Isolation of Shiga Toxin-Producing Escherichia coli O157 and Non-O157 from Retail Imported Frozen Beef Marketed in Saudi Arabia Using Immunomagnetic Separation and Multiplex PCR
Abstract
Introduction
Methods
Beef sample collection
Beef samples enrichment
Immunomagnetic beads (IMB) procedures
Genomic DNA extraction
Multiplex PCR assay for virulence genes
ERIC-PCR DNA fingerprinting analysis
Results
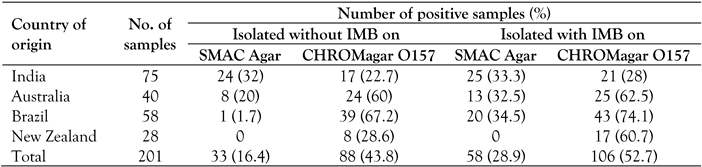
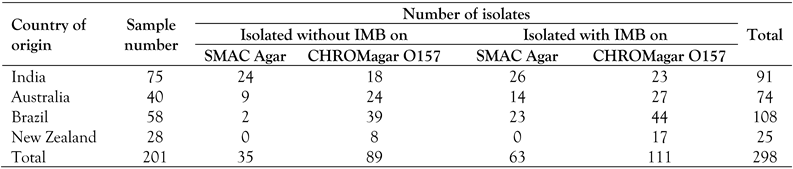
Immunomagnetic beads (IMB) separation of E. coli O157
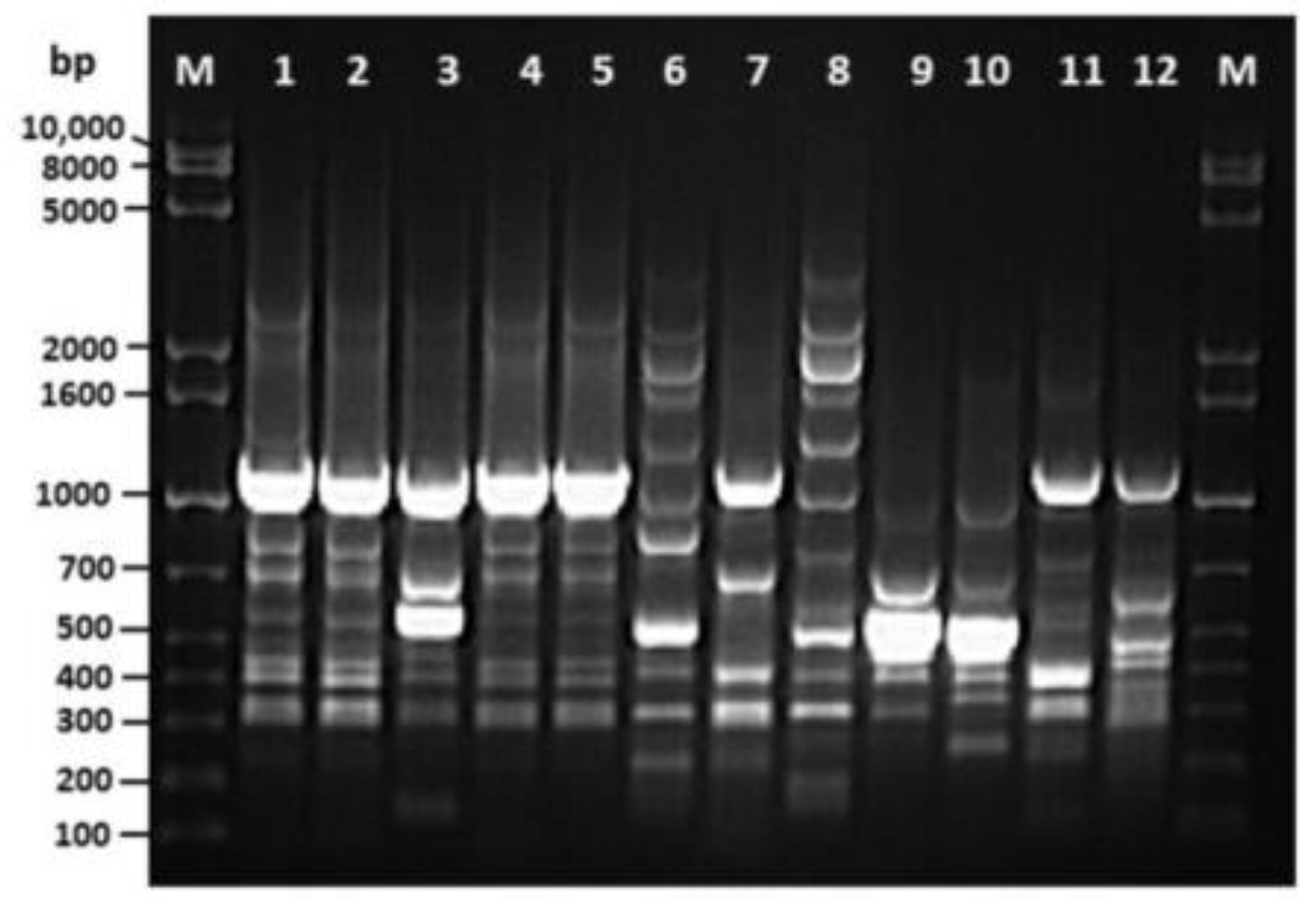
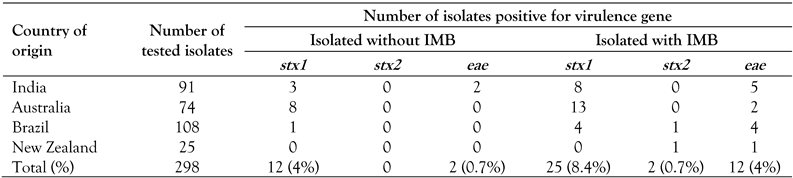
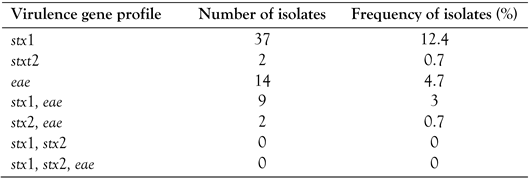
Multiplex PCR assay for virulence genes
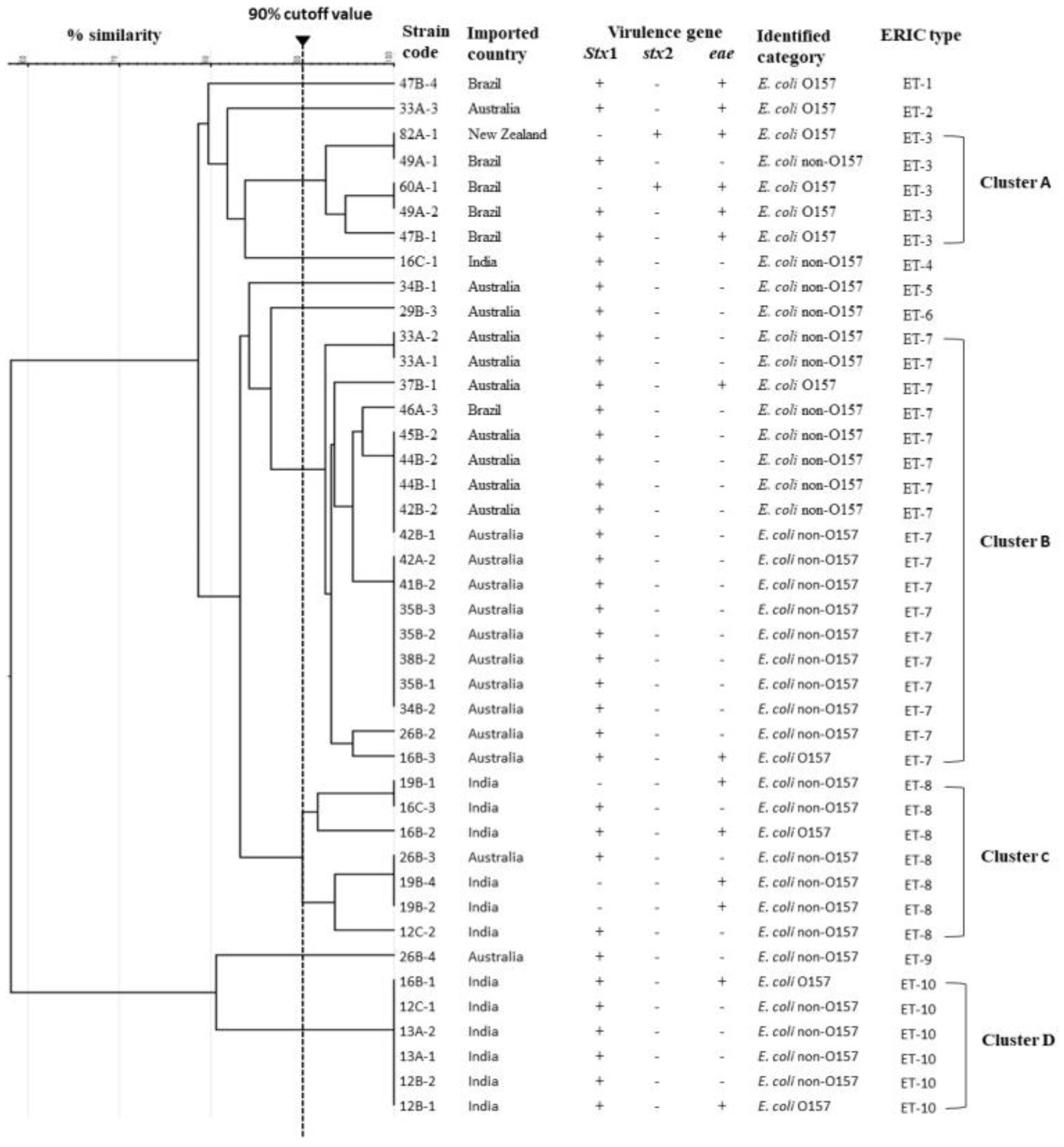
ERIC-PCR typing
Discussion
Conclusions
Author Contributions
Funding
Conflicts of Interest
Notes
References
- Pokharel, P.; Dhakal, S.; Dozois, C.M. The diversity of Escherichia coli pathotypes and vaccination strategies against this versatile bacterial pathogen. Microorganisms. 2023, 11, 344. [Google Scholar] [CrossRef]
- Croxen, M.A.; Law, R.J.; Scholz, R.; Keeney, K.M.; Wlodarska, M.; Finlay, B.B. Recent advances in understanding enteric pathogenic Escherichia coli. Clin Microbiol Rev. 2013, 26, 822–880. [Google Scholar] [CrossRef]
- Mengistu, D.Y.; Mengesha, Y. New approaches for severity intervention and rapid diagnosis of enterohemorrhagic Escherichia coli: a review. All Life. 2023, 16, 2218582. [Google Scholar] [CrossRef]
- Majowicz, S.E.; Scallan, E.; Jones-Bitton, A.; et al. Global incidence of human Shiga toxin-producing Escherichia coli infections and deaths: a systematic review and knowledge synthesis. Foodborne Pathog Dis. 2014, 11, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Law, D. Virulence factors of Escherichia coli O157 and other Shiga toxin-producing E. coli. J Appl Microbiol. 2000, 88, 729–745. [Google Scholar] [CrossRef]
- Etcheverría, A.I.; Padola, N.L. Shiga toxin-producing Escherichia coli: factors involved in virulence and cattle colonization. Virulence. 2013, 4, 366–372. [Google Scholar] [CrossRef]
- Bruyand, M.; Mariani-Kurkdjian, P.; Gouali, M.; et al. Hemolytic uremic syndrome due to Shiga toxin-producing Escherichia coli infection. Med Mal Infect. 2018, 48, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Canpolat, N. Hemolytic uremic syndrome. Turk Pediatri Ars. 2015, 50, 73–82. [Google Scholar] [CrossRef]
- Mühlen, S.; Dersch, P. Treatment strategies for infections with Shiga toxin-producing Escherichia coli. Front Cell Infect Microbiol. 2020, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Withenshaw, S.M.; Smith, R.P.; Davies, R.; Smith, A.E.O.; Gray, E.; Rodgers, J. A systematized review and qualitative synthesis of potential risk factors associated with the occurrence of non-O157 Shiga toxin-producing Escherichia coli (STEC) in the primary production of cattle. Compr Rev Food Sci Food Saf. 2022, 21, 2363–2390. [Google Scholar] [CrossRef]
- Kovac, J. Precision food safety: a paradigm shift in detection and control of foodborne pathogens. mSystems. 2019, 4, e00164-19. [Google Scholar] [CrossRef]
- Xiong, Q.; Cui, X.; Saini, J.K.; et al. Development of an immunomagnetic separation method for efficient enrichment of Escherichia coli O157: H7. Food Control. 2014, 37, 41–45. [Google Scholar] [CrossRef]
- Sarimehmetoglu, B.; Aksoy, M.H.; Ayaz, N.D.; Ayaz, Y.; Kuplulu, O.; Kaplan, Y.Z. Detection of Escherichia coli O157: H7 in ground beef using immunomagnetic separation and multiplex PCR. Food Control. 2009, 20, 357–361. [Google Scholar] [CrossRef]
- Qin, Y.; Puthiyakunnon, S.; Zhang, Y.; et al. Rapid and specific detection of Escherichia coli O157: H7 in ground beef using immunomagnetic separation combined with loop-mediated isothermal amplification. Pol J Food Nutr Sci. 2018, 68, 115–123. [Google Scholar] [CrossRef]
- Jung, Y.; Porto-Fett, A.C.S.; Parveen, S.; et al. Recovery rate of cells of the seven regulated serogroups of Shiga toxin-producing Escherichia coli from raw veal cutlets, ground veal, and ground beef from retail stores in the Mid-Atlantic region of the United States. J Food Prot. 2021, 84, 220–232. [Google Scholar] [CrossRef]
- Toro, M.; Rivera, D.; Jiménez, M.F.; Díaz, L.; Navarrete, P.; Reyes-Jara, A. Isolation and characterization of non-O157 Shiga toxin-producing Escherichia coli (STEC) isolated from retail ground beef in Santiago, Chile. Food Microbiol. 2018, 75, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Castro, V.S.; Teixeira, L.A.C.; Rodrigues, D.D.P.; Dos Santos, L.F.; Conte-Junior, C.A.; Figueiredo, E.E.S. Occurrence and antimicrobial resistance of E. coli non-O157 isolated from beef in Mato Grosso, Brazil. Trop Anim Health Prod. 2019, 51, 1117–1123. [Google Scholar] [CrossRef]
- Marder, E.P.; Cui, Z.; Bruce, B.B.; et al. Risk factors for non-O157 Shiga toxin-producing Escherichia coli infections, United States. Emerg Infect Dis. 2023, 29, 1183–1190. [Google Scholar] [CrossRef]
- Onyeka, L.O.; Adesiyun, A.A.; Keddy, K.H.; Madoroba, E.; Manqele, A.; Thompson, P.N. Shiga toxin-producing Escherichia coli contamination of raw beef and beef-based ready-to-eat products at retail outlets in Pretoria, South Africa. J Food Prot. 2020, 83, 476–484. [Google Scholar] [CrossRef]
- Alotaibi, K.; Hikal, A.F.; Sung, K.; Zhang, G.; Khan, A.A. Draft genome sequences of nine non-O157 Shiga toxin-producing Escherichia coli in ready-to-eat food from supermarkets in Argentina. Microbiol Resour Announc. 2023, 12, e0042923. [Google Scholar] [CrossRef]
- Hessain, A.M.; Al-Arfaj, A.A.; Zakri, A.M.; et al. Molecular characterization of Escherichia coli O157: H7 recovered from meat and meat products relevant to human health in Riyadh, Saudi Arabia. Saudi J Biol Sci. 2015, 22, 725–729. [Google Scholar] [CrossRef]
- Al-Zogibi, O.G.; Mohamed, M.I.; Hessain, A.M.; El-Jakee, J.K.; Kabli, S.A. Molecular and serotyping characterization of shiga toxogenic Escherichia coli associated with food collected from Saudi Arabia. Saudi J Biol Sci. 2015, 22, 438–442. [Google Scholar] [CrossRef]
- Bosilevac, J.M.; Gassem, M.A.; Al Sheddy, I.A.; et al. Prevalence of Escherichia coli O157:H7 and Salmonella in camels, cattle, goats, and sheep harvested for meat in Riyadh. J Food Prot. 2015, 78, 89–96. [Google Scholar] [CrossRef]
- Elabbasy, M.T.; Hussein, M.A.; Algahtani, F.D.; et al. MALDI-TOF MS based typing for rapid screening of multiple antibiotic resistance E. coli and virulent non-O157 shiga toxin-producing E. coli isolated from the slaughterhouse settings and beef carcasses. Foods. 2021, 10, 820. [Google Scholar] [CrossRef] [PubMed]
- Alhadlaq, M.A.; Mujallad, M.I.; Alajel, S.M.I. Detection of Escherichia coli O157:H7 in imported meat products from Saudi Arabian ports in 2017. Sci Rep. 2023, 13, 4222. [Google Scholar] [CrossRef] [PubMed]
- Almejhim, M.; Aljeldah, M.; Elhadi, N. Improved isolation and detection of toxigenic Vibrio parahaemolyticus from coastal water in Saudi Arabia using immunomagnetic enrichment. PeerJ. 2021, 9, e12402. [Google Scholar] [CrossRef]
- Kim, Y.B.; Okuda, J.; Matsumoto, C.; et al. Isolation of an Escherichia coli O157:H7 strain producing Shiga toxin 1 but not Shiga toxin 2 from a patient with hemolytic uremic syndrome in Korea. FEMS Microbiol Lett. 1998, 166, 43–48. [Google Scholar] [CrossRef]
- Rajendran, P.; Ajjampur, S.S.R.; Chidambaram, D.; et al. Pathotypes of diarrheagenic Escherichia coli in children attending a tertiary care hospital in South India. Diagn Microbiol Infect Dis. 2010, 68, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Versalovic, J.; Koeuth, T.; Lupski, R. Distribution of repetitive DNA sequences in eubacteria and application to finerpriting of bacterial enomes. Nucleic Acids Res. 1991, 19, 6823–6831. [Google Scholar] [CrossRef]
- Alsultan, A.; Elhadi, N. Evaluation of ERIC-PCR method for determining genetic diversity among Escherichia coli isolated from human and retail imported frozen shrimp and beef. Int J Food Contam. 2022, 9, 12. [Google Scholar] [CrossRef]
- Elhadi, N. Clonal relationship among the Vibrio parahaemolyticus isolates from coastal water in Saudi Arabia. Egypt J Aquat Res. 2018, 44, 131–137. [Google Scholar] [CrossRef]
- Heras, J.; Domínguez, C.; Mata, E.; et al. GelJ--a tool for analyzing DNA fingerprint gel images. BMC Bioinformatics. 2015, 16, 270. [Google Scholar] [CrossRef]
- Mathusa, E.C.; Chen, Y.; Enache, E.; Hontz, L. Non-O157 Shiga toxin-producing Escherichia coli in foods. J Food Prot. 2010, 73, 1721–1736. [Google Scholar] [CrossRef]
- Sirikaew, S.; Sukkua, K.; Rattanachuay, P.; Khianngam, S.; Sukhumungoon, P. High level of shiga toxin-producing Escherichia coli and occurrence of STX-Negative E. coli O157 from raw meats: characterization of virulence profile and genetic relatedness. Southeast Asian J Trop Med Public Health. 2016, 47, 1008–1019. [Google Scholar]
- Lewis, G.L.; Cernicchiaro, N.; Moxley, R.A. Performance of chromogenic agar media for isolation of Shiga toxin-producing Escherichia coli from ground beef. J Food Prot. 2020, 83, 1149–1154. [Google Scholar] [CrossRef]
- Hunduma, D.; Amenu, K.; Desta, H.; Grace, D.; Agga, G.E.; Kerro Dego, O. Prevalence and antimicrobial resistance of Escherichia coli O157:H7 and Salmonella, and the prevalence of Staphylococcus aureus in dairy cattle and camels under pastoral production system. Antibiotics (Basel). 2023, 13, 26. [Google Scholar] [CrossRef]
- Adzitey, F.; Huda, N.; Ali, G.R.R. Molecular techniques for detecting and typing of bacteria, advantages and application to foodborne pathogens isolated from ducks. 3 Biotech. 2013, 3, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, A.A. Bacterial typing methods from past to present: a comprehensive overview. Gene Reports. 2022, 29, 101675. [Google Scholar] [CrossRef]
- Simar, S.R.; Hanson, B.M.; Arias, C.A. Techniques in bacterial strain typing: past, present, and future. Curr Opin Infect Dis. 2021, 34, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Yamani, L.Z.; Elhadi, N. Virulence characteristics, antibiotic resistance patterns and molecular typing of enteropathogenic producing Escherichia coli (EPEC) isolates in Eastern province of Saudi Arabia: 2013-2014. Infect Drug Resist. 2022, 15, 6763–6772. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, R.; Karami, A.; Farshad, S.; Giammanco, G.M.; Mammina, C. Typing methods used in the molecular epidemiology of microbial pathogens: a how-to guide. New Microbiol. 2014, 37, 1–15. [Google Scholar] [PubMed]
- Szczuka, E.; Kaznowski, A. Typing of clinical and environmental Aeromonas sp. strains by random amplified polymorphic DNA PCR, repetitive extragenic palindromic PCR, and enterobacterial repetitive intergenic consensus sequence PCR. J Clin Microbiol. 2004, 42, 220–228. [Google Scholar] [CrossRef] [PubMed]
 |
 |
 |
 |
 |
© GERMS 2024.
Share and Cite
Almulhim, A.; Alomar, A.; Alhabib, I.; Yamani, L.Z.; Elhadi, N. Isolation of Shiga Toxin-Producing Escherichia coli O157 and Non-O157 from Retail Imported Frozen Beef Marketed in Saudi Arabia Using Immunomagnetic Separation and Multiplex PCR. GERMS 2024, 14, 352-361. https://doi.org/10.18683/germs.2024.1445
Almulhim A, Alomar A, Alhabib I, Yamani LZ, Elhadi N. Isolation of Shiga Toxin-Producing Escherichia coli O157 and Non-O157 from Retail Imported Frozen Beef Marketed in Saudi Arabia Using Immunomagnetic Separation and Multiplex PCR. GERMS. 2024; 14(4):352-361. https://doi.org/10.18683/germs.2024.1445
Chicago/Turabian StyleAlmulhim, Ahlam, Amer Alomar, Ibrahim Alhabib, Lamya Zohair Yamani, and Nasreldin Elhadi. 2024. "Isolation of Shiga Toxin-Producing Escherichia coli O157 and Non-O157 from Retail Imported Frozen Beef Marketed in Saudi Arabia Using Immunomagnetic Separation and Multiplex PCR" GERMS 14, no. 4: 352-361. https://doi.org/10.18683/germs.2024.1445
APA StyleAlmulhim, A., Alomar, A., Alhabib, I., Yamani, L. Z., & Elhadi, N. (2024). Isolation of Shiga Toxin-Producing Escherichia coli O157 and Non-O157 from Retail Imported Frozen Beef Marketed in Saudi Arabia Using Immunomagnetic Separation and Multiplex PCR. GERMS, 14(4), 352-361. https://doi.org/10.18683/germs.2024.1445




