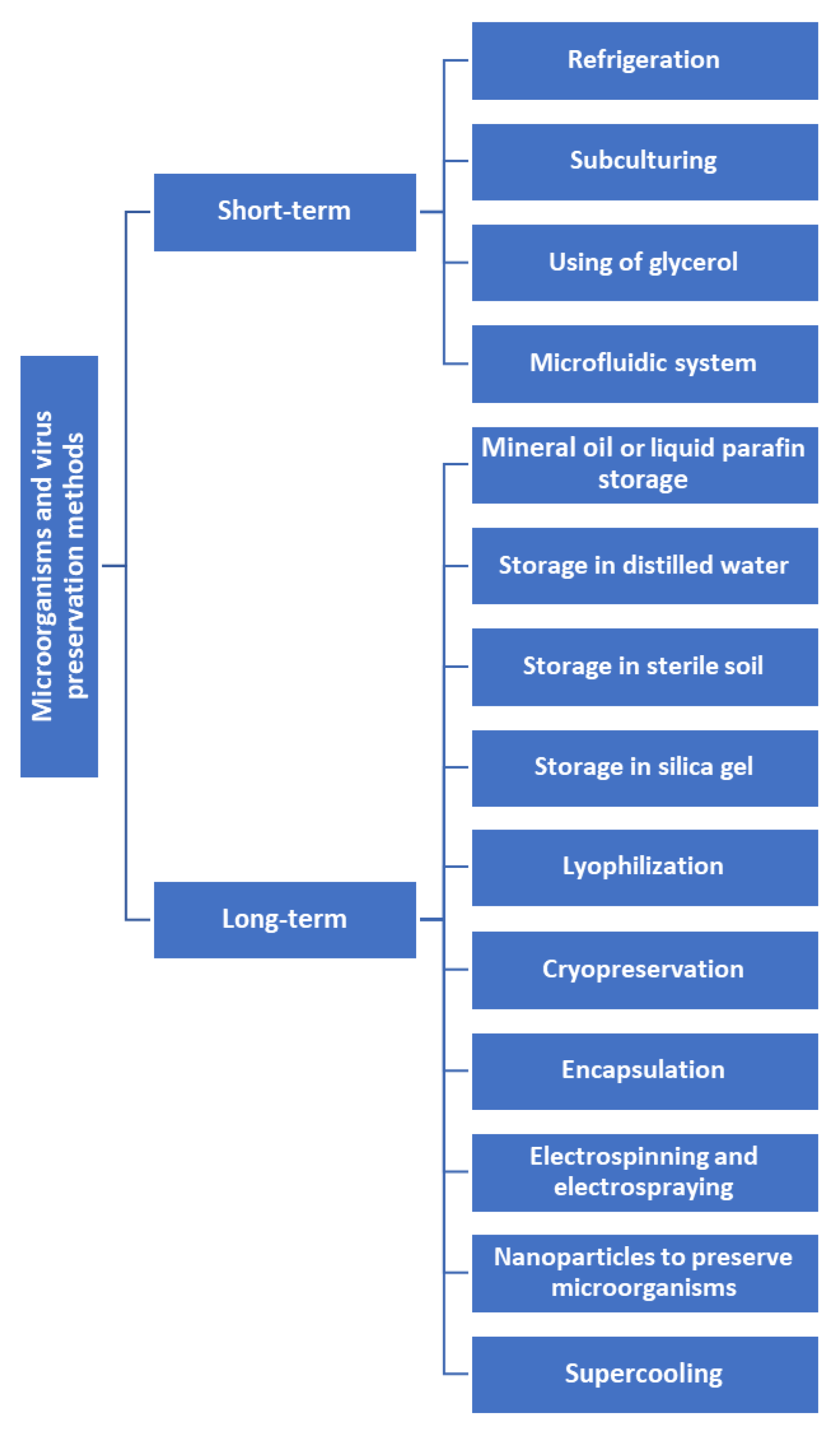
Here, we have reviewed the short-term and long-term microbial and viral preservation methods and compared and contrasted the novel and emerging technologies with previous methods.
Microorganism and virus preservation methods
Microorganism preservation can be broadly divided into short-term and long-term preservation (
Figure 1).
Short-term preservation methods for microorganisms are designed to maintain their viability for periods ranging from a few days to one year.
Refrigeration involves storing the microorganisms at temperatures between 2-8°C, which can slow their metabolic rate, preventing or delaying their growth and prolonging their viability [
3,
4].
S. pneumoniae,
H. influenzae, and
N. meningitidis are highly susceptible to low temperatures. The cold temperature negatively affects the metabolic processes, enzymatic reactions, and overall cellular functions required for the survival of the bacterium [
7].
Influenza viruses, including seasonal influenza strains and the more well-known strains like H1N1 and H3N2, are highly labile and require specialized storage methods such as ultra-low temperature freezers or preservation in liquid nitrogen for long-term viability [
3].
Subculturing, which involves transferring a small amount of the original culture to a sterile and fresh medium, is another commonly used method for the short-term preservation of microorganisms [
1,
4].
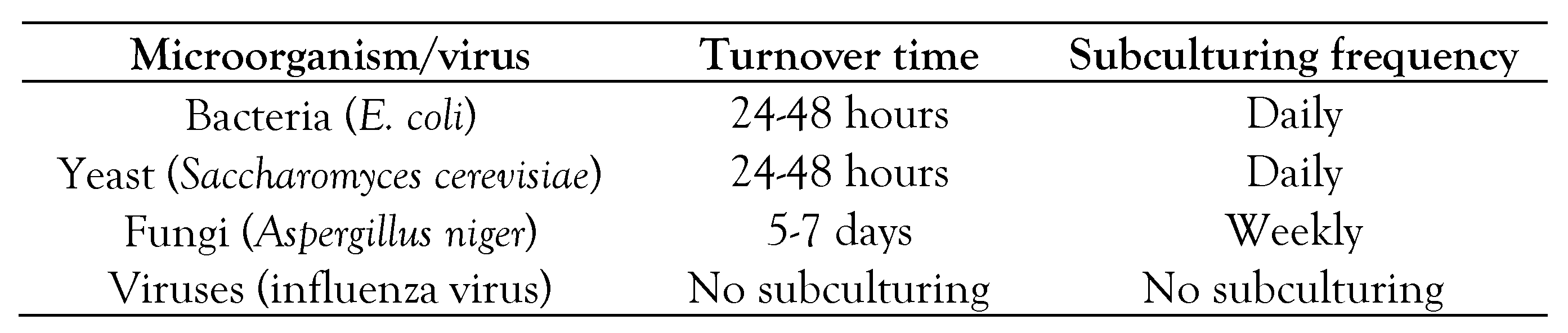
Table 1 displays the turnover time and subculturing frequency of commonly used microbes.
Preservation can be challenging when working with fastidious bacteria with specific nutritional and environmental requirements [
8,
9]. Subculturing under antibiotic pressure can result in the emergence of antibiotic-resistant subpopulations due to random mutations or horizontal gene transfer. Additionally, subculturing without specific nutrients can adapt bacterial subpopulations that can utilize alternative resources. The genetic changes during subculturing can affect the expression or functionality of virulence factors in pathogens, resulting in subpopulations with different levels of pathogenicity [
8].
At 4°C, most filamentous fungi may survive for at least one to two years. Superficial fungi such as
Malassezia furfur can be sub-cultured on Dixon's Agar or Leeming-Notman agar. They should be incubated at 30°C for 5 to 7 days to promote optimal growth. For dimorphic fungi such as
Histoplasma capsulatum, subcultures are typically grown on Sabouraud-dextrose agar (SDA) at room temperature (25°C) for a period of 2 to 4 weeks [
10].Dermatophytes, including
Microsporum audouinii, are commonly sub-cultured on Sabouraud glucose agar at a temperature of 26°C. The cultures should be incubated for 2 to 4 weeks. Yeast species like
Cryptococcus neoformans can be subcultured on SDA and incubated at 30°C for 48 to 72 hours [
10]. Opportunistic fungi, such as
Candida albicans, are sub-cultured on SDA and incubated at 30°C for 24 to 72 hours [
11].
Some common types of cultures used for virus preservation include cell cultures, embryonated eggs, animal models, insects, and artificial matrices. Cell cultures can be used for many types of viruses, including herpes simplex virus (HSV), cytomegalovirus, human papillomavirus, hepatitis B virus, and human immunodeficiency virus. Influenza virus is commonly preserved using embryonated eggs. Animal models can preserve rabies virus by maintaining it in animal models, such as mice or hamsters. Dengue virus and yellow fever virus can be preserved by maintaining them in mosquito hosts [
1,
12].
The viability of different organisms in glycerol can vary.
E. coli and
S. pneumoniae can remain viable for up to 5 months [
4],while
H. influenzae can remain viable for up to 4 months. On the other hand,
N. meningitidis can remain viable for 6 weeks, and
N. gonorrhoeae remains viable for 3 weeks.
Listeria monocytogenes can be stored in 20% glycerol at -80°C [
13].
The concentration of glycerol used is typically between 10-20% v/v [
14].Examples of fungi that can be preserved using glycerol are
Aspergillus spp. and
Penicillium spp. [
15].
The higher glycerol concentration might have a virucidal effect because it dehydrates the viruses. Therefore, optimizing the glycerol concentration for each virus and cell type is important.
The RNA of the rabies virus was successfully preserved in 50% glycerol at -70°C for up to one year [
16]. Glycerol is effective in preserving the viability of a wide range of viruses, including enveloped viruses, HSV-I, and non-enveloped viruses, poliovirus type 1 and the concentration of 85% glycerol appears to be preferred to preserve them because it does not affect the breakdown of extracellular DNA [
12,
13].
Microfluidic systems allow for the creation of microenvironments that support the growth and proliferation of specific microorganisms and the isolation of individual cells or small populations of microorganisms for studying microbial behavior and interactions [
17]. One example of a microfluidic system for microbial preservation is the microfluidic cell trap array, which uses microfabricated channels and traps to capture and preserve individual microorganisms. While microfluidic systems have the potential to provide more precise and efficient preservation methods compared to traditional techniques, more research is needed to optimize these systems and ensure their long-term viability for microbial preservation. Bacteria and fungi can be preserved using this technique, such as
E. coli and
Saccharomyces cerevisiae, ranging from days to weeks or even longer [
18].
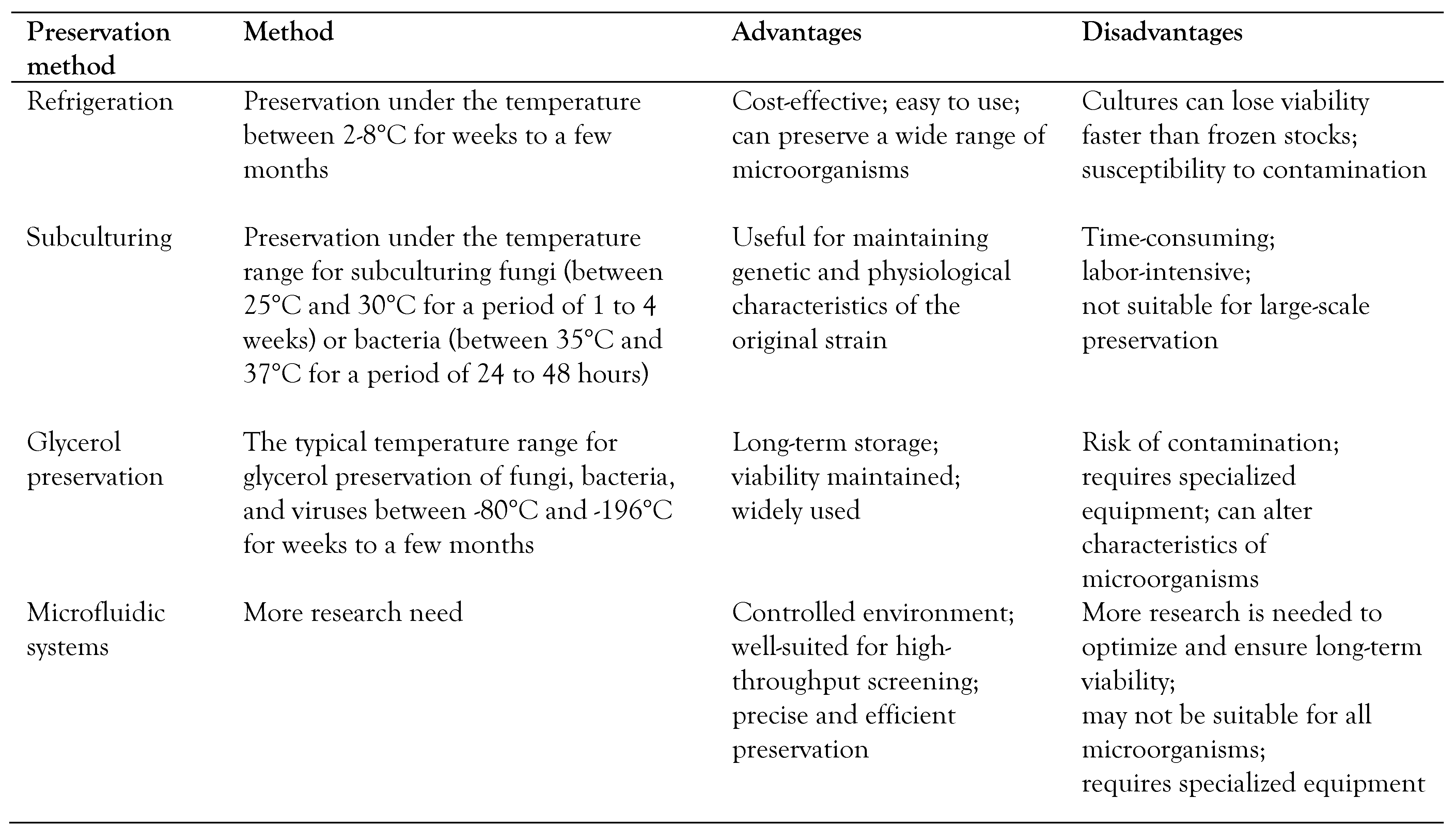
Short-term preservation methods of microbes, with advantages and disadvantages, are displayed in
Table 2.
- 2.
Long-term preservation methods
Long-term preservation of microorganisms involves storing the organisms for an extended period, ranging from months to decades while retaining their viability and genetic stability.
The method involves the addition of mineral oil or liquid paraffin to the culture medium, covering the top layer of the medium and the microorganisms, forming a barrier between the organisms and oxygen. This creates an oxygen-free environment that prevents the growth and multiplication of microorganisms, preserving their viability for an extended period [
16].
One of the most significant benefits is its ability to maintain the viability of microorganisms for months to years. When stored under mineral oils, pathogenic bacteria, such as
E. coli and
S. aureus, may lose virulence or pathogenicity over time [
19].
Using this method, some bacterial genera, such as
Bacillus spp., can be preserved for up to 8-12 years, and
Mycobacterium spp. and
Azotobacter spp. for 7-10 years [
11,
20].
However, it is important to note that preserving bacterial cultures using paraffin oil is not foolproof. Therefore, it is crucial to maintain a clean and sterile environment when working with bacterial cultures, regardless of the preservation method used [
20].
At room temperature or between 15° and 20°C, cultures can be stored even for a few years (2, 4 or, in extreme circumstances, up to 32 years). This approach is suitable for mycelial or nonsporulating fungi cultures that cannot be frozen or dried using freeze-drying technology. The main drawback of the method is that the fungi keep growing, which opens the possibility of selection for mutants [
18].
A 10 mm layer of paraffin or oil covers fungi cultures grown on agar slants. Periodically check the oil level in the tubes, add additional oil if needed.
Pleurotus spp.,
Aspergillus spp.,
Trichophyton mentagrophytes, and
C. albicans can be preserved using the paraffin oil [
18,
21].
Sterile distilled water is a medium for the long-term preservation of microorganisms because it is sterile and free from any nutrients that might encourage the growth of the microorganisms. However, over time, the distilled water can become depleted of oxygen and nutrients, leading to a decline in the viability of the microorganisms [
22].
The researchers found that bacteria could survive longer in water with a neutral pH (pH 7) compared to acidic or alkaline water. A wide range of bacteria can be stored, including
P. fluorescens, Erwinia spp.,
Xanthomonas campestris,
Salmonella spp.,
Yersinia enterocolitica,
Escherichia coli O157:H7,
Listeria monocytogenes,
S. aureus, Rhizobium spp. and
Pseudomonas spp. Suspending the organisms before inoculation in a screw-capped tube containing phosphate-buffered saline at pH 7.2 would be useful [
8,
11,
21].
The main concerns with this technique are using an appropriate volume of water to inoculum blocks, which should be at least 40 times more significant, and avoiding evaporative water loss from poorly sealed storage tubes by periodically adding sterile water to maintain the necessary water level. Fungi such as
C. albicans, Cladosporium spp.,
Penicillium spp.,
Cephalosporium spp.,
A. niger, and
A. fumigatus can be preserved [
17,
23].
The method of preserving spore-forming bacteria and fungi by storing them in sterile soil is widely accepted. This method involves placing the microorganisms in a dormant state within the sterile soil. The soil is sterilized, and a spore suspension is added using aseptic techniques. Studies have shown that this method is highly effective in preserving the viability of spore-forming microorganisms, with some organisms remaining viable for up to 70-80 years [
24].
This technique has been used to store some bacteria species that produce spores, particularly Clostridia. Additionally, it has been utilized to store bacilli and
Azotobacter spp. non-sporulating bacteria that may not survive well in the lyophilization process can also be stored in soil using this method (
E. coli).
Bacillus subtilis, Pseudomonas fluorescens, and
Rhizobium spp. can be preserved using this method [
16,
23].
This low-maintenance and economical technique is suitable for
Fusarium spp.,
Penicillium spp.,
Alternaria spp.,
Rhizopus spp.,
Septoria spp.,
Rhizoctonia spp., and
Pseudocercosporella spp. Dryness-induced dormancy, however, might take time to develop, and morphological differences in some fungi can be observed [
14,
24].
Sterile silica gel is a widely used medium for storing bacteria and fungi. The process involves sterilizing screw-cap tubes partially filled with silica gel in an oven. After cooling, a skim-milk suspension containing spores and cells of the target microorganism is added over the silica gel and cooled again. The tubes are then dried at 25°C, followed by cooling, and stored in closed containers containing desiccants to prevent moisture absorption. Organisms can survive for 1-2 years [
25].
P. denitrificans, E. coli, and Azotobacter vinelandii can be preserved using this silica gel method [
12,
16].
Fusarium spp.,
S. cerevisiae, and
A. nidulans can be stored [
14,
24,
25]. Fungal spores stored on silica gel have the ability to remain viable for over ten years, making this method inexpensive, simple, and reliable for several fungi, including entomopathogens [
14,
24].
Lyophilization, also known as freeze-drying, can be divided into three steps: freezing, sublimation, and desorption [
26]. It involves removing the water from the microorganisms by subjecting them to a vacuum and low temperature. This process helps to prevent damage caused by ice crystal formation and allows the microorganisms to be stored for long periods at room temperature [
1,
13,
26].
Common ingredients used to maintain viability during lyophilization include mannitol, skim milk, and bovine serum albumin [
27].Cryoprotectants, such as sucrose and trehalose, help preserve the biomolecules' structure during the process [
28]. Typically, a basic freeze-drying solution is made using 20% skim milk and 5-10% sucrose. Probiotics, including
Bifidobacteria, can be preserved using this freeze-drying method [
28]. Certain bacteria, such as
Clostridium botulinum, Aquaspirillum serpens, and Helicobacter pylori, cannot be preserved using lyophilization [
1]. These bacteria have unique physiological and structural characteristics that make them particularly vulnerable to the stresses induced by lyophilization.
The freeze-drying method suits coelomycetes, hyphomycetes, basidiomycetes, ascomycetes, and some thermophilic fungi such as
Thermothelomyces spp.
and Humicola spp. [
29]. However, it is generally ineffective for fungi with large vacuolar volumes and contains a lot of water, such as some Oomycetes and Entomophthorales. It does not adequately preserve non-sporulating fungi (vegetative hyphae), and some species of yeast (
Brettanomyces, Dekkera, Lipomyces, Leucosporidium Bulleera, Sporobolomyces) [
1]. Store the lyophilized ampoules at approximately 4°C, although they can be kept at ambient temperature, if necessary, their viability will likely decline sooner than if refrigerated [
13,
17,
29].
This is the most satisfactory method of keeping viruses alive for a very long time. Depending on the unique design of the freeze-drying equipment, there are several changes in the technical procedures. The most straightforward and efficient method only requires one vacuum stage for small volumes of virus and small numbers of samples because the glass ampules are inserted directly onto the branching exhaust manifold of the freeze dryer [
12].
The preservation of biological materials at cryogenic temperatures, typically -80°C or -196°C, is referred to as cryopreservation (liquid nitrogen). Cryopreservation is useful for the long-term preservation of microorganisms that are difficult to culture or grow and those that are sensitive to low temperatures. Cryopreservation also requires cryoprotective agents to protect the microorganisms from damage caused by ice crystal formation during freezing. Low temperatures protect proteins and DNA from denaturation and damage while slowing cellular water movement. As a result, the biochemical and physiological activities of the cells are effectively halted, and the cells are protected for extended periods. Cell preservation at -20°C is not recommended to be used for long-term storage. A reservation at -80°C is adequate, but -196°C is ideal because it largely prevents DNA mutations from ocurring [
22,
30].
The bacterial cryopreservation protocol involves preparing the bacterial culture in a suitable medium, washing the cells, suspending them in a cryoprotective agent, and freezing them in small vials. The cryoprotective agent helps to protect the cells from damage during the freezing process [
31].
To select the appropriate freezing medium, researchers should consider the growth requirements of the bacteria and the medium's composition. Additionally, they should take precautions to prevent contamination during the cryopreservation process, such as using sterile techniques and thoroughly cleaning equipment [
27,
28].
In commercial cryopreservation systems, polystyrene beads can be used as carriers to cryopreserve sporulating
A. fumigatus cultures at -80°C and conidia of entomopathogenic fungi. For
S. cerevisiae cultures, porous ceramic beads can be employed at -70°C. This technique can be used to preserve fungi that are difficult to keep alive, such as unculturable, obligate parasites and mutualists such as.
Halophythophthora,
Saprolegnia, and
Aphanomyces spp., as well as some Basidiomycota and Glomeromycota members [
23,
26].
Viruses can be cryopreserved at -20°C to -70°C and in liquid nitrogen. Viruses can remain infectious at temperatures as low as -60°C, and to preserve their infectivity, it is best to rapidly freeze small volumes (0.1-0.5 mL) of virus suspension. However, if retaining infectivity is not necessary, the sample can be stored at -20°C for many years without losing its antigenicity, though infectivity may decrease. A useful method for preserving specific virus antigens for diagnostic purposes is to store acetone-fixed virus-infected cells on glass coverslips at -20°C for long-term storage. Baculoviruses or pox viruses can be preserved at -20°C. Virus-like particles, certain DNA viruses, and non-enveloped viruses can be kept stable at 4°C for an extended period. Because RNA and most encapsulated viruses are extremely heat-labile, long-term storage requires rapid freezing and storing at -80°C [
15,
26,
32].
Encapsulation involves coating the microorganism with a protective layer to improve its stability and prevent loss of activity during storage and transportation. The protective layer can comprise various materials, such as alginate, gelatin, polyethylene glycol or polyvinyl alcohol [
26,
33].
The techniques used for immobilizing cells through encapsulation can be broadly categorized into two types depending on the size of the polymeric bead produced: macro-encapsulation and microencapsulation. Macroencapsulation involves trapping cells within polymeric beads ranging in size from a few millimeters to centimeters. Microencapsulation produces beads within the size range of 1-1000 µm. In the case of microencapsulation, it has been observed that the viability of cells at the center of larger beads tends to decrease over time [
33].
Encapsulation can protect microorganisms from environmental stresses such as temperature, pH, and osmotic pressure, improving their stability during storage and transportation. However, the encapsulation process can reduce the microorganisms' activity, and the capsule may limit the diffusion of substrates and products in and out of the capsule, which can affect the performance of the biosensor.
Fluorescent dyes, such as SYTO-green, ethidium bromide, and 6-carboxyfluorescein, have been used to assess the viability of encapsulated cells. These dyes stain the nucleic acid of dead bacteria, allowing differentiation from live bacteria with intact cytoplasmic membranes.
Several types of microorganisms can be effectively encapsulated using different encapsulation techniques. Some bacteria suitable for encapsulation include
Pantoea agglomerans,
E. coli, B. subtilis,
Pseudomonas spp.,
Lactobacillus, and
Bifidobacterium spp. Yeast strains like
Saccharomyces boulardii,
Saccharomyces cerevisiae, and
Hirsutella rhossiliensis, an endoparasitic fungus, can also be encapsulated successfully. The shelf life of encapsulated viruses is highly dependent on the stability of the viral particles and the encapsulation method employed [
15,
26,
33].
Electrospinning and electrospraying are emerging microencapsulation techniques used to preserve the viability of sensitive microorganisms. They involve atomization processes using an electrically charged jet of polymer solution to create nanoscale and microscale fibers or particles. These techniques have shown promise in food applications and as efficient methods for preserving microorganisms. A polymer solution containing microorganisms is placed in a syringe with a needle at the tip, and a high-voltage electric field is applied, which causes the polymer solution to form a charged jet that is pulled toward a collector, forming nanofibers. The microorganisms become encapsulated within these fibers, which act as protective carriers during storage. The electrospraying electric field causes the solution to break into fine droplets, forming nanoparticles or microcapsules that can protect the microorganisms [
34].
Both electrospinning and electrospraying provide high surface area-to-volume ratios and high permeability, making them practical for preserving microorganisms. The thin polymeric fibers produced in electrospinning processes allow the trapped cells to exchange nutrients and metabolites while maintaining their metabolic activity [
34,
35].Electrospinning has been successfully used to encapsulate bifidobacterial strains, maintaining high microbial viability despite osmotic changes and electrostatic fields.
Electrospraying involves atomizing a liquid flow into droplets and has also been used to encapsulate probiotic strains onto protein-based or polysaccharide-based matrices. Microencapsulation through electrospraying with whey protein concentrate can prolong bifidobacterial cells survival, even under conditions of high humidity [
34,
35,
36].
Nanoparticles are tiny particles with diameters in the range of 1-100 nanometers. They have unique physicochemical properties, including a high surface area-to-volume ratio, which can make them effective at preserving microorganisms. Using nanoparticles for microbial preservation can involve coating the microorganisms with a layer of nanoparticles, which can protect them from environmental stresses such as high or low temperatures, radiation, or oxidative stress. The nanoparticles can also act as a slow-release vehicle for antimicrobial compounds or other chemicals that can help maintain the viability of the microorganisms during storage [
36,
37].
Supercooling is a method of cooling liquids or solutions below their freezing point without forming ice crystals [
38,
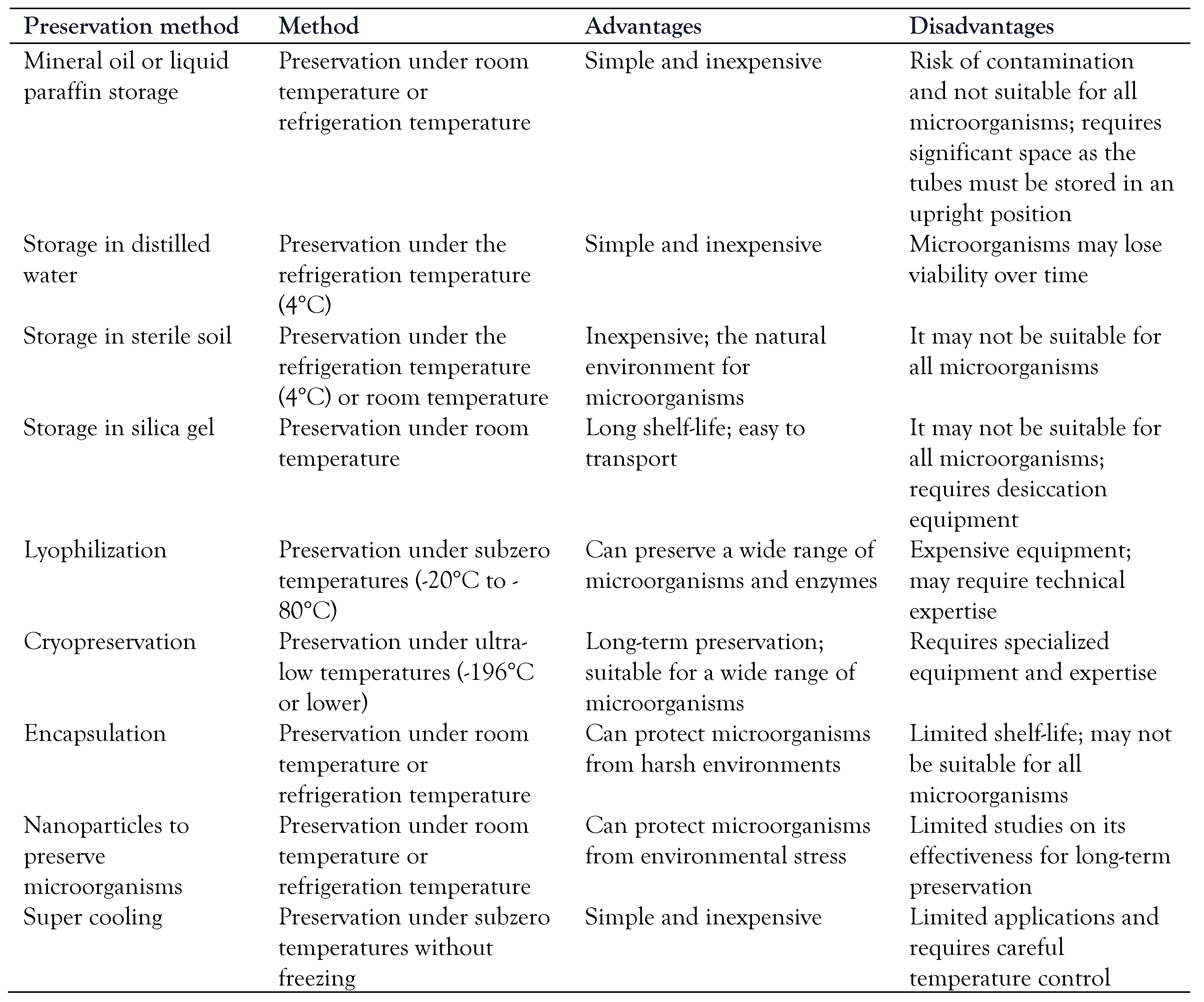
39]. By avoiding the formation of ice crystals, supercooling can help preserve the viability of microorganisms that might otherwise be damaged by freezing. In one approach to using supercooling for microbial preservation, microorganisms are suspended in a solution containing cryoprotective agents (such as glycerol or DMSO) and then supercooled to temperatures below their freezing point. The supercooled suspension can then be stored at low temperatures, such as -80°C, without forming ice crystals, which can help maintain the microorganisms' viability. Long-term preservation methods with advantages and disadvantages are displayed in
Table 3.
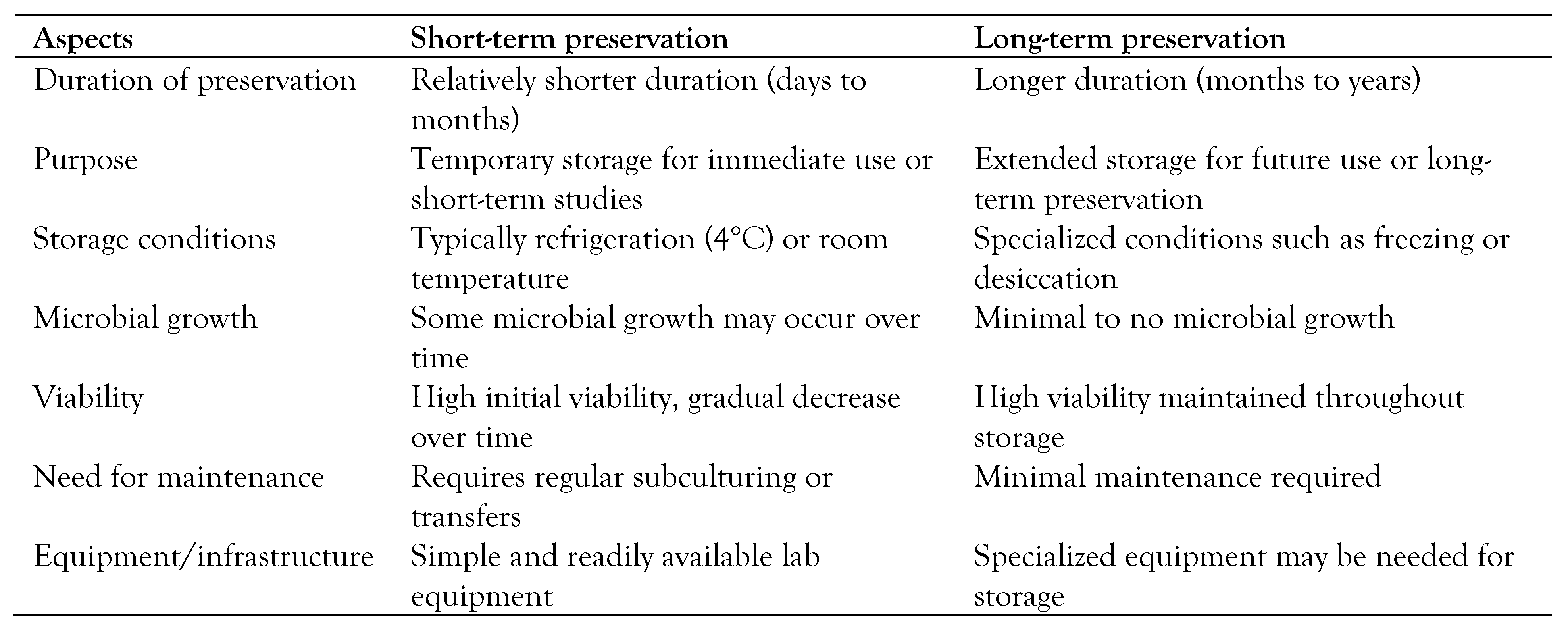
Table 4 compares short-term and long-term microbial and viral preservation methods in terms of microbial growth, viability, maintenance, and equipment.
- 3.
Assessing the viability of preserved microbes, including viruses
Following the preservation of microbes and different viruses, their viability is assessed using different methods. Microscopic examination using vital stains: Cells are observed microscopically in a counting chamber after staining with vital stains. Vital stains can distinguish between live and dead cells based on specific staining patterns. Nowadays, molecular techniques such as PCR (polymerase chain reaction) or QPCR (quantitative PCR/real-time PCR) can detect specific genetic markers to determine the presence or absence of viable microorganisms [
24,
25,
26,
27,
35].