Abstract
Vaccination is the most successful application of immunological principles to human health. Vaccine efficacy needs to be reviewed from time to time and its safety is an overriding consideration. DNA vaccines offer simple yet effective means of inducing broad-based immunity. These vaccines work by allowing the expression of the microbial antigen inside host cells that take up the plasmid. These vaccines function by generating the desired antigen inside the cells, with the advantage that this may facilitate presentation through the major histocompatibility complex. This review article is based on a literature survey and it describes the working and designing strategies of DNA vaccines. Advantages and disadvantages for this type of vaccines have also been explained, together with applications of DNA vaccines. DNA vaccines against cancer, tuberculosis, Edwardsiella tarda, HIV, anthrax, influenza, malaria, dengue, typhoid and other diseases were explored.
Introduction
Communicable diseases represent a worldwide problem. The prevention of communicable diseases is a public health priority. The primary goal of vaccine research progress in developing new vaccines is based on improved understanding of the molecular pathology of human disease and of the immune response in mammals. DNA (deoxyribonucleic acid) vaccination is a relatively new technology which utilizes genetically engineered DNA to produce an immunologic response. An important strategy to achieve this aim is to use DNA plasmids having antigens encoded on them. This antigen-encoding DNA plasmid can induce humoral and cellular immune response against parasites, bacteria and disease-producing viruses [1,2,3]. The expression of the antigen-encoding gene can be controlled by a strong mammalian promoter which can be used on a plasmid backbone of bacterial DNA [1,2,4]. Moreover various promoters, enhancers, and other elements were designed to elevate expression of the encoded protein in vaccine recipients. A number of vectors and the DNA transfer technologies have been reported by Khan also [5,6,7,8].
When transfected with DNA vaccines, cells transcribe, translate, and express the encoded proteins in the context of self-major histocompatibility complex (MHC) [1,2,9]. An important role in inducing immunity is played by professional antigen presenting cells (APCs), which are known to migrate to the primary lymphoid organs when directly transfected in the skin or muscle. In these organs they initiate an immune response [10,11,12] and cross-present antigen produced by transfected non-immune cells such as muscle cells [2,13,14,15,16,17].
Still experiments are in progress on nucleic acid vaccines. This technology has been applied on various bacterial, virus and parasitic models of disease. Moreover it was also utilized on several tumor models [18]. DNA vaccination emerged as a strong and efficient means of eliciting cell mediated and humoral responses in small animal models against a number of antigens from parasites and also from bacteria and viruses [19,20]. In humans as well as in large outbred animals, the efficiency of this vaccination has not been so encouraging. It continues to remain an immunological problem that has to be overcome [21].
This article is based on the modern as well as the traditional methods for literature survey. The search engines and databases used were ScienceDirect, PubMed, Google Scholar. The keywords used for the literature review were “DNA vaccine(s)”, “DNA vaccines, diseases” or DNA vaccines, applications”. The keywords were used alone or along with other related topics (cancer, HIV, dengue, malaria, typhoid, etc). The author used books as well as journals for the preparation of this manuscript. The papers cited in this article were not limited to a particular region; articles in English, dating back five years, including the year 2012, were considered, along with some older papers of historic value.
The aim of this paper was to create awareness about DNA vaccines, to describe the construction, working and designing strategies of DNA vaccines. This article has also explored the advantages, disadvantages and applications of this type of vaccines.
Construction of DNA vaccines
Genes of required interest coupled with a suitable promoter are injected directly into muscle or coated into gold micro particles and “shot” into the skin by pressurized gas using a gene gun. This can induce cellular and humoral immunity in experimental animals for a longer period of time. The mechanism appears to be through uptake and expression of the DNA in antigen presenting cells (APCs) [22]. The diagrammatic representation of DNA vaccines has been shown in Figure 1.
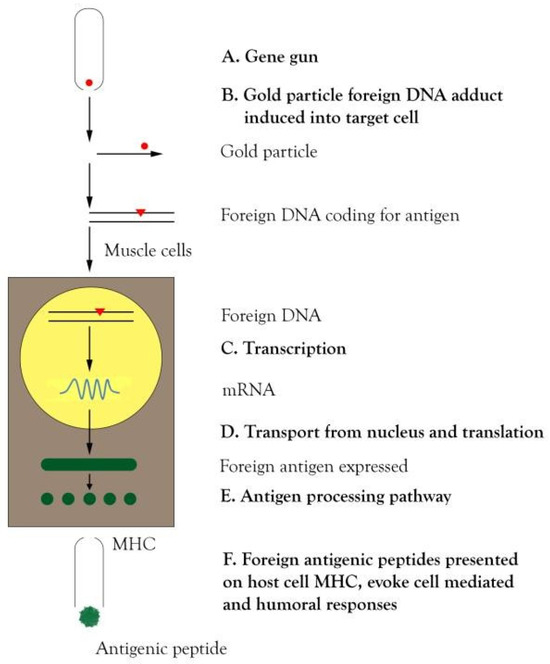
Figure 1.
Diagrammatic representation for the production of DNA vaccine. A) A gene gun is an instrument containing gold particles having DNA coated on it for DNA vaccines. B) The gold particle fired from the gene gun into the target cell gets integrated into the DNA of the target cell. C) Transcription takes place leading to the formation of mRNA. D) mRNA is transported from the nucleus for translation to the form of protein antigen. E) The expressed antigen enters into the antigen-processing pathway. F) The foreign antigenic peptides presented on host cell MHC evoke cell mediated and humoral responses. DNA deoxyribonucleic acid; MHC major histocompatibility complex; mRNA messenger ribonucleic acid.
The host’s response to administration of DNA vaccines is interconnected with the main aim of the vaccine: to act on the immune system and provide immunity to the host. The humoral immune response is the host defense that is mediated by antibodies present in the plasma, lymph and tissue fluids. It protects against extracellular bacteria and foreign macromolecules. The cell mediated immune response depends on antigen-specific T cells and on various non specific cells of the immune system. It protects against intracellular bacteria.
DNA vaccines raise both humoral and cellular immunity. The injected gene of the concerned DNA vaccine is expressed in the injected muscle cell and also in nearby APCs. The protein is made up of peptides which, after being processing as endogenous antigens through the MHC class I pathway, form the protein encoded by the concerned DNA and are expressed on the surface of both cell types. Cells that present the antigen in the context of class I MHC molecules stimulate development of cytotoxic T cells. The protein encoded by the injected DNA is also expressed as a soluble, secreted protein. This is taken up and finally processed, and presented through class II MHC molecules. This pathway provokes B-cell immunity and generates antibodies and B-cell memory against the protein. This response serves to defend the host from the concerned microorganism for which the particular DNA vaccine has been made [23].
The researchers later proposed three different mechanisms that contribute to the immunogenicity of DNA vaccines. First, the antigens encoded by the DNA are presented by somatic cells (myocytes or keratinocytes) to CD8 T cells through their MHC class I pathway. Second, the DNA immunization results in direct transfection of professional antigen presenting cells (APC) (e.g., dendritic cells). Third, cross-priming results from transfected somatic cells are phagocytosed by professional APCs which then present the antigens to T cells. As the muscle cells are not up to the mark at presenting antigens through MHC class I, the latter two mechanisms appear to be more appropriate to DNA vaccines [24].
Currently, attempts are also underway to incorporate DNA into the nasal tissue by using nasal drops. It should be noted that once inside the cells of the recipient, the plasmid does not replicate, but only expresses itself, and protein is produced. Usually, bacterial plasmids are used, and a gene encoding the antigen is inserted into the control of mammalian promoter and this chimeric plasmid is then introduced into the recipient. The recipient cell then expresses the foreign antigenic protein coded by the introduced DNA into the host. The immune system then responds to the antigen as to any other antigen entering the body [25].
Strategies for DNA vaccines
A number of features have to be kept in mind while designing a DNA vaccine. The selection of antigens, vector, delivery route, dose, timing, adjuvants, and boosting agents will all affect the outcome of vaccination. The reason behind this is that they affect the magnitude and quality of immunity elicited. The selection of target antigens should be given the first priority while designing a DNA vaccine. An individual must select the genes from the pathogen and also the form of the gene, whether the gene is mutated or wild type, intracellular or membrane-bound or secreted. After the selection of the desired gene, one can proceed for its modification to achieve the immunogenicity of the DNA vaccine.
The vectors used for expression of the antigen can also have a large impact on immunogenicity. Promoters, enhancers, and introns can affect the level of antigen expression. Most DNA vaccine studies use plasmids carrying promoters that constitutively yield high levels of protein in most mammalian tissues. Additional modifications can be made to increase protein production in transfected host cells. The most effective of these is codon optimization.
In order for a DNA vaccine to work, it is essential to incorporate DNA coding an appropriate antigen, to elicit the required antibody response of the immune system. A variety of factors may affect the route of choice. DNA vaccines can be easily injected with needles. They can be easily prepared in saline. The main advantages of biolistic technology, such as Gene Gun (Bio-Rad, USA) or Biojector 2000 (Bioject Medical Technologies, USA) lie in the fact that the technology possesses high efficiency [26].
Advantages and disadvantages of DNA vaccines
DNA vaccines appear to have certain advantages over conventional vaccines, for example the ability to induce a wider range of types of immune response. A number of advantages and disadvantages are listed in Table 1 and Table 2, respectively.

Table 1.
Advantages of DNA vaccines.

Table 2.
Disadvantages of DNA vaccines.
Applications of DNA vaccines
Tests of DNA vaccines in animal models have shown that these vaccines are able to induce protective immunity against a number of pathogens including influenza and rabies viruses. At present, human trials are under way with several DNA vaccines, including those for malaria, AIDS, influenza, Ebola and herpesvirus. The author describes the current studies on DNA vaccines in a number of diseases.
DNA vaccines against cancer
Cancer is a worldwide leading cause of death, and several malignancies are incurable by conventional therapies. Therefore, new anti-tumor immunotherapies are necessary to improve the outcome of patients with advanced cancer, and DNA vaccines are reliable forms of immunotherapy. DNA vaccines are a valuable form of antigen-specific immunotherapy, as they are safe, stable and can be easily produced. Moreover, tumor-specific antigens are expressed for a longer period of time as compared to RNA or protein-based vaccines [31].
DNA vaccination has become an effective strategy for the development of vaccines against cancer, including cervical carcinoma (CC). Persistent infection with human papillomaviruses (HPV) is the main etiological factor in cervical cancer, the second most common cancer in women worldwide [32]. The formation of CC is associated with HPV infection. Viral E6 and E7 oncoproteins are suitable targets for therapeutic vaccination. In this context, DNA vaccine against HPV type 16 was reported [33].
DNA vaccines against tuberculosis
Tuberculosis (TB) remains a major worldwide health problem [34]. TB is driven by the acquired immune response to the tubercle bacillus Mycobacterium tuberculosis. Use of therapeutic DNA vaccines is a promising strategy against TB. DNA vaccine expression of IL-2 and the HSP65 fusion gene was studied. It elevated the immunogenicity and protective as well as therapeutic effects of the HSP65-DNA vaccine against TB in mice. This was achieved by improving the Th1-type response [34]. Addition of immunostimulatory motifs in the transcribed region of a plasmid DNA vaccine elevated Th1 immune responses and the therapeutic effect against Mycobacterium tuberculosis in murine models [35]. Recent studies have described the efficacy of T-bet as Th1-inducing adjuvant in the context of Ag85B DNA-based vaccination. It could also prove to be a promising candidate for DNA vaccine development against TB [36]. A novel TB DNA vaccine was reported to have been synthesized. This vaccine utilizes an HIV-1 p24 protein backbone. It confers protection against Mycobacterium tuberculosis and simultaneously elicits humoral and cellular response to HIV-1 [37].
DNA vaccines against Edwardsiella tarda
Edwardsiella tarda is a Gram-negative bacterium of the family Enterobacteriaceae. It is a pathogen with a broad host range that includes humans, animal, and fish [38,39]. As a human pathogen, E tarda is known to cause gastroenteritis and is implicated in septicemia, meningitis, and wound infections [40]. Eta6 and FliC are the antigens found in E tarda. These two antigens are homologues to an ecotin precursor and the FliC flagellin, respectively. They were identified as a chimeric DNA vaccine. With the above information, pCE6 was constructed, which encodes an Eta6 fused in-frame to FliC. pCE6 was observed to elicit elevated levels of protection as compared to pEta6 [40].
DNA vaccines against HIV
Human immunodeficiency virus (HIV) causes acquired immunodeficiency syndrome (AIDS) and remains one of the most serious threats to global health. Today there are no vaccines to prevent HIV infection. As far as the knowledge of this author is concerned, all of the candidates explored so far are in the experimental stage. HIV-negative people were used to study the effect of preventive vaccine candidates to see if they can prevent infection [41]. The safety, stability, and ability for repeated homologous vaccination encourage the DNA vaccine platform as important candidate for an effective HIV-1 vaccine. The immunogenicity of DNA vaccines for HIV has been increased through improvement of the DNA vector, through the inclusion of molecular adjuvants, heterologous prime-boost strategies, and delivery with electroporation [42]. The principle behind electroporation is that it applies a small electric field across the site of injection that causes temporary membrane instability and produces an electric gradient, which elevates the cellular uptake of DNA. It is a useful technique as it increases the transfection efficiency of DNA vaccines in vivo [42]. Nanoparticles as drug-delivery systems have also been explained by the Editor-in-chief of this Journal in a previous editorial [43]. The study of nanoparticles provides a strong platform to combining protein- and DNA-based vaccines/antiretrovirals which can help the production, preclinical evaluation and the clinical testing in the near future [41].
DNA vaccines against anthrax
Anthrax is an infectious zoonotic disease caused by Bacillus anthracis, a spore-forming encapsulated bacteria. In human beings, three forms of anthrax have been recognized. They are cutaneous, gastroenteritis and pulmonary forms [44]. This disease is not common in western countries but the countermeasures against this disease are important because the spores of B anthracis can be used as bio-terror weapons [45]. DNA vaccination resulted in varying degrees of protection and appears to be a promising approach in this field [46]. The immunogenicity and efficacy of an anthrax/plague DNA fusion vaccine in a murine model has been described [47].
DNA vaccines against influenza
Each year, particularly in the months of February and September, the World Health Organization (WHO) recommends the influenza viruses to be included in influenza vaccines for the forthcoming winters in the Northern and Southern hemispheres respectively. Generally, influenza vaccines are often updated so as to be most effective against newly emerging strains of human influenza viruses that are likely to circulate in the forthcoming influenza season [48]. Influenza viruses A and B are associated with significant morbidity and mortality in humans. Influenza virions contain two major surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA), and these are the predominant antigens of these viruses. Several influenza genes have been evaluated as potential DNA vaccine candidates, including HA, NA, matrix protein (M1), nucleoprotein (NP) or nonstructural protein (NS1) [49]. An epidermal DNA vaccine for influenza, immunogenic in humans, has been reported [49]. Intramuscular influenza HA DNA vaccines have been shown to be immunogenic in preclinical models [50]. Preparation and immunological effectiveness of a swine influenza DNA vaccine encapsulated in chitosan nanoparticles has also been reported [51]. Complete protection against a H5N2 avian influenza virus by a DNA vaccine expressing a fusion protein of H1N1 HA and M2e has been described [52].
DNA vaccine and malaria
Malaria is a major cause of disease and death. Approximately half of the world’s population is at risk of malaria [53]. The United States National Institute of Health is supporting about ten International Centers of Excellence for Malaria Research throughout the world [54]. In South Asia, India has more than three million square kilometers of land, and vast amounts of these lands are well suited for the breeding of mosquitoes, leading to the propagation of malaria parasites [54]. Various strategies have been developed to prevent this burden, aimed at diagnosis, treatment, and vector control. DNA vaccination is one of the novel approaches for developing new generation vaccines against malaria. Coated DNA vaccines have been shown to exhibit good immunogenicity and show protective levels of antigen-specific IgG, an elevated proportion of CD4+, CD8+ T cells, INF-γ and IL-12 levels in the serum and cultured splenocyte supernatant, as well as INF-γ-producing cells in the spleen. An effective delivery system for malaria vaccination has been described for an NP-coated, MSP-1 DNA-based vaccine which confers protection against lethal Plasmodium yoelii infection in mouse models across various routes of administration [55]. Molecular adjuvants for malaria DNA vaccines based on the modulation of host-cell apoptosis have been described [56]. Field literature describes Vaxfectin (Vical, USA) as having the ability to elevate antibody response and T cell response to each component of a 5-gene Plasmodium falciparum plasmid DNA vaccine mixture [57]. It has been hypothesized by some of the researchers that a malaria therapeutical vaccine targeting the erythrocyte stage of the parasite through erythrocyte sickling can lower the parasite density and also control the progression and severity of this disease [58].
DNA vaccine against dengue
Dengue is a mosquito-transmitted infectious disease. It also has an important impact on human health globally. This disease has increased dramatically in the past century throughout the globe, and is now among the most common causes of febrile illness in travelers [59]. The human immune system produces antibodies against a number of dengue proteins, namely C, prM, E, NS1, NS3, NS4B and NS5. Most of the anti-dengue neutralizing antibody epitopes have been mapped to the E protein. That is why the E gene has been chosen for constructing DNA vaccines. It has also been reported that the prM gene is essential for the proper processing and folding of the E protein and hence the prM gene has also been included [60,61]. A number of DNA dengue vaccine have also been studied and presented [62] and a West Nile virus CD4+ T cell epitope appears to improve the immunogenicity of dengue virus serotype 2 vaccines [63]. Immunogenicity and protective efficacy of a Vaxfectin-adjuvanted tetravalent dengue DNA vaccine has also been discussed [64].
DNA vaccine against typhoid
Salmonella infection is a food borne infection [65]. Typhoid fever is a prolonged febrile illness caused by bacterium Salmonella typhi. It has a global distribution and is a worldwide problem as described by Khan et al. [66,67,68,69,70]. Typhoid can be treated by using antibiotics [66,70]. Vaccination [66,70] and herbal drugs [66,67,68,69,71,72,73,74,75,76] also showed interesting results [64]. A number of plants have been reviewed by this author for their medicinal assessment [72,77,78]. Recently, a number of vaccines against Salmonella have been developed including live-attenuated as well as DNA vaccines and their clinical trials exhibited promising results [79].
Other diseases
A recent study has reported the efficacy of DNA vaccine-generated duck polyclonal antibodies as post-exposure prophylaxis to prevent hantavirus pulmonary syndrome [80]. The development of DNA vaccines against foot-and-mouth disease has also been studied in detail [30]. The efficacy of Leishmania donovani ribosomal P1 gene as DNA vaccine in experimental visceral leishmaniasis has also been reported [81]. Development of a DNA vaccine targeting Merkel cell polyomavirus has also been studied [82]. A number of DNA vaccines against different antigens and the concerned system in which the vaccine was tested are listed in Table 3.

Table 3.
DNA vaccines against different antigens.
Guidance on prophylactic DNA vaccines
The Center for Biologics Evaluation and Research of the US Food and Drug Administration (CBER/FDA) governs the progress of clinical development of DNA vaccines. The function of CBER is to set and also to implement vaccine policy by keeping in consideration a number of laws and guidelines [84]. Before proceeding for a human clinical trial, a DNA vaccine has to be declared safe and immunogenic. The CBER policy is to observe and accumulate preclinical data in relevant animal models. Mice can be used as a model for preclinical study to confirm the immunogenicity of the vaccine. Rabbits can be also used as an animal model to study the acute and chronic toxicity of the DNA vaccine.
A guideline was released in 1996 by the FDA to assist the people engaged in developing DNA vaccines. The name of the guidance document is “Points to consider on plasmid DNA vaccines for preventive infectious disease indications” [78]. The document provided necessary information regarding the pre-clinical and clinical issues concerned with the development of DNA vaccines. Moreover it also raised the safety concerns to be taken into account by workers before the starting of clinical trials. Further, the guidance was revised in the year 2007 to understand more preclinical and clinical issues for DNA vaccine manufacturing [84].
The main aim of FDA guidance is to have a full watch on the methods, processes and facilities availed to manufacture vaccines so that the vaccine be pure and potent. It also checks the safety of vaccine before it goes on to clinical trial.
The guidance document framed during the year 2007 explained that there is no requirement of sponsor to perform a preclinical trail to assess the effect of a vaccine on autoimmunity. It further concluded that the established clinical monitoring procedures were enough to asses any adverse effect. Moreover the adverse effect also includes autoimmune disease. Once a claim has been made that the vaccination induces protection in adults, preclinical studies in appropriate animal models can help to study in forward direction in younger individuals.
Conclusion
The field of DNA vaccination has recorded significant progress during the past decades. Better-designed constructs and promoters, as well as novel delivery technologies have been tested in animal models and advanced in the clinic. The author explored the strategies for construction and working of DNA vaccines. The applications of DNA vaccines in different diseases were highlighted. Much stress has to be required by the researcher to develop DNA vaccines against various diseases. It is also the requirement of the present time to develop ways and means to develop the vaccine in a limited period of time, in order to help eradicate emerging infectious diseases.
Acknowledgments
The author, Dr. KH Khan, Assistant Professor (Senior) wishes to thank VIT University, Vellore-14, Tamil Nadu, India for providing the facility for writing this manuscript.
Conflicts of Interest
None to declare.
References
- Wolff, J.A.; Malone, R.W.; Williams, P.; et al. Direct gene transfer into mouse muscle in vivo. Science 1990, 247 Pt 1, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Chow, Y.H.; Chiang, B.L.; Lee, Y.L.; et al. Development of Th1 and Th2 populations and the nature of immune responses to hepatitis B virus DNA vaccines can be modulated by codelivery of various cytokine genes. J. Immunol. 1998, 160, 1320–1329. [Google Scholar] [CrossRef]
- Ulmer, J.B.; Donnelly, J.J.; Parker, S.E.; et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 1993, 259, 1745–1749. [Google Scholar] [CrossRef]
- Klinman, D.M.; Yamshchikov, G.; Ishigatsubo, Y. Contribution of CpG motifs to the immunogenicity of DNA vaccines. J Immunol. 1997, 158, 3635–3639. [Google Scholar]
- Khan, K.H. Vectors Used in Gene Manipulation—A retrospective. Advanced Biotech Journal—Online. Tutor. Rev. 2009, 9, 1–8. [Google Scholar]
- Khan, K.H. Gene transfer technologies in plants: Roles in improving crops. Recent Res. Sci. Technol. 2009, 1, 116–123. [Google Scholar]
- Khan, K.H. Gene transfer technologies leading to transgenic animals. J. Ecobiotechnology 2009, 1, 32–40. [Google Scholar]
- Khan, K.H. Gene Transfer Technologies and their Applications: Roles in Human Diseases. Asian J. Exp. Biol. Sci. 2010, 1, 208–218. [Google Scholar]
- Manthorpe, M.; Cornefert-Jensen, F.; Hartikka, J.; et al. Gene therapy by intramuscular injection of plasmid DNA: Studies on firefly luciferase gene expression in mice. Hum Gene Ther. 1993, 4, 419–431. [Google Scholar] [CrossRef]
- Condon, C.; Watkins, S.C.; Celluzzi, C.M.; et al. DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996, 2, 1122–1128. [Google Scholar] [CrossRef]
- Mor, G.; Klinman, D.M.; Shapiro, S.; et al. Complexity of the cytokine and antibody response elicited by immunizing mice with Plasmodium yoelii circumsporozoite protein plasmid DNA. J Immunol. 1995, 155, 2039–2046. [Google Scholar] [PubMed]
- Casares, S.; Inaba, K.; Brumeanu, T.D.; et al. Antigen presentation by dendritic cells after immunization with DNA encoding a major histocompatibility complex class II-restricted viral epitope. J Exp Med. 1997, 186, 1481–1486. [Google Scholar] [CrossRef]
- Corr, M.; Lee, D.J.; Carson, D.A.; et al. Gene vaccination with naked plasmid DNA: Mechanism of CTL priming. J Exp Med. 1996, 184, 1555–1560. [Google Scholar] [CrossRef]
- Doe, B.; Selby, M.; Barnett, S.; et al. Induction of cytotoxic T lymphocytes by intramuscular immunization with plasmid DNA is facilitated by bone marrow-derived cells. Proc Natl Acad Sci USA 1996, 93, 8578–8583. [Google Scholar] [CrossRef]
- Ulmer, J.B.; Deck, R.R.; Dewitt, C.M.; et al. Generation of MHC class I-restricted cytotoxic T lymphocytes by expression of a viral protein in muscle cells: Antigen presentation by non-muscle cells. Immunology 1996, 89, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Corr, M.; von Damm, A.; Lee, D.J.; et al. In vivo priming by DNA injection occurs predominantly by antigen transfer. J Immunol. 1999, 163, 4721–4727. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.M.; Ulmer, J.B.; Caulfield, M.J.; et al. Priming of cytotoxic T lymphocytes by DNA vaccines: Requirement for professional antigen presenting cells and evidence for antigen transfer from myocytes. Mol Med. 1997, 3, 362–371. [Google Scholar] [CrossRef]
- Cheng, W.F.; Hung, C.F.; Chen, C.A. Characterization of DNA vaccines encoding the domains of calreticulin for their ability to elicit tumor-specific immunity and antiangiogenesis. Vaccine 2005, 23, 3864–3874. [Google Scholar] [CrossRef]
- Alarcon, J.B.; Waine, G.W.; McManus, D.P. DNA vaccines: Technology and application as anti-parasite and antimicrobial agents. Adv Parasitol. 1999, 42, 343–410. [Google Scholar] [CrossRef]
- Smooker, P.M.; Rainczuk, A.; Kennedy, N.; et al. DNA vaccines and their application against parasites--promise, limitations and potential solutions. Biotechnol Annu Rev. 2004, 10, 189–236. [Google Scholar] [CrossRef]
- Kennedy, N.J.; Spithill, T.W.; Tennent, J. DNA vaccines in sheep: CTLA-4 mediated targeting and CpG motifs enhance immunogenicity in a DNA prime/protein boost strategy. Vaccine 2006, 24, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Male, D.; Brostoff, J.; Roth, D.B.; Roitt, I. Immunology, 7th Edition, Vaccination, Chapter 18 (p325-40), MOSBY Elsevier, 2006.
- Kindt, T.J.; Goldsby, R.A.; Osborne, B.A. Sixth Edition, Kuby Immunology. Vaccines Chapter 19 (p475-92), W.H.Freeman and Co, New York. 2007.
- Li, L.; Saade, F.; Petrovsky, N. The future of human DNA vaccines. J Biotechnol. 2012, 162, 171–182. [Google Scholar] [CrossRef]
- Khan, F.A. The elements of immunology. Vaccines, Chapter 16 (p343-59). Chennai Microprint, India, 2009.
- Doria-Rose, N.A.; Haigwood, N.L. DNA vaccine strategies: Candidates for immune modulation and immunization regimens. Methods 2003, 31, 207–216. [Google Scholar] [CrossRef]
- Sasaki, S.; Takeshita, F.; Xin, K.Q.; et al. Adjuvant formulations and delivery systems for DNA vaccines. Methods 2003, 31, 243–254. [Google Scholar] [CrossRef]
- Robinson, H.L.; Pertmer, T.M. DNA vaccines for viral infections: Basic studies and applications. Adv Virus Res. 2000, 55, 1–74. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hu, Y.H.; Liu, C.S.; et al. Construction and analysis of an experimental Streptococcus iniae DNA vaccine. Vaccine 2010, 28, 3905–3912. [Google Scholar] [CrossRef] [PubMed]
- Fowler, V.L.; Barnett, P.V. Progress in the development of DNA vaccines against foot-and-mouth disease. Expert Rev Vaccines. 2012, 11, 481–493. [Google Scholar] [CrossRef]
- Kim, D.; Hung, C.F.; Wu, T.C.; et al. DNA vaccine with αgalactosylceramide at prime phase enhances anti-tumor immunity after boosting with antigen-expressing dendritic cells. Vaccine 2010, 28, 7297–7305. [Google Scholar] [CrossRef]
- Bharadwaj, M.; Hussain, S.; Nasare, V.; et al. HPV & HPV vaccination: Issues in developing countries. Indian J Med Res. 2009, 130, 327–333. [Google Scholar]
- Poláková, I.; Pokorná, D.; Dusková, M.; et al. DNA vaccine against human papillomavirus type 16: Modifications of the E6 oncogene. Vaccine 2010, 28, 1506–1513. [Google Scholar] [CrossRef]
- Changhong, S.; Hai, Z.; Limei, W.; et al. Therapeutic efficacy of a tuberculosis DNA vaccine encoding heat shock protein 65 of Mycobacterium tuberculosis and the human interleukin 2 fusion gene. Tuberculosis (Edinb). 2009, 89, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ma, H.; Qu, Q. Incorporation of immunostimulatory motifs in the transcribed region of a plasmid DNA vaccine enhances Th1 immune responses and therapeutic effect against Mycobacterium tuberculosis in mice. Vaccine 2011, 29, 7624–7630. [Google Scholar] [CrossRef]
- Hu, D.; Wu, J.; Zhang, R.; et al. T-bet acts as a powerful adjuvant in Ag85B DNA-based vaccination against tuberculosis. Mol Med Report. 2012, 6, 139–144. [Google Scholar] [CrossRef]
- Li, X.; Xu, W.; Xiong, S. A novel tuberculosis DNA vaccine in an HIV-1 p24 protein backbone confers protection against Mycobacterium tuberculosis and simultaneously elicits robust humoral and cellular responses to HIV-1. Clin Vaccine Immunol. 2012, 19, 723–730. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. Infections associated with the genus Edwardsiella: The role of Edwardsiella tarda in human disease. Clin Infect Dis. 1993, 17, 742–748. [Google Scholar] [CrossRef]
- Slaven, E.M.; Lopez, F.A.; Hart, S.M.; et al. Myonecrosis caused by Edwardsiella tarda: A case report and case series of extraintestinal E. tarda infections. Clin Infect Dis. 2001, 32, 1430–1433. [Google Scholar] [CrossRef]
- Jiao, X.D.; Zhang, M.; Hu, Y.H. Construction and evaluation of DNA vaccines encoding Edwardsiella tarda antigens. Vaccine 2009, 27, 5195–5202. [Google Scholar] [CrossRef]
- Boyapalle, S.; Mohapatra, S.; Mohapatra, S. Nanotechnology applications to HIV vaccines and microbicides. J Glob Infect Dis. 2012, 4, 62–68. [Google Scholar] [CrossRef]
- Hutnick, N.A.; Myles, D.J.; Bian, C.B.; et al. Selected approaches for increasing HIV DNA vaccine immunogenicity in vivo. Curr Opin Virol. 2011, 1, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Streinu-Cercel, A. Nanoparticles. GERMS 2012, 2, 90. [Google Scholar] [CrossRef] [PubMed]
- Munang’andu, H.M.; Banda, F.; Chikampa, W. Risk analysis of an anthrax outbreak in cattle and humans of Sesheke district of Western Zambia. Acta Trop. 2012, 124, 162–165. [Google Scholar] [CrossRef]
- Chitlaru, T.; Altboum, Z.; Reuveny, S. Progress and novel strategies in vaccine development and treatment of anthrax. Immunol Rev. 2011, 239, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Bhatnagar, R. Recent progress in the development of anthrax vaccines. Recent Pat Biotechnol. 2011, 5, 148–159. [Google Scholar] [CrossRef]
- Albrecht, M.T.; Eyles, J.E.; Baillie, L.W. Immunogenicity and efficacy of an anthrax/plague DNA fusion vaccine in a mouse model. FEMS Immunol Med Microbiol. 2012, 65, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Klimov, A.I.; Garten, R.; Russell, C.; et al. WHO recommendations for the viruses to be used in the 2012 Southern Hemisphere Influenza Vaccine: Epidemiology, antigenic and genetic characteristics of influenza A(H1N1)pdm09, A(H3N2) and B influenza viruses collected from February to September 2011. Vaccine 2012, 30, 6461–6471. [Google Scholar] [CrossRef]
- Drape, R.J.; Macklin, M.D.; Barr, L.J.; et al. Epidermal DNA vaccine for influenza is immunogenic in humans. Vaccine 2006, 24, 4475–4481. [Google Scholar] [CrossRef]
- Donnelly, J.J.; Friedman, A.; Martinez, D.; et al. Preclinical efficacy of a prototype DNA vaccine: Enhanced protection against antigenic drift in influenza virus. Nat Med. 1995, 1, 583–587. [Google Scholar] [CrossRef]
- Zhao, K.; Shi, X.; Zhao, Y.; et al. Preparation and immunological effectiveness of a swine influenza DNA vaccine encapsulated in chitosan nanoparticles. Vaccine 2011, 29, 8549–8556. [Google Scholar] [CrossRef]
- Park, K.S.; Seo, Y.B.; Lee, J.Y.; et al. Complete protection against a H5N2 avian influenza virus by a DNA vaccine expressing a fusion protein of H1N1 HA and M2e. Vaccine 2011, 29, 5481–5487. [Google Scholar] [CrossRef]
- WHO Media Centre. Malaria. Fact sheet No.94. 2012. Accessed on: November 2, 2012. Available at: http://www.who.int/mediacentre/factsheets/fs094/en/.
- Kumar, A.; Chery, L.; Biswas, C.; et al. Malaria in South Asia: Prevalence and control. Acta Trop. 2012, 121, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Cherif, M.S.; Shuaibu, M.N.; Kurosaki, T.; et al. Immunogenicity of novel nanoparticle-coated MSP-1 C-terminus malaria DNA vaccine using different routes of administration. Vaccine 2011, 29, 9038–9050. [Google Scholar] [CrossRef]
- Bergmann-Leitner, E.S.; Leitner, W.W.; Duncan, E.H.; et al. Molecular adjuvants for malaria DNA vaccines based on the modulation of host-cell apoptosis. Vaccine 2009, 27, 5700–5708. [Google Scholar] [CrossRef]
- Sedegah, M.; Rogers, W.O.; Belmonte, M.; et al. Vaxfectin enhances both antibody and in vitro T cell responses to each component of a 5-gene Plasmodium falciparum plasmid DNA vaccine mixture administered at low doses. Vaccine 2010, 28, 3055–3065. [Google Scholar] [CrossRef]
- Solomons, H.D.; Joubert, C.G.; Beichter, B.; et al. The winding road to developing a malaria vaccine. Study hypothesis. GERMS 2012, 2, 91–94. [Google Scholar] [CrossRef]
- Wright, W.F.; Pritt, B.S. Update: The diagnosis and management of dengue virus infection in North America. Diagn Microbiol Infect Dis. 2012, 73, 215–220. [Google Scholar] [CrossRef]
- Lorenz, I.C.; Allison, S.L.; Heinz, F.X.; et al. Folding and dimerization of tick-borne encephalitis virus envelope proteins prM and E in the endoplasmic reticulum. J Virol. 2002, 76, 5480–5491. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A structural perspective of the flavivirus life cycle. Nat Rev Microbiol. 2005, 3, 13–22. [Google Scholar] [CrossRef]
- Danko, J.R.; Beckett, C.G.; Porter, K.R. Development of dengue DNA vaccines. Vaccine 2011, 29, 7261–7266. [Google Scholar] [CrossRef]
- Hughes, H.R.; Crill, W.D.; Davis, B.S.; et al. A West Nile virus CD4 T cell epitope improves the immunogenicity of dengue virus serotype 2 vaccines. Virology 2012, 424, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Porter, K.R.; Ewing, D.; Chen, L.; et al. Immunogenicity and protective efficacy of a vaxfectin-adjuvanted tetravalent dengue DNA vaccine. Vaccine 2012, 30, 336–341. [Google Scholar] [CrossRef]
- Dhanashekar, R.; Akkinepalli, S.; Nellutla, A. Milk-borne infections. An analysis of their potential effect on the milk industry. GERMS 2012, 2, 101–109. [Google Scholar] [CrossRef]
- Khan, K.H. Recent trends in typhoid research-a review. Int. J. Biosci. 2012, 2, 110–120. [Google Scholar]
- Khan, K.H. Emblica officinalis reduces the initiation of oxidative stress by Salmonella typhimurium in mice and can be used in typhoid. Lat Am J Pharm. 2010, 29, 171–177. [Google Scholar]
- Khan, K.H. Protective effect of Emblica officinalis against S typhimurium through its antioxidant activity. Recent Res. Sci. Technol. 2010, 2, 29–36. [Google Scholar]
- Khan, K.H. Immunomodulatory activity of Terminalia chebula against Salmonella typhimurium in mice. Recent Res. Sci. Technology. 2009, 1, 211–216. [Google Scholar]
- Khan, K.H.; Ganjewala, D.; Jain, S.K. Pretreatment with Emblica officinalis can reduce typhoid risk. Trendz BioTech 2008, 4, 15–21. [Google Scholar]
- Khan, K.H. Terminalia chebula reduces the oxidative stress induced by salmonella typhimurium in mice and may reduce the risk of getting typhoid. Adv. Biol. Res. 2009, 3, 1–8. [Google Scholar]
- Khan, K.H. Roles of Emblica officinalis in medicine—A review. Bot. Res. Int. 2009, 2, 218–228. [Google Scholar]
- Khan, K.H. The effect of regular intake of Terminalia chebula on oxidative stress in mice originated from Salmonella typhimurium. EurAsia J BioSci. 2009, 3, 113–121. [Google Scholar] [CrossRef]
- Khan, K.H.; Jain, S.K. Regular intake of Terminalia chebula can reduce the risk of getting typhoid fever. Adv. Biotech. 2009, 8, 10–15. [Google Scholar]
- Khan, K.H.; Ganjewala, D. Recent advancement in typhoid research—A review. Adv. Biotech. 2008, 7, 35–41. [Google Scholar]
- Khan, K.H.; Prasad, B.N.; Ganjewala, D.; Jain, S.K. Efficacy of lyophilized juice of Emblica officinalis against experimentally induced salmonellosis. Recent Adv. Biotechnol. 2008, 136–143. [Google Scholar]
- Khan, K.H.; Madani, A.; Jain, S.K. Infectious diseases and herbal drugs. Compendium of Invited lectures. World Ayurveda Congr. 2002, 64–76. [Google Scholar]
- Khan, K.H.; Jain, S.K. Medicinal Plants a retrospective. Hamdard Med. 2003, XLVI, 23–33. [Google Scholar]
- Marathe, S.A.; Lahiri, A.; Negi, V.D.; et al. Typhoid fever & vaccine development: A partially answered question. Indian J Med Res. 2012, 135, 161–169. [Google Scholar]
- Brocato, R.; Josleyn, M.; Ballantyne, J.; et al. DNA vaccine-generated duck polyclonal antibodies as a postexposure prophylactic to prevent hantavirus pulmonary syndrome (HPS). PLoS ONE 2012, 7, e35996. [Google Scholar] [CrossRef] [PubMed]
- Masih, S.; Arora, S.K.; Vasishta, R.K. Efficacy of Leishmania donovani ribosomal P1 gene as DNA vaccine in experimental visceral leishmaniasis. Exp Parasitol. 2011, 129, 55–64. [Google Scholar] [CrossRef]
- Zeng, Q.; Gomez, B.P.; Viscidi, R.P.; et al. Development of a DNA vaccine targeting Merkel cell polyomavirus. Vaccine 2012, 30, 1322–1329. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, M.; Liu, C.; et al. A divalent DNA vaccine based on Sia10 and OmpU induces cross protection against Streptococcus iniae and Vibrio anguillarum in Japanese flounder. Fish Shellfish Immunol. 2012, 32, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Klinman, D.M.; Klaschik, S.; Tross, D. FDA guidance on prophylactic DNA vaccines: Analysis and recommendations. Vaccine 2010, 28, 2801–2805. [Google Scholar] [CrossRef] [PubMed]
© GERMS 2013.