Abstract
Introduction: Adverse drug reactions associated with efavirenz (EFV) therapy are poorly described beyond the first year of treatment. We aimed to describe the incidence and predictors of EFV-related adverse drug reactions (ADRs) in a cohort of adult Nigerian HIV-infected patients on antiretroviral therapy (ART). Methods: This retrospective cohort study utilized clinical data of HIV-1 infected adults (aged ≥15 years), commenced on efavirenz containing-regimen between January 2004 and December 2011. The time-dependent occurrence of clinical adverse events as defined by the World Health Organization was analyzed by Cox regression analysis. Results: A total of 2920 patients with baseline median (IQR) age of 39 (33-46) years, largely made up of men (78%) were included in the study. During 8834 person-years of follow up, 358 adverse drug events were reported; the incidence rate was 40.3 ADRs per 1000 person-years of treatment. Lipodystrophy and neuropsychiatric disorders were the most common ADRs with incidences of 63 and 30 per 1000 patients respectively. About one-third of the neuropsychiatric adverse events were within 12 months of commencement of ART. The risk of neuropsychiatric ADRs was independently predicted for women [adjusted hazard ratio (aHR) 9.05; 95% CI: 5.18-15.82], those aged <40 years (aHR 2.59; 95% CI: 1.50-4.45), advanced HIV disease (WHO stage 3 or 4) [aHR 2.26; 95% CI: 1.37-3.72], and zidovudine [aHR 2.21; 95% CI: 1.27-3.83. or stavudine [aHR 4.22; 95% CI: 1.99-8.92] containing regimen compared to tenofovir. Conclusion: Neuropsychiatric adverse drug events associated with efavirenz-based ART had both early and late onset in our clinical cohort of patients on chronic EFV therapy. Continuous neuropsychiatric assessment for improved detection and management of neuropsychiatric ADRs is recommended in resource-limited settings where the use of efavirenz-based regimens has been scaled up.
Introduction
Efavirenz (EFV) has demonstrated high antiretroviral efficacy in several clinical trials and is currently a first-line drug for treatment of human immunodeficiency virus (HIV) infection in several countries [1,2]. Despite its reported efficacy[2,3]. and wide acceptance in the management of HIV infection, EFV use has been associated with an array of adverse effects [1,4,5]. Neuropsychiatric disorders are the most common and significant adverse effects associated with EFV therapy. Others include rash, lipodystrophy, and gynecomastia [4,5,6]. EFV-associated adverse events may compromise adherence to treatment and lead to treatment discontinuation. Some studies have reported treatment discontinuation rates ranging from 4% to 46% related to neuropsychiatric side effects of EFV [7,8].
Studies have reported ethnic variability in the incidence of neuropsychiatric disorders associated with EFV therapy [9,10]. In US and European cohorts, one-half of patients have neuropsychiatric symptoms after initiating EFV therapy, but these symptoms usually resolve within one month [11]. People of African ancestry with a variant of hepatic enzyme CYP2B6 may experience slower clearance of EFV from plasma and increased neurotoxicity [9,10].
Efavirenz plus tenofovir (TDF) and lamivudine (3TC) or emtricitabine (FTC) was recommended as the preferred first-line regimen for the treatment and prevention of HIV in adults and adolescents by the World Health Organization (WHO) in its 2013 consolidated guideline for the management of HIV infection [12]. The implementation of this public health strategy for the management of HIV infection is expected to result in rapid scale-up in the use of EFV-based regimens, especially in resource-limited settings. There is, therefore, an overarching need to conduct locally-relevant epidemiological studies to inform programmatic decisions given the shift towards EFV-containing regimens for the management of HIV infection [12]. Such studies are imported against the background of reported ethnic variability in the incidence of neuropsychiatric disorders associated with EFV therapy [9,10]. The current study utilizes longitudinal data spanning a period of eight years and aims to describe the incidence, type and predictors of EFV-related adverse drug reactions (ADRs) in a clinical cohort of adult Nigerian patients on antiretroviral therapy (ART).
Methods
Study setting
The study was carried out at the HIV clinic at the Jos University Teaching Hospital (JUTH), Jos, Nigeria. The clinic provides comprehensive HIV treatment; care and support services to HIV-infected persons within Jos and the neighboring states (provinces). Since 2004, the clinic has been supported by the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR). During the period covered by the study, the clinic had about 14,000 patients in care and over 9000 on ART.
Study design
A retrospective cohort study was conducted utilizing clinical data of HIV-1 infected adults (aged ≥15 years), who received EFV as part of their initial antiretroviral therapy between January 2004 and December 2011. Patients’ data up to December 31, 2012 were included in the study to allow for at least one year of follow-up.
Based on the Nigerian National ART guidelines [13], HIV-positive patients were initiated on ART at a CD4 T-cell count <200 cells/cmm or a CD4 count of 200-350 cells/cmm with HIV-related symptoms. First-line ART combinations were non-nucleoside reversetranscriptase inhibitors (NNRTI) (nevirapine [NVP] or efavirenz [EFV]) plus 2 nucleoside reverse-transcriptase inhibitors (NRTIs), often stavudine (d4T), didanosine (DDI), zidovudine (AZT), tenofovir (TDF), or abacavir (ABC) plus lamivudine (3TC). The ART guideline used during the study period did not recommend EFV-based therapy for women of childbearing age because of the risk of teratogenicity [13]. Patients on a non-EFV-based regimen were excluded from the study. Patients’ demographic, clinical, pharmacy and laboratory data were captured in an electronic database (FileMaker Pro, v10; File Maker, Inc, Santa Clara, California, USA) designed by the Harvard
PEPFAR/APIN program and utilized in the clinic. Dispensing of antiretroviral drugs (ARVs) as well as monitoring of ART efficacy and toxicity was done monthly and at scheduled clinical visits and documented in real time in the electronic database. Monitoring of ART efficacy and toxicity was performed based on standard clinical procedures. However, most ADR reports were based on spontaneous reports by patients. The definition and grading of ADRs were according to WHO severity classification and pharmacovigilance definitions [14]. Grade 1 ADRs were defined as “mild” events, with no limitation of daily activities; Grade 2, “moderate” ADRs with mild to moderate limitation of activities; Grade 3, “severe” ADR with marked limitation of activities and Grade 4, “life-threatening” ADR with extreme limitation of activities and significant medical intervention.
Hepatitis B serum antigen (HBsAg) was determined using Enzyme immunoassay (EIA) (Monolisa HBsAg Ultra3; BioRad, Hercules, CA, USA), while flow cytometry was used to determine CD4+ T-cell counts (Partec, GmbH, Munster, Germany).
All patients included in the study provided written informed consent for the use of their data for research as approved by the institutional review board at the Jos University Teaching Hospital. The use of secondary data was approved by the Harvard School of Public Health.
Study endpoints
Patient’s data were analyzed from the time of ART initiation (baseline) up to the date of an ADR or the end of the study period, which was pre-defined as December 31, 2012. Patients were censored at the date of their last ARV pick-up, transfer to other treatment facilities, withdrawal from treatment, or death. The incidence rate of ADR was expressed as the number of patients with at least one occurrence of the given event per 1000 person-years.
Statistical analysis
Descriptive analyses including median [interquartile range (IQR)] for numerical variables, frequencies and proportions for categorical variables were performed. Bivariate analyses to assess factors associated with ADRs were performed using Pearson Chi-square test. Variables that were significantly associated with ADR (p-value < .10) in the bivariate analysis were fitted into a Cox regression model and those with a p-value < .05 were considered significant in the multivariable analysis, calculating hazard ratios (HR) and 95% confidence intervals (CI). Missing data were assumed to be missing at random and were not included in the bivariate and multivariable analysis. IBM SPSS Statistics for Windows, Version 20 (IBM Corp, Armonk, New York, USA) was used for the statistical analysis.
Results
Cohort description
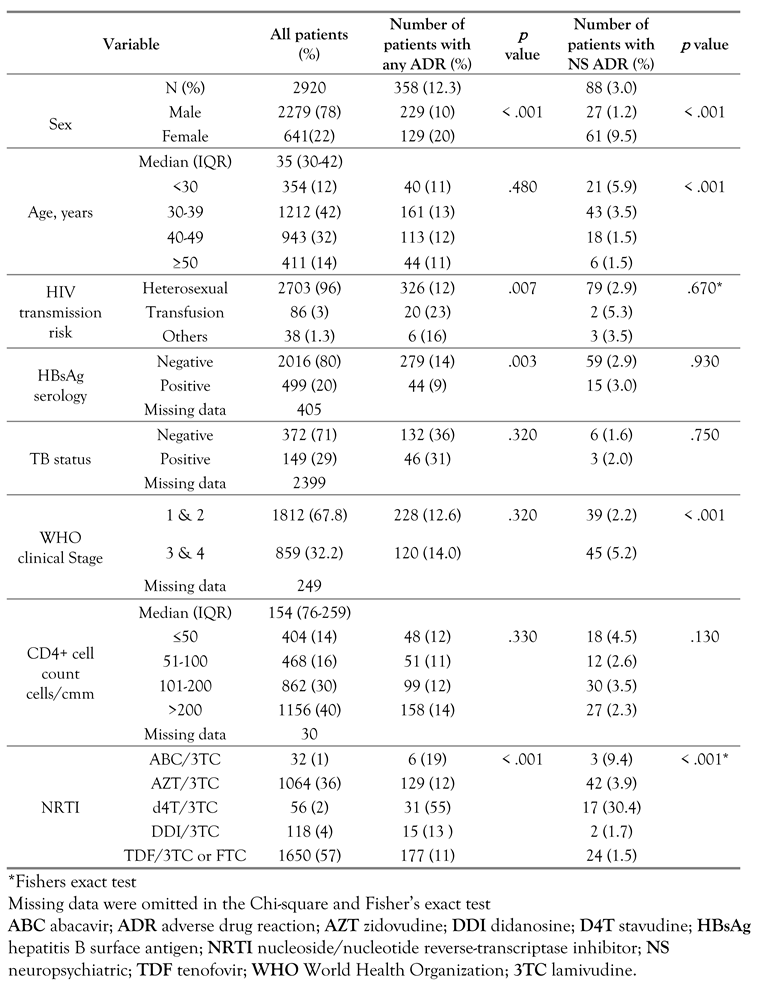
A total of 2920 patients made up of mostly men (78%) were followed for a median (IQR) duration of 2.9 (1-5) years. Cohort characteristics are shown in Table 1. At treatment initiation, the median (IQR) age was 39 (33-46) years, CD4 cell count was 154 (76-259) cells/cmm, and about 68% were at WHO clinical stage 1 and 2.

Table 1.
Baseline and clinical characteristics of patients on efavirenz-containing regimen and bivariate association with occurrence of adverse drug reactions.
Incidence and severity of adverse drug reactions
A total of 358 adverse drug events were reported during 8834 person-years of follow-up, with an incidence rate (IR) of 40.5 ADRs per 1000 person-years (py) on EFV-based regimens. In terms of onset, 195 out of 358 (55%) ADRs were reported within 12 months of treatment initiation, while 50 (14%), 36 (10.1%), and 77 (21.5%) were within 13 to 24, 25 to 36 and greater than 36 months of commencement of treatment. The majority (55%) of the ADRs were Grade 3 in terms of severity while Grade 2 and Grade 1 ADRs accounted for 31.3% and 12.3% respectively. The proportion of life-threatening (Grade 4) ADRs was 1.4%. The incidence of ADRs varied across the NRTIs, and was 151 (31 events), 51.7 (6 events), 40.2 (176 events), 34.7 (128 events) and 33.8 (15 events) per 1000 py for d4T, ABC, TDF, AZT and DDI containing regimens respectively. Of those that experienced an ADR, 48.9% (n=175) had EFV substituted with nevirapine (NVP).
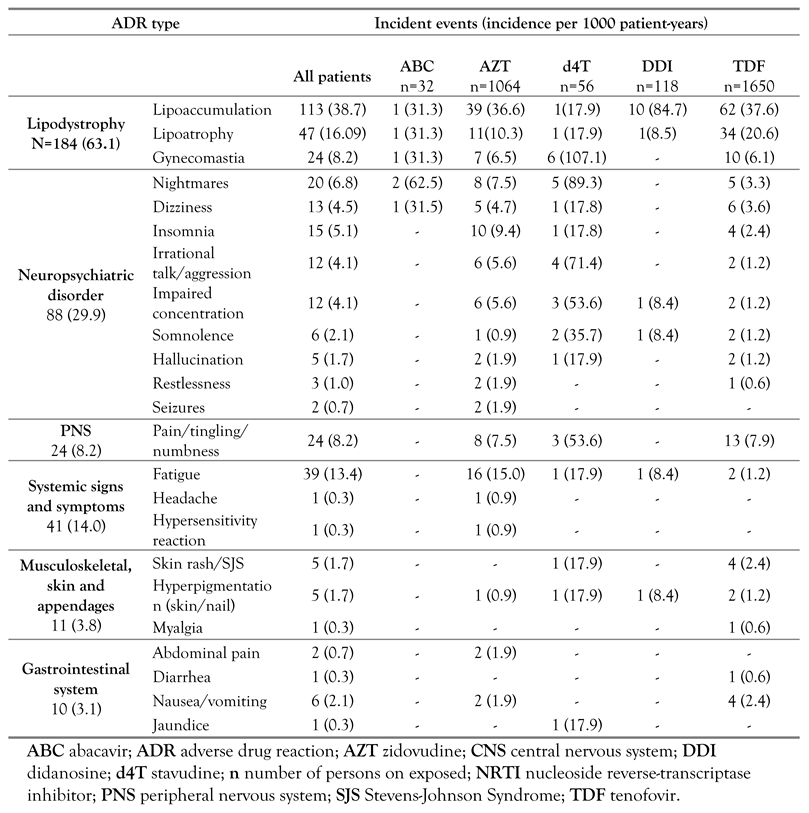
The most common ADR was lipodystrophy, with an incidence of 63.1 per 1000 py (184 events) followed by neuropsychiatric symptoms with an incidence of 29.9 per 1000 py (88 events). A little above one-third (33 out of 88) of the neuropsychiatric adverse events occurred within 12 months of commencement of ART, while 12 (13.6%), 13 (14.8%) and 30 (34.1%) were within 13 to 24, 25 to 36 and greater than 36 months of treatment. The profiles of reported ADRs for different NRTIs are summarized in Table 2. Lipoaccumulation was the most common lipodystrophy syndrome reported, while nightmares were the most frequently reported neuropsychiatric symptom (incidence of 6.8 per 1000). Twenty-four incidents of gynecomastia were recorded, mainly in patients who received regimens containing d4T (incidence 107 per 1000 py), while seizures were rare, with only two incidents reported among patients on AZT-based NRTI.

Table 2.
Incidence of adverse drug reactions per 1000 patient-years on efavirenz-based antiretroviral therapy in Jos, 2004-2012.
Predictors of adverse drug reaction
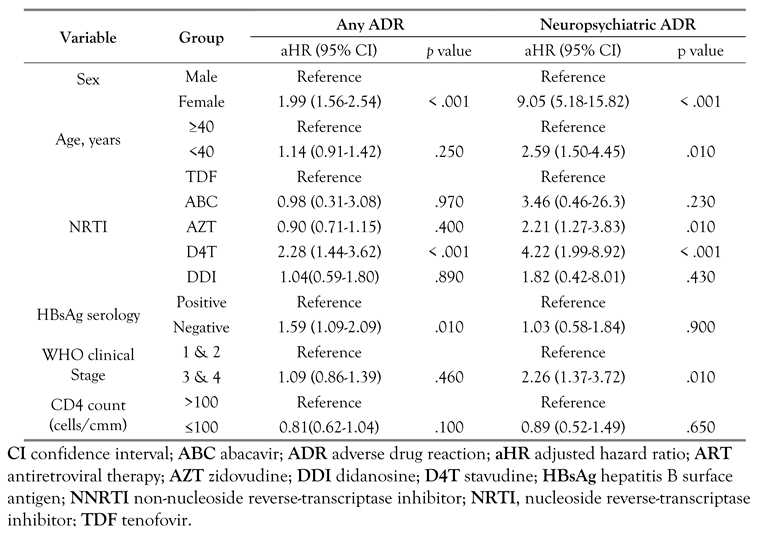
In the bivariate analysis (Table 1), the proportion of females with reported ADRs was higher than males (20% versus 10%, p < .001). Also neuropsychiatric symptoms were more common in females (9.5% versus 1.2%, p < .001). Across the different age groups, the percentage of reported neuropsychiatric ADRs declined with increasing age and was 5.9, 3.5, 1.5 and 1.5% for age groups <30, 30-39, 40-49, and ≥50 years respectively (p < .001). A higher proportion of neuropsychiatric ADRs were reported among those who initiated treatment at WHO clinical stage 3 or 4 compared to those who commenced treatment at WHO clinical stage 1 or 2, (p < .001). A significantly higher proportion of patients on ARV regimen containing d4T reported adverse drug events compared to those on the other NRTI backbones. After adjustment for confounders in the Cox multivariable analysis (Table 3), the risk of neuropsychiatric ADRs was about nine times higher in females compared to males [adjusted hazard ratio (aHR) 9.05; 95% CI:5.18-15.82], three-fold higher in patients aged <40 years, and double in advanced HIV disease (WHO stage 3 or 4) (aHR 2.26; 95% CI: 1.37-3.72). Across the NRTIs, the risk of neuropsychiatric ADRs increased about 2- and 4fold in patients on AZT and stavudine (d4T) containing NRTIs respectively compared to those on TDF.

Table 3.
Multivariate Cox regression analysis for predictors of adverse drug reactions among patients on efavirenz-based ART in Jos, Nigeria.
Discussion
This study observed early and late onset neuropsychiatric symptoms among patients on chronic efavirenz-based ART. EFV-related ADRs were significantly associated with female gender, younger age, advanced HIV disease, and use of AZT or d4T.
The incidence of neuropsychiatric symptoms observed in this study was lower than those reported in previous studies where between 40 to 70% of patients on chronic EFV therapy had at least one neuropsychiatric adverse effect within four weeks of treatment initiation [15,16]. ADRs reported in our study were based on spontaneous self-reporting and may under-report ADRs or increase the likelihood of more clinically significant ADRs being reported. Secondly, lack of active screening using assessment tools that are suitable for detecting EFV treatment-associated complications at a subclinical level could potentially result in under-reporting of the incidence of EFV central nervous system (CNS) symptoms in this study. In terms of time of onset of adverse events, early and late onset CNS symptoms were observed in this study. This finding is consistent with other studies where the most severe toxicity effects of EFV treatment have been consistently reported within the first 2–4 weeks after treatment initiation and symptoms generally cease after 6– 8 weeks [17,18]. There are also reports suggesting that as many as half of patients may develop delayed neuropsychiatric disorders with EFV [19,20]. Although the incidence of EFV-associated ADRs was low in this study, they were severe enough to result in the substitution of EFV in almost half of the patients with reported ADR. The proportion of patients who discontinued EFV due to ADRs in this study was higher than findings from Haiti and Côte d’Ivoire where only 4 to 10% of patients discontinued EFV as a result of toxicity [21,22].
We found an increased risk of neuropsychiatric ADRs in women compared to men. Increased risk of neuropsychiatric symptoms in women has been documented in earlier studies [23,24]. The reason for this trend has not been completely elucidated, but genderspecific toxicity might occur because EFV dosing is not based on body weight [24]. Other studies of HIV-infected women have also reported greater tendency of women to perceive side effects [25,26]. The significance of this finding is magnified by the fact that EFV-containing regimens are widely used in the prevention of mother-to-child transmission (PMTCT) of HIV in resourcelimited settings [14]. It is therefore important to intensify monitoring of women initiated on EFV-containing regimens and provide timely interventions to mitigate the negative impact of drug toxicity such as poor adherence to treatment, treatment discontinuation, poor health outcomes, and reduction in the quality of life.
Although a weak association was observed between the age of patients and the overall reporting of ADRs in our cohort, we found that patients younger than 40 years had a higher risk of CNS symptoms. This finding is important as the majority of our patient cohort fell within this age bracket. We suggest that EFV should be avoided in patients younger than 40 years where there are alternatives, and those that receive EFV should receive adequate counseling and monitoring.
An important finding of this study is the modifying effect of NRTIs on the ADR profile of EFV. The risk of neuropsychiatric symptoms was higher with AZT and d4T containing regimens compared to TDF. While reports of psychiatric symptoms associated with d4T are not typical [27], there are anecdotal reports of psychiatric symptoms, including mania and depression, in patients with no previous psychiatric history treated with AZT [28,29]. The mechanisms involved in AZT-associated psychiatric effects are not clear. However, its CNS penetration may explain the confusion, agitation, and insomnia reported in up to 5% of people who took AZT for one year [30].
The higher risk of CNS ADRs observed in patients with advanced HIV disease (WHO stage 3 or 4) in this study might be related to the pathology of HIV. In a minority of patients, CNS HIV-1 infection progresses to encephalitis during the late stages of systemic infection, which compromises brain function and presents clinically as acquired immunodeficiency syndrome dementia complex (ADC) [31]. CNS symptoms in advanced HIV disease could be amplified by EFV-related ADRs as observed in this study.
A limitation of this study was the fact that neuropsychiatric assessment was not performed at the time of ART initiation and within the first month of treatment which has been suggested by most studies as the period of greatest risk of CNS adverse effects [18,19]. Spontaneous reporting, which was the primary method of ADR reporting, may also have underestimated the burden of ADRs in our patients on EFVcontaining ART. However, the data may reflect the experience of many ART treatment centers in resource-limited settings implementing these ART regimens.
Conclusion
Neuropsychiatric adverse drug events associated with efavirenz-based ART had both early and late onset in our clinical cohort of patients on chronic EFV therapy. Continuous ADR monitoring in females, in patients younger than 40 years and in those with advanced HIV disease initiated on EFV-based ART is recommended in resource-limited settings where the use of EFV has been scaled up.
Author Contributions
IOA designed the study, drafted the manuscript. and conducted the statistical analyses. IAF, CWD, and KDF collected the data and performed the background literature review for the manuscript. MA, EOA MEA, OOA, PO, JI and PK provided intellectual support and critically edited the work. All authors reviewed and approved the final version of the manuscript.
Funding
This publication was facilitated, in part, by the US Department of Health and Human Services, Health Resources and Services Administration (U51HA02522- 0101) which funded HIV/AIDS treatment and care services at APIN, JUTH, Jos. Data analysis and writing of this paper was supported by the Medical Education Partnership Initiative in Nigeria (MEPIN) project funded by Fogarty International Center, the Office of AIDS Research, and the National Human Genome Research Institute of the National Institute of Health, the Health Resources and Services Administration (HRSA) and the Office of the US Global AIDS Coordinator under Award Number R24TW008878. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations.
Acknowledgments
We thank APIN, JUTH for permission to use the patients’ data.
Conflicts of Interest
All authors – none to declare.
References
- Staszewski, S.; Morales-Ramirez, J.; Tashima, K.T.; et al. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. Study 006 Team. N Engl J Med 1999, 341, 1865–1873. [Google Scholar] [CrossRef]
- Maggiolo, F. Efavirenz: A decade of clinical experience in the treatment of HIV. J Antimicrob Chemother 2009, 64, 910–928. [Google Scholar] [CrossRef]
- Staszewski S, Gallant J, Pozniak, A.L.; et al. Efficacy and safety of tenofovir DF (TDF) versus stavudine (d4T) when used in combination with lamivudine and efavirenz in antiretroviral-naive patients: 96-week preliminary interim results. In: Program and abstracts of the 10th Conference on Retroviruses and Opportunistic Infections; February 1014, 2003; Boston. Abstract 564b.
- Fumaz, C.R.; Tuldra, A.; Ferrer MJet, a.l. Quality of life, emotional status, and adherence of HIV-1-infected patients treated with efavirenz versus protease inhibitor-containing regimens. J Acquir Immune Defic Syndr 2002, 29, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, T.; Geist, C.; Young Bet, a.l. Comparison of neuropsychiatric side effects in an observational cohort of efavirenz- and protease inhibitor-treated patients. HIV Clin Trials 2005, 6, 187–196. [Google Scholar] [CrossRef]
- Agbaji, O.O.; Agaba, P.A.; Ekeh, P.N.; et al. Efavirenz-induced gynecomastia in HIV-infected Nigerian men: A report of six cases. J Med Med Sci 2011, 2, 1221–1224. [Google Scholar]
- Bartlett, J.A.; Chen, S.S.; Quinn, J.B. Comparative efficacy of nucleoside/nucleotide reverse transcriptase inhibitors in combination with efavirenz: Results of a systematic overview. HIV Clin Trials 2007, 8, 221–226. [Google Scholar] [CrossRef]
- Spire, B.; Carrieri, P.; Garzot, M.A.; L'henaff, M.; Obadia, Y. ; TRT-5 Group. Factors associated with efavirenz discontinuation in a large community-based sample of patients. AIDS Care 2004, 16, 558–564. [Google Scholar]
- Barrett, J.S.; Joshi, A.S.; Chai, M.; Ludden, T.M; Fiske, W.D.; Pieniaszek, H.J.; Jr. Population pharmacokinetic meta-analysis with efavirenz. Int J Clin Pharmacol Ther 2002, 40, 507–519.
- Pfister, M.; Labbe, L.; Hammer, S.M.; et al. Population pharmacokinetics and pharmacodynamics of efavirenz, nelfinavir, and indinavir: Adult AIDS Clinical Trial Group Study 398. Antimicrob Agents Chemother 2003, 47, 130–137. [Google Scholar] [CrossRef]
- Molina, J.M.; Ferchal, F.; Rancinan, C.; et al. Once-daily combination therapy with emtricitabine, didanosine, and efavirenz in human immunodeficiency virus-infected patients. J Infect Dis 2000, 182, 599–602. [Google Scholar] [CrossRef]
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach, June 2013. Available online: http://apps.who.int/iris/bitstream/10665/ 85321/1/9789241505727_eng.pdf (accessed on 6 May 2014).
- Federal Ministry of Health. National guidelines for HIV and AIDS treatment and care in adolescents and adults. Abuja, Nigeria. 2007. Available online: http://www.who.int/hiv/amds/Nigeria_ adult_2007.pdf (accessed on 18 February 2014).
- World Health Organization. A practical handbook on the pharmacovigilance of antiretroviral medicines. 9 June. Available online: http://www.who.int/hiv/ topics/pharmacovigilance/arv_pharmacovigilance_handbo ok.pdf?ua=1 (accessed on day month year).
- Puzantian, T. Central nervous system adverse effects with efavirenz: Case report and review. Pharmacotherapy 2002, 22, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Blanch, J.; Martínez, E.; Rousaud, A.; et al. Preliminary data of a prospective study on neuropsychiatric side effects after initiation of efavirenz. J Acquir Immune Defic Syndr 2001, 27, 336–343.
- Arendt, G.; de Nocker, D.; von Giesen, H.J.; Nolting, T. Neuropsychiatric side effects of efavirenz therapy. Expert Opin Drug Saf 2007, 6, 147–154. [Google Scholar] [CrossRef]
- Cespedes, M.S.; Aberg, J.A. Neuropsychiatric complications of antiretroviral therapy. Drug Saf 2006, 29, 865–874.
- Hawkins, T.; Geist, C.; Young, B.; et al. Comparison of neuropsychiatric side effects in an observational cohort of efavirenz- and protease inhibitor-treated patients. HIV Clin Trials 2005, 6, 187–196. [Google Scholar] [CrossRef]
- Gutiérrez, F.; Navarro, A.; Padilla, S.; et al. Prediction of neuropsychiatric adverse events associated with long-term efavirenz therapy, using plasma drug level monitoring. Clin Infect Dis 2005, 41, 1648–1653.
- Bartlett, J.A.; Chen, S.S.; Quinn, J.B. Comparative efficacy of nucleoside/nucleotide reverse transcriptase inhibitors in combination with efavirenz: Results of a systematic overview. HIV Clin Trials 2007, 8, 221–226. [Google Scholar] [CrossRef]
- Severe, P.; Leger, P.; Charles, M.; et al. Antiretroviral therapy in a thousand patients with AIDS in Haiti. N Engl J Med 2005, 353, 2325–2334. [Google Scholar] [CrossRef]
- Clifford, D.B.; Evans, S.; Yang, Y.; et al. Impact of efavirenz on neuropsychological performance and symptoms in HIV-infected individuals. Ann Intern Med 2005, 143, 714–721. [Google Scholar] [CrossRef] [PubMed]
- von Giesen, H.J.; Köller, H.; de Nocker, D.; Haslinger, B.A.; Arendt, G. Long-term safety and efficacy of NNRTI within the central nervous system. HIV Clin Trials 2003, 4, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Cederfjäll, C.; Langius-Eklöf, A.; Lidman, K.; Wredling, R. Gender differences in perceived health-related quality of life among patients with HIV infection. AIDS Patient Care STDS 2001, 15, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Ofotokun, I.; Pomeroy, C. Sex differences in adverse reactions to antiretroviral drugs. Top HIV Med 2003, 11, 55–59. [Google Scholar] [PubMed]
- Turjanski, N.; Lloyd, G.G. Psychiatric side-effects of medications: Recent developments. Adv Psychiatr Treat 2005, 11, 58–70. [Google Scholar] [CrossRef]
- Foster, R.; Olajide, D.; Everall, I.P. Antiretroviral therapy-induced psychosis: Case report and brief review of the literature. HIV Med 2003, 4, 139–144. [Google Scholar] [CrossRef]
- Maxwell, S.; Scheftner, W.A.; Kessler, H.A.; Busch, K. Manic syndrome associated with zidovudine treatment. JAMA 1988, 259, 3406–3407. [Google Scholar] [CrossRef]
- Rachlis, A.; Fanning, M.M. Zidovudine toxicity. Clinical features and management. Drug Saf 1993, 8, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Price, R.W.; Spudich, S. Antiretroviral therapy and central nervous system HIV type 1 infection. J Infect Dis 2008, 197 Suppl 3, S294–S306. [Google Scholar] [CrossRef]
© GERMS 2015.