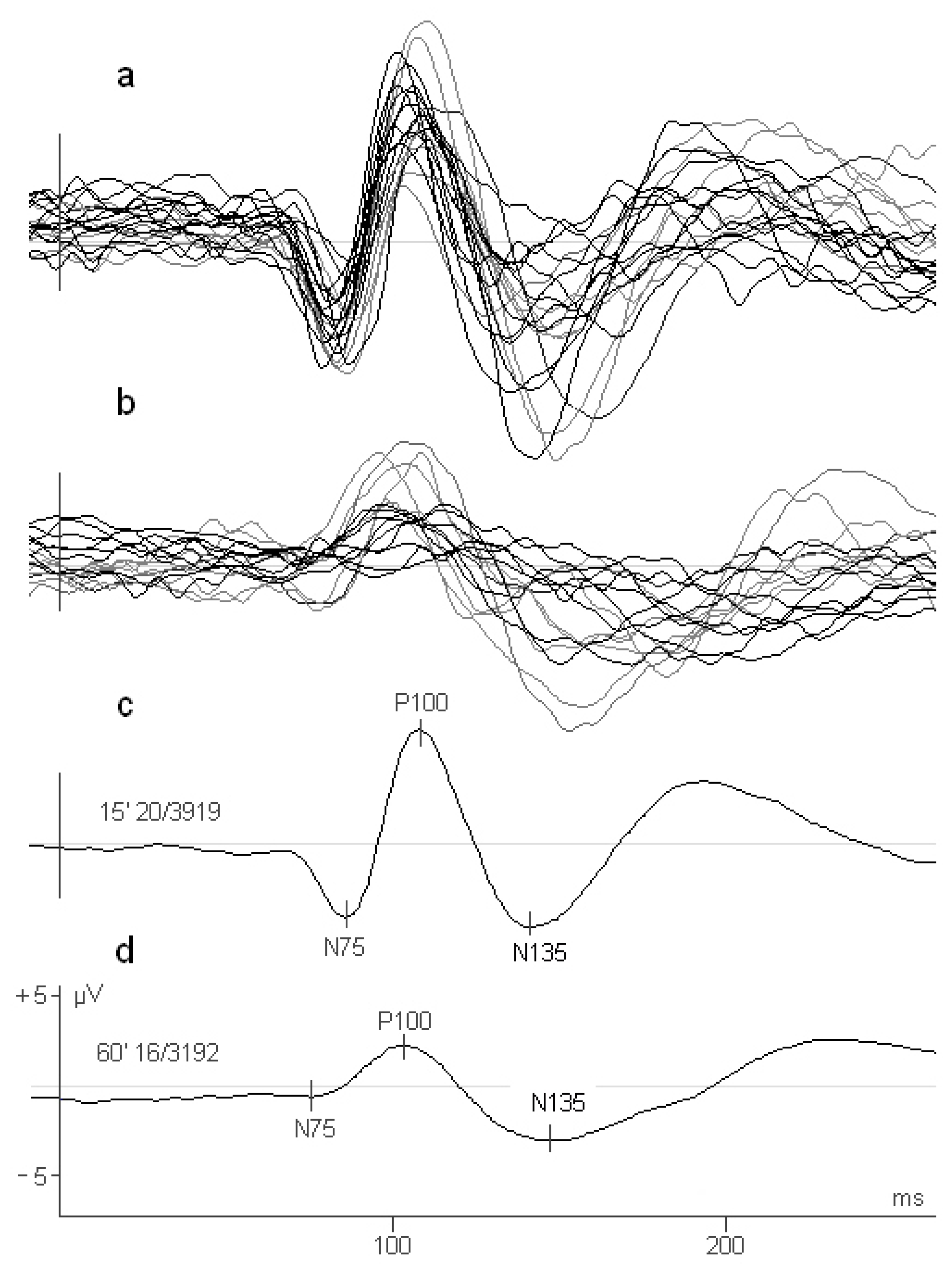
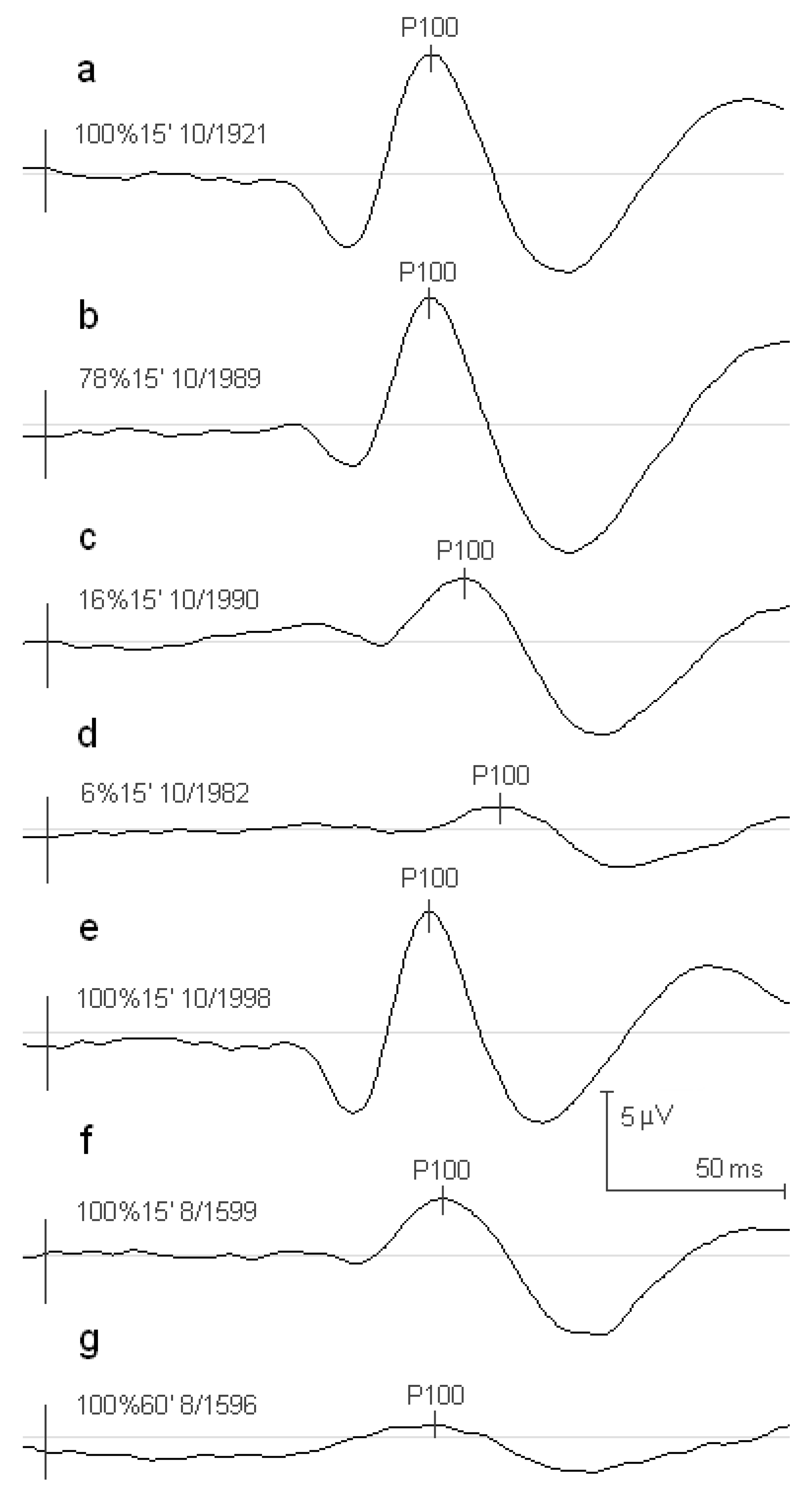
2. Materials and Methods
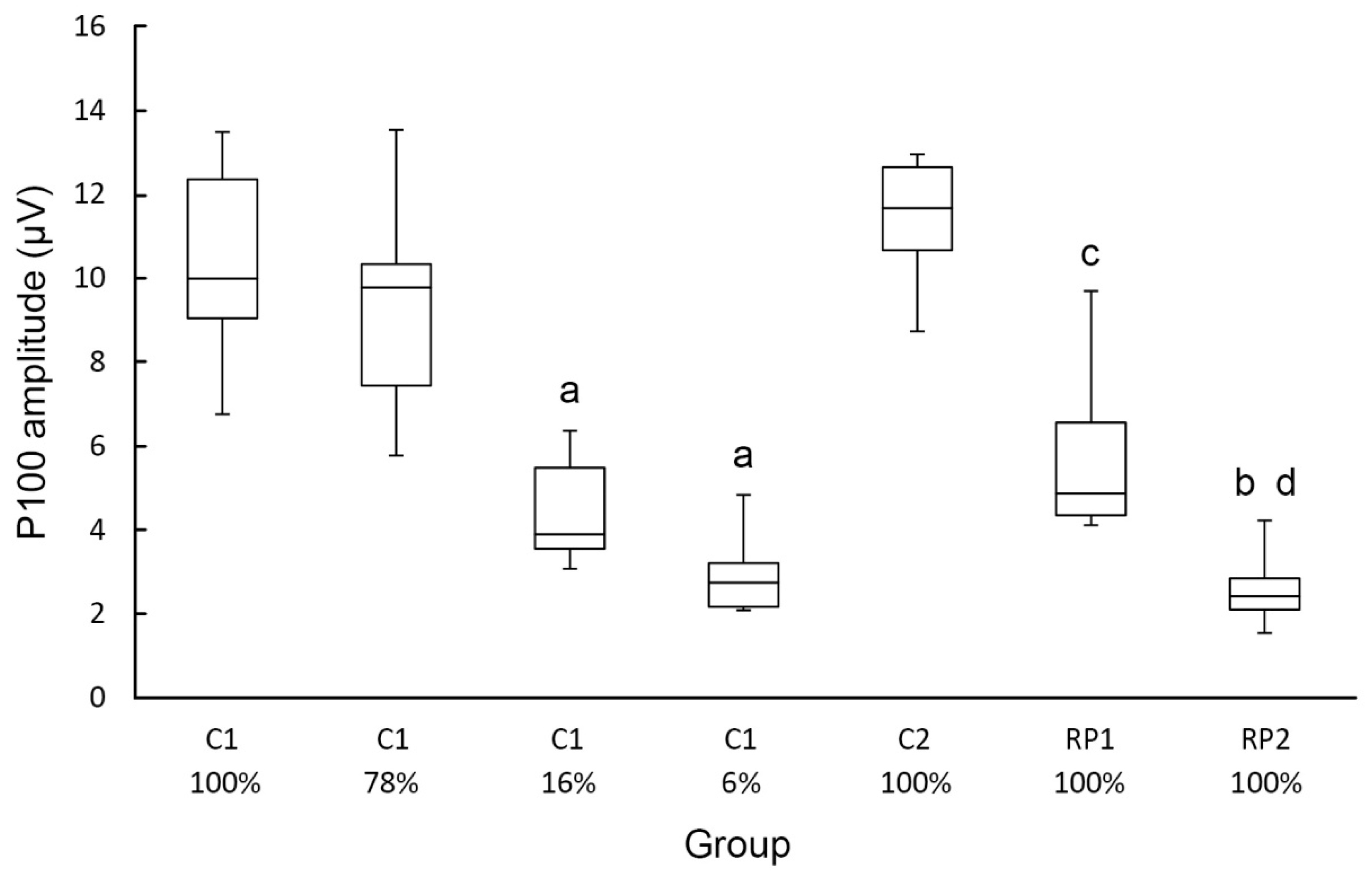
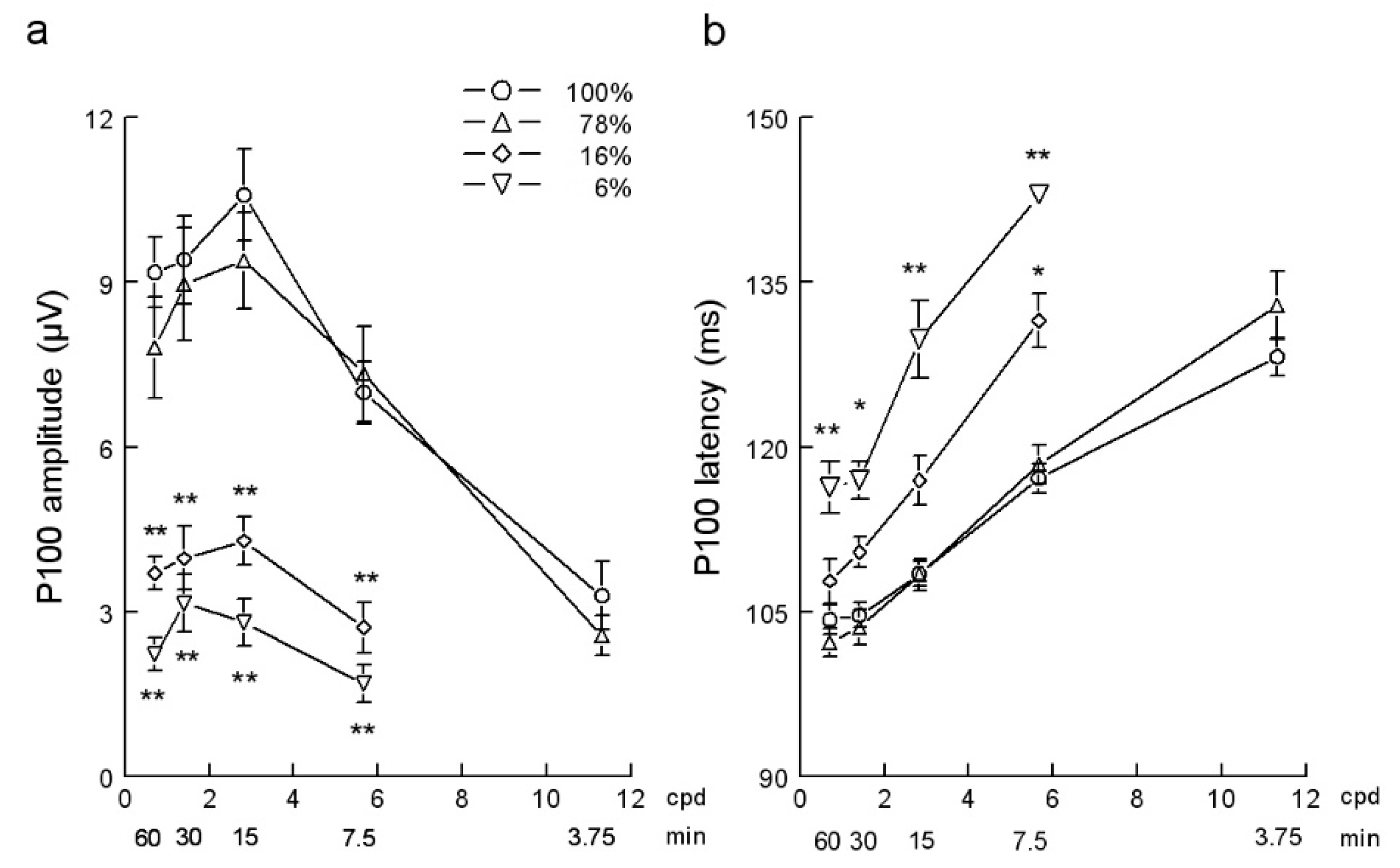
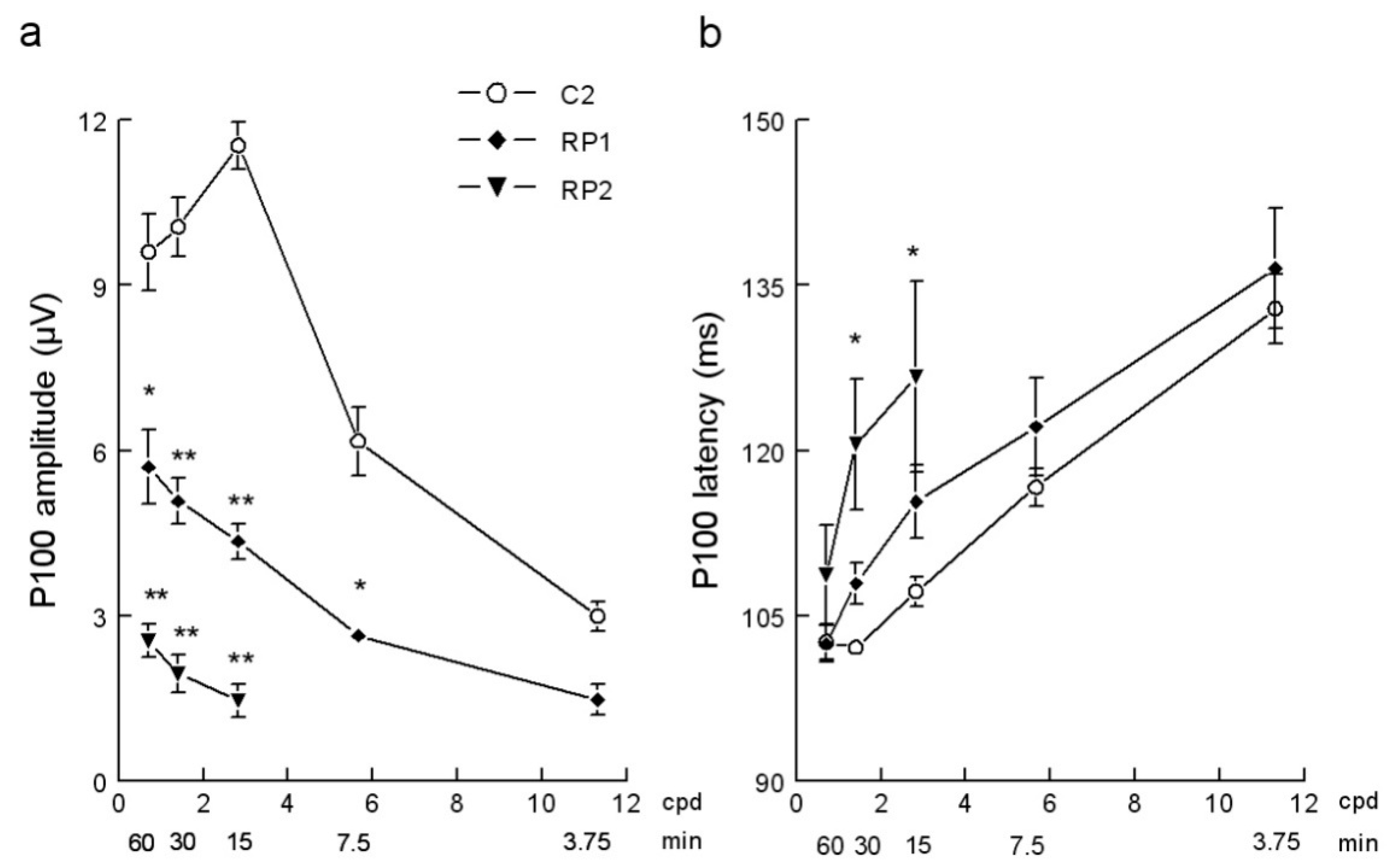
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fahim, A.T.; Daiger, S.P.; Weleber, R.G. Nonsyndromic Retinitis Pigmentosa Overview. GeneReviews 2017, Updated 19 January 2017. Available online: http://www.ncbi.nlm.nih.gov/books/NBK1417 (accessed on 16 October 2020).
- Vezinaw, C.M.; Fishman, G.A.; McAnany, J.J. Visual impairment in retinitis pigmentosa. Retina 2020, 40, 1630–1633. [Google Scholar] [CrossRef] [PubMed]
- Birtel, J.; Gliem, M.; Oishi, A.; Müller, P.L.; Herrmann, P.; Holz, F.G.; Mangold, E.; Knapp, M.; Bolz, H.J.; Issa, P. Genetic testing in patients with retinitis pigmentosa: Features of unsolved cases. Clin. Exp. Ophthalmol. 2020, 47, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Vaccarella, L.; Olatunji, S.; Cebulla, C.; Christoforidis, J. Diagnostic Challenges in Retinitis Pigmentosa: Genotypic Multiplicity and Phenotypic Variability. Curr. Genom. 2011, 12, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.R.L.; Kim, H.J.; Ueda, X.K.; Zhao, X.Z.; Owji, A.P.; Yang, X.T.; Stephen, H.; Tsang, S.H.; Sparrow, J.R. Effects of deficiency in the RLBP1-encoded visual cycle protein CRALBP on visual dysfunction in humans and mice. J. Biol. Chem. 2020, 295, 6767–6780. [Google Scholar] [CrossRef]
- Goureau, O.; Orieux, G. Photoreceptor cell transplantation for future treatment of retinitis pigmentosa. Med. Sci. 2020, 36, 600–606. [Google Scholar] [CrossRef]
- Moore, S.M.; Skowronska-Krawczyk, D.; Chao, D.L. Targeting of the NRL Pathway as a Therapeutic Strategy to Treat Retinitis Pigmentosa. J. Clin. Med. 2020, 9, 2224. [Google Scholar] [CrossRef]
- Özmert, E.; Arslan, U. Management of retinitis pigmentosa by Wharton’s jelly derived mesenchymal stem cells: Preliminary clinical results. Stem Cell Res. Ther. 2020, 11, 25. [Google Scholar] [CrossRef]
- Sahni, J.N.; Angi, M.; Irigoyen, C.; Semeraro, F.; Romano, M.R.; Permeggiani, F. Therapeutic challenges to retinitis pigmentosa: From neuroprotection to gene therapy. Curr. Genom. 2011, 12, 276–284. [Google Scholar] [CrossRef]
- Markand, O.N. Visual Evoked Potentials. In Clinical Evoked Potentials; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Odom, J.V.; Bach, M.; Brigell, M.; Holder, G.E.; McCulloch, D.L.; Mizota, A.; Tormene, A.P. ISCEV standard for clinical visual evoked potentials: (2016 update). Doc. Ophthalmol. 2016, 133, 1–9. [Google Scholar] [CrossRef]
- Fhale, M.; Bach, M. Origin of the visual evoked potentials. In Principles and Practice of Clinical Electrophysiology of Vision; MIT Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Paranhos, F.R.; Katsum, O.; Arai, M.; Nehemy, M.B.; Hirose, T. Pattern reversal visual evoked response in retinitis pigmentosa. Doc. Ophthalmol. 1998, 96, 321–331. [Google Scholar] [CrossRef]
- Janaky, M.; Palffy, A.; Horvath, G.; Tuboly, G.; Benedek, G. Pattern-reversal electroretinograms and visual evoked potentials in retinitis pigmentosa. Doc. Ophthalmol. 2008, 117, 27–36. [Google Scholar] [CrossRef]
- Hassankarimi, H.; Jafarzadehpur, E.; Mohammadi, A.; Mohammad Reza Noori, S. Low-contrast Pattern-reversal Visual Evoked Potential in Different Spatial Frequencies. JOVR 2020, 15, 362–371. [Google Scholar] [CrossRef]
- Nakamura, M.; Kakigi, R.; Okussa, T.; Hoshiyama, M.; Watanabe, K. Effects of check size on pattern reversal visual evoked magnetic field and potential. Brain Res. 2000, 872, 77–86. [Google Scholar] [CrossRef]
- Papathanasopoulos, P.G.; Papakostopoulos, D. Pattern reversal visual evoked potentials in retinitis pigmentosa. Int. J. Psychophysiol. 1994, 16, 245–250. [Google Scholar] [CrossRef]
- Porciatti, V. Electrophysiological assessment of retinal ganglion cell function. Exp. Eye Res. 2015, 141, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Rimmer, S.; Iragui, V.; Klauber, M.R.; Karz, B. Retinocortical time exhibits spatial selectivity. IOVS 1989, 30, 2045–2049. [Google Scholar] [CrossRef]
- Sanes, J.R.; Masland, R.H. The types of retinal ganglion cells: Current status and implications for neuronal classification. Annu. Rev. Neurosci. 2015, 38, 221–246. [Google Scholar] [CrossRef] [PubMed]
- Atilgan, N.; Yu, S.M.; He, S. Visual crowding effect in the parvocellular and magnocellular visual pathways. J. Vis. 2020, 20, 6. [Google Scholar] [CrossRef]
- Silveira, L.C.; Saito, C.A.; Lee, B.B.; Kremers, J.; Filho, M.; Kilavik, B.E.; Yamada, E.S.; Perry, V.H. Morphology and physiology of primate M- and P-cells. Prog. Brain Res. 2004, 144, 21–46. [Google Scholar] [CrossRef]
- Jones, B.W.; Pfeiffer, R.L.; Ferrell, W.D.; Watt, C.D.; Marmor, M.; Marc, R.E. Retinal remodeling in human retinitis pigmentosa. Exp. Eye Res. 2016, 150, 149–165. [Google Scholar] [CrossRef]
- Alexander, K.R.; Rajagopalan, A.S.; Seiple, W.; Zemon, V.M.; Fishman, G.A. Contrast response properties of magnocellular and parvocellular pathways in retinitis pigmentosa assessed by the visual evoked potential. IOVS 2005, 46, 2967–2973. [Google Scholar] [CrossRef]
- Hillyard, S.A.; Anllo-Vento, L. Event-related brain potentials in the study of visual selective attention. Proc. Natl. Acad Sci. USA 1998, 95, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Noesselt, T.; Hillyard, S.A.; Woldorff, M.G.; Schoenfeld, A.; Hagner, T.; Hinrichs, H.; Heinze, H. Delayed striate cortical activation during spatial attention. Neuron 2002, 35, 575–587. [Google Scholar] [CrossRef]
| Patient | Sex | Age | Visual | Inheritance |
|---|---|---|---|---|
| (Years) | Acuity | Pattern | ||
| Group RP1 | ||||
| 1 | Female | 54 | 0.40 | S |
| 2 | Female | 51 | 0.67 | AR |
| 3 | Male | 35 | 1.00 | AR |
| 4 | Male | 25 | 0.67 | AD |
| 5 | Female | 23 | 1.00 | AD |
| 6 | Female | 50 | 0.40 | S |
| 7 | Female | 35 | 0.40 | AD |
| 8 | Male | 30 | 0.40 | AD |
| Group RP2 | ||||
| 9 | Male | 52 | 0.40 | S |
| 10 | Female | 40 | 0.40 | S |
| 11 | Female | 48 | 0.20 | AD |
| 12 | Female | 29 | 0.10 | AR |
| 13 | Female | 52 | 0.05 | AR |
| 14 | Female | 57 | 0.40 | AR |
| 15 | Female | 38 | 0.67 | S |
| 16 | Female | 48 | 0.29 | S |
| CheckWidth | Group | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (cpd) | C1 | C2 | RP1 | RP2 | |||||||||||||||||
| 100% | 78% | 16% | 6% | 100% | 100% | 100% | |||||||||||||||
| Amplitude (µV) | |||||||||||||||||||||
| 0.71 | 9.18 | ± | 2.05 | 7.81 | ± | 2.89 | 3.70 | ± | 0.97 a | 2.22 | ± | 0.96 a | 9.60 | ± | 2.18 | 5.70 | ± | 1.93 d | 2.54 | ± | 0.84 c,f |
| 1.41 | 9.41 | ± | 2.53 | 8.97 | ± | 3.27 | 3.97 | ± | 1.84 a | 3.15 | ± | 1.68 a | 10.06 | ± | 1.70 | 5.08 | ± | 1.15 c | 1.95 | ± | 0.89 c,e |
| 2.83 | 10.59 | ± | 2.66 | 9.39 | ± | 2.79 | 4.29 | ± | 1.36 a | 2.80 | ± | 1.14 a | 11.54 | ± | 1.37 | 4.35 | ± | 0.90 c | 1.46 | ± | 0.59 c,e |
| 5.66 | 6.99 | ± | 1.81 | 7.33 | ± | 2.72 | 2.71 | ± | 1.31 a | 1.69 | ± | 0.60 a | 6.17 | ± | 1.97 | 2.64 | ± | 0.45 d | |||
| 11.31 | 3.29 | ± | 1.85 | 2.56 | ± | 1.09 | 2.99 | ± | 0.81 | 1.47 | ± | 0.57 | |||||||||
| Latency (ms) | |||||||||||||||||||||
| 0.71 | 104.27 | ± | 4.40 | 102.22 | ± | 4.05 | 107.79 | ± | 6.45 | 116.31 | ± | 7.57 a | 102.61 | ± | 4.91 | 102.44 | ± | 4.60 | 108.71 | ± | 12.69 |
| 1.41 | 104.66 | ± | 3.52 | 103.58 | ± | 5.17 | 110.39 | ± | 4.43 | 116.95 | ± | 5.56 b | 102.12 | ± | 2.62 | 107.93 | ± | 5.29 | 120.57 | ± | 15.68 d |
| 2.83 | 108.45 | ± | 3.46 | 108.37 | ± | 4.61 | 116.93 | ± | 7.04 | 129.77 | ± | 9.40 a | 107.19 | ± | 4.19 | 115.36 | ± | 9.50 | 126.70 | ± | 17.38 d |
| 5.66 | 117.13 | ± | 4.14 | 118.41 | ± | 5.65 | 131.49 | ± | 6.96 b | 143.00 | ± | 1.47 a | 116.66 | ± | 5.37 | 122.15 | ± | 10.82 | |||
| 11.31 | 128.22 | ± | 5.11 | 132.89 | ± | 9.33 | 132.86 | ± | 9.37 | 136.53 | ± | 10.97 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancebo-Azor, R.; Sáez-Moreno, J.A.; Rodríguez-Ferrer, J.M. Cortical Contrast Processing in Retinitis Pigmentosa: Evidence of PVEPs Spatial Functions. Eur. J. Investig. Health Psychol. Educ. 2020, 10, 1010-1019. https://doi.org/10.3390/ejihpe10040071
Mancebo-Azor R, Sáez-Moreno JA, Rodríguez-Ferrer JM. Cortical Contrast Processing in Retinitis Pigmentosa: Evidence of PVEPs Spatial Functions. European Journal of Investigation in Health, Psychology and Education. 2020; 10(4):1010-1019. https://doi.org/10.3390/ejihpe10040071
Chicago/Turabian StyleMancebo-Azor, Rafael, José Antonio Sáez-Moreno, and José Manuel Rodríguez-Ferrer. 2020. "Cortical Contrast Processing in Retinitis Pigmentosa: Evidence of PVEPs Spatial Functions" European Journal of Investigation in Health, Psychology and Education 10, no. 4: 1010-1019. https://doi.org/10.3390/ejihpe10040071
APA StyleMancebo-Azor, R., Sáez-Moreno, J. A., & Rodríguez-Ferrer, J. M. (2020). Cortical Contrast Processing in Retinitis Pigmentosa: Evidence of PVEPs Spatial Functions. European Journal of Investigation in Health, Psychology and Education, 10(4), 1010-1019. https://doi.org/10.3390/ejihpe10040071





