Introduction
Fungi, as well as bacteria, communicate through quorum-sensing molecules (QSMs), signaling molecules that regulate a wide range of cellular functions, including the expression of virulence factors. As
Candida spp. are opportunistic pathogens, the expression of virulence factors is crucial for the occurrence of the disease. Farnesol, tyrosol, tryptophol, and phenylethanol are the main QSMs used by
Candida spp. [
1].
Farnesol is a metabolite of the mevalonate/sterol synthesis pathway [
2], an acyclic sesquiterpene alcohol with anti-inflammatory properties [
3]. Tyrosol is a phenylethanoid involved in germ tube formation, adherence, and biofilm formation. Both farnesol and tyrosol modulate a wide range of cellular functions in
Candida spp. [
4] and despite being considered potential adjuvants in preventing and treating
Candida infections, their role is still incompletely elucidated. As virulence is multifactorial, how yeast cells communicate and regulate their cellular processes can be considered a virulence factor [
5].
The study aims to analyze the effects of farnesol and tyrosol on the growth rate and biofilm formation ability of five Candida spp., as well as to study the effects of the mentioned QSMs on the expression of ALS3, SAP2 and HSP70, virulence genes in C. albicans.
Besides studying C. albicans, the current study fills a gap in the literature by including non-albicans species like Candida parapsilosis, Candida guilliermondii, Candida krusei, and Candida auris, as most studies focus on C. albicans, the most isolated Candida spp.
Methods
The growth rate of Candida spp. in the presence of farnesol and tyrosol
To assess the growth rate, a 0.5 McFarland inoculum was created by mixing fresh cultures of
C. albicans ATCC 10231,
C. krusei ATCC 6258,
C. parapsilosis ATCC 22019,
C. auris CBS 10913, and
C. guilliermondii IC184 (Cantacuzino Institute, Romania) in saline solution (approximately 1-3 × 10
6 fungal cells/mL). From each inoculum, 10 µL were transferred in Sabouraud dextrose liquid medium (Oxoid, UK) with or without added substances. The QSM farnesol (95%, Sigma-Aldrich, USA) and tyrosol, 2-4(-hydroxyphenyl)ethanol (98%, Sigma-Aldrich, USA), were added in a final concentration of 100 µM. The 100 µM concentration was chosen for comparability with other studies [
6,
7].
All samples were incubated for 48 hours at 37°C. The optical density (OD) was read spectrophotometrically, by using the Eppendorf BioPhotometer® D30 (Eppendorf, Austria), immediately after creating the mix (H0), after 6 hours (H1), after 9 hours (H2), after 12 hours (H3), after 24 hours (H4) and after 48 hours of incubation (H5). Before every reading, each sample was vortexed for 10 seconds. Blank probes for the spectrophotometer consisted of Sabouraud medium mixed with the farnesol and tyrosol, in the studied concentrations and without added substances for the control. The experiment was performed in triplicate.
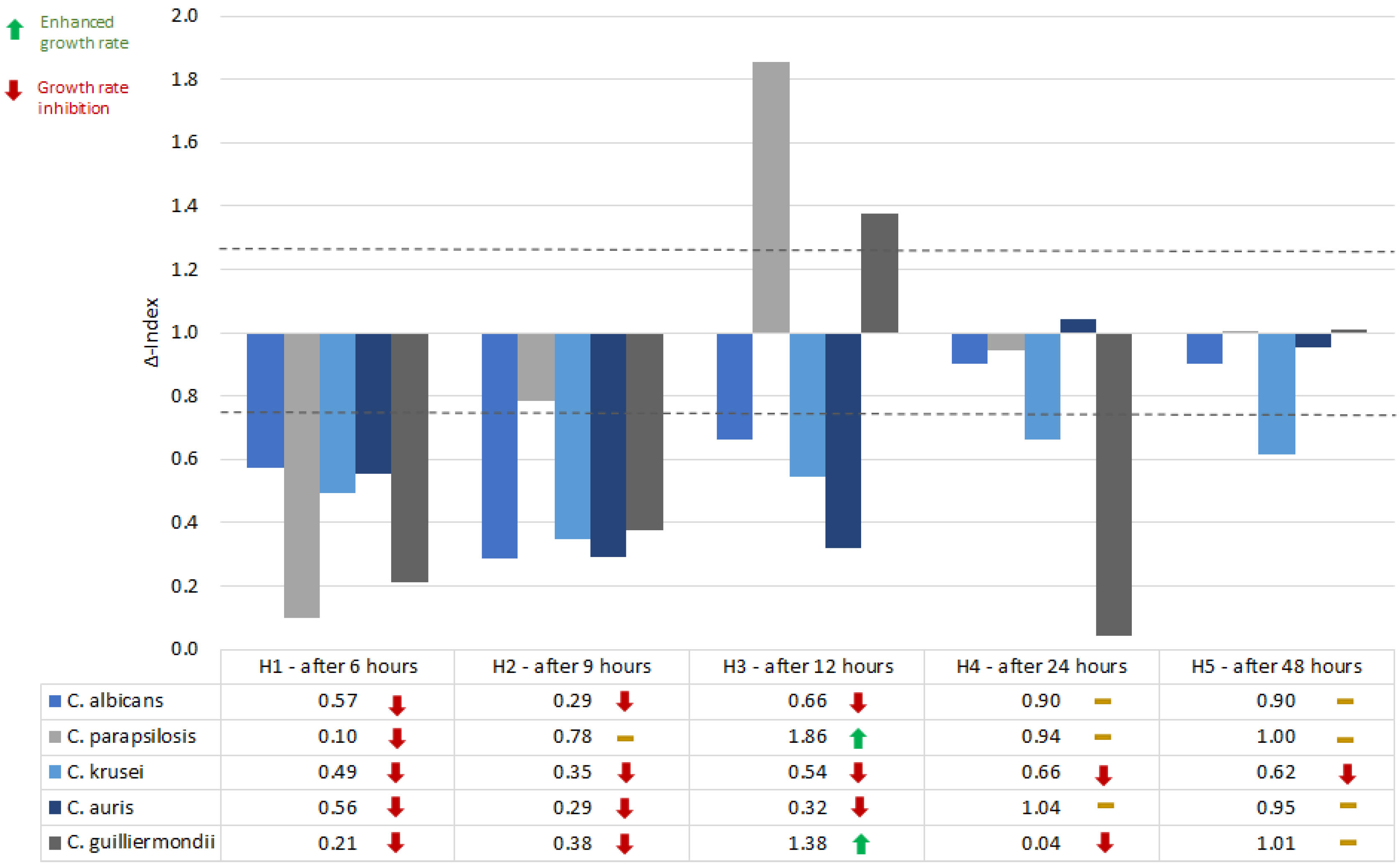
The mean OD of the samples was compared to the mean OD of growth control (Δ-Index). A Δ-Index value that exceeds or equals the threshold of 1.25 was considered an indicator of growth stimulation, while an Δ-Index value ≤0.75 is equivalent to growth inhibition. If the Δ-Index values ranged between 0.75 and 1.25, the influence of the studied QSMs on the growth rate was considered insignificant.
C. albicans virulence gene expression in the presence of farnesol and tyrosol
Cells from a fresh culture of C. albicans ATCC 10231 were incubated overnight in a shaking water-bath, at 37°C, in Sabouraud liquid medium (Oxoid, UK) (approximately 3×107 cells/mL). From this inoculum, 1 mL was distributed in a 1.5 mL sterile tube. The mixture was centrifuged at 5000 rpm for 5 minutes. The sediment was resuspended in 1.5 mL of culture medium with the tested substances (with the final concentrations of 100 µM) and incubated for 3 hours, at 37°C in the thermomixer, to allow the substances to exert an effect over the yeast cells. After the incubation, the samples were centrifuged (5000 rtm/5 minutes) and the sediment was resuspended in TE buffer (500 µL). The total fungal RNA was extracted after physical and chemical lysis, by using column purification.
Because of the thick yeast cell wall, the samples were heated at 95°C, then frozen at -70°C in two freeze-thaw cycles of 10 minutes each. Lyticase (150 units per each sample) (from Arthrobacter luteus, Sigma-Aldrich, USA) was added for the chemical digestion of the fungal cell wall. To allow the digestion to take place, the samples were incubated at 37°C for one hour. The reaction was stopped by heating the tubes at 94°C for 10 minutes. Silica-based spin columns were further used, according to the producer’s protocols (IndiSpin Pathogen Kit, Indical Bioscience, Germany). After purification, a volume of 25 µL elute was obtained. The samples were then treated with 1 µL DNase I (Thermo Scientific, UK), according to the producer’s instructions (the elute, the reaction buffer and the enzyme were incubated for 30 minutes at 37°C; 1 µL EDTA was added to each sample after the incubation period and the samples were incubated again at 65°C, for 10 minutes).
The purity and the concentration of the extracted RNA, required for adapting the volume of the elute to participate in the reaction, were evaluated by nanodrop reading (Eppendorf BioPhotometer D30, Eppendorf Austria GmbH). The RNA was adapted to 1 µg/µL for each sample. For reverse transcription, the GoScript Reverse Transcription System (Promega, USA) was used. Each sample was incubated with 1 µL of oligoNT primers for 5 minutes at 70°C and the samples were allowed to chill on ice for 6 minutes. During this time, a mix containing MgCl2, Reaction Buffer, PCR Nucleotide Mix, and Reverse Transcriptase was prepared. Fifteen microliters from the mix were incubated with 5 µL of the RNA-oligoNT mixture for 5 minutes at 25°C (annealing), 60 minutes at 42°C (extension), and 15 minutes at 70°C (the inactivation of the enzyme). RT-PCR was performed using the following primers: ACT1 (FW 5'–TTG TTG ACC GAA GCT CCA ATG–3', REV 5'–ACG TGA GTA ACA CCA TCA CCA–3'), ALS3 (FW 5'–CCA CTT CAC AAT CCC CAT C–3', REV 5'–CAG CAG TAG TAG TAA CAG TAG TAG TTT CAT C–3'), HSP70 (FW 5'–TGG TAT TCC ACC AGC TCC AAG–3', REV 5'–CAA CTT CTT CAA CAG TTG GTC CAC–3'), SAP2 (FW 5'–GAT GCT GCC ACG GGA CAA AT–3', REV 5'–AGA AGC AGC AAA TTC GGA AGC–3'). A sample with added substances served as the positive control. Samples without added substances served as a negative control. The experiment was performed in triplicate. To quantify the expression of the virulence genes, the threshold cycle (CT) value of the targeted gene was normalized against a housekeeping gene (here, ACT1). If the 2^ΔΔCt value (2^ΔCt of the housekeeping gene/ ΔCt of the analyzed virulence gene) is ˂0.5, the QSM inhibits the gene expression; a value ≥0.5 and ≤1.5 equals no effect on the gene expression, while a 2^ΔΔCt value >1.5 stands for the stimulation in terms of gene expression.
Results
Farnesol (100 µM) generally inhibited the growth rate of the studied
Candida spp. in the first 6-9 hours. After 12 hours of incubations, the response to farnesol is species-dependent (inhibition for
C. albicans,
C. krusei and
C. auris, and growth stimulation for
C. parapsilosis and, to a lower extent
C. guilliermondii). After 12-24 hours, the inhibitory effect of farnesol was consistent for
C. krusei (
Figure 1).
Tyrosol (100 µM) lowered the growth rate of C.
albicans at all the time points, but the Δ-Index was below the threshold of significance. C.
parapsilosis and
C. krusei growth rates were inconsistently affected by tyrosol, while C.
auris growth was inhibited by tyrosol, with the higher inhibition being observed after 9 and 12 hours of incubation. The effect of tyrosol on
C. guilliermondii was discontinuous (
Figure 2).
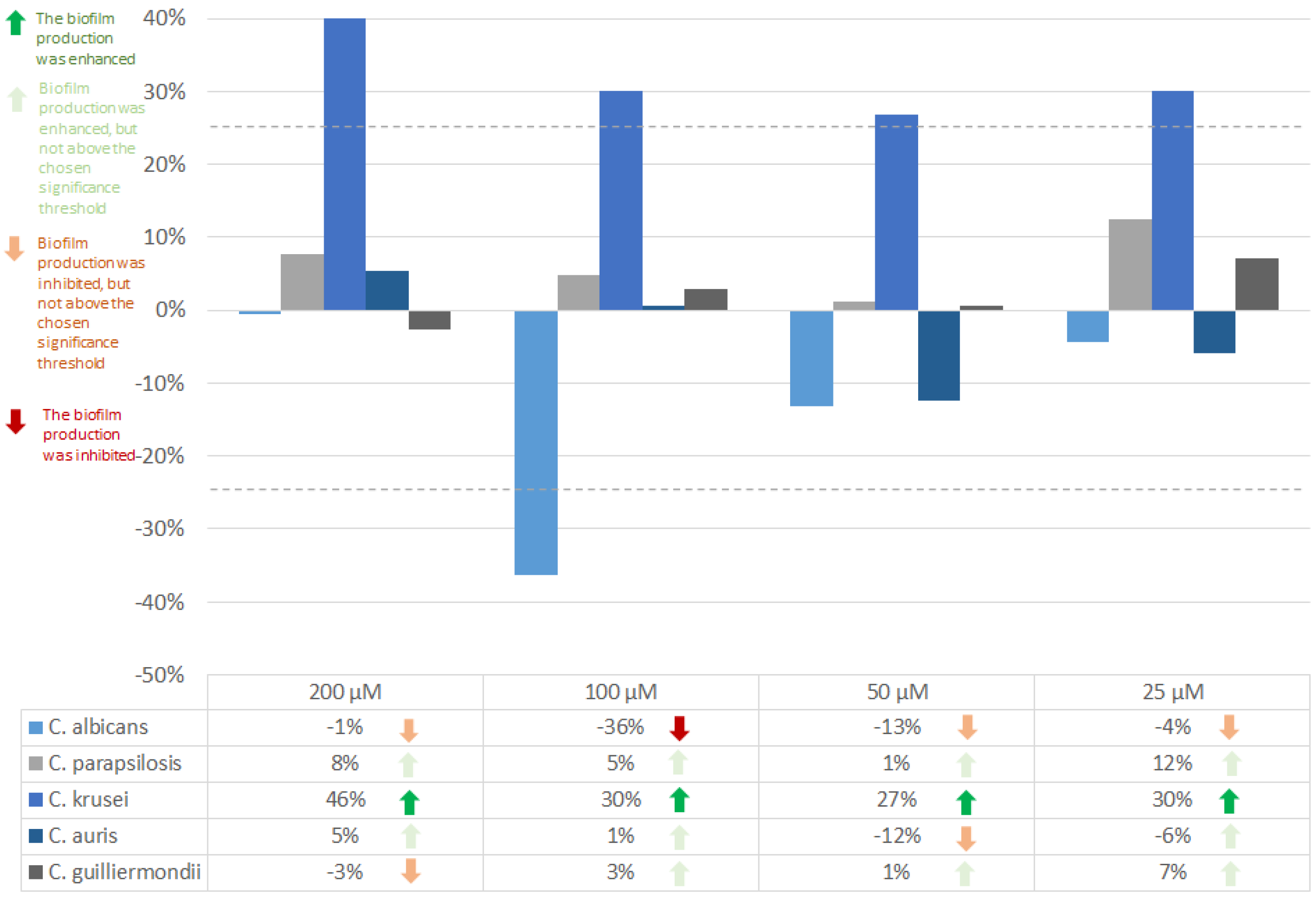
Farnesol in a concentration of 25 µM does not affect the biofilm formation ability of
C. albicans. Higher concentrations (50, 100, 200 µM) inhibited the formation of
C. albicans biofilms. A concentration of 100 µM was observed to have the highest inhibitory effect (26%).
C. auris biofilms were slightly inhibited by 200 µM, and the inhibition decreases with the concentration (a 50 µM concentration of farnesol has almost no effect on
C. auris biofilm formation rate) (
Figure 3).
C. albicans biofilm formation ability was inhibited by 36% tyrosol in a concentration of 100 µM, while for
C. krusei, tyrosol exerted a stimulating effect on the biofilm production (27-46%) (
Figure 4).
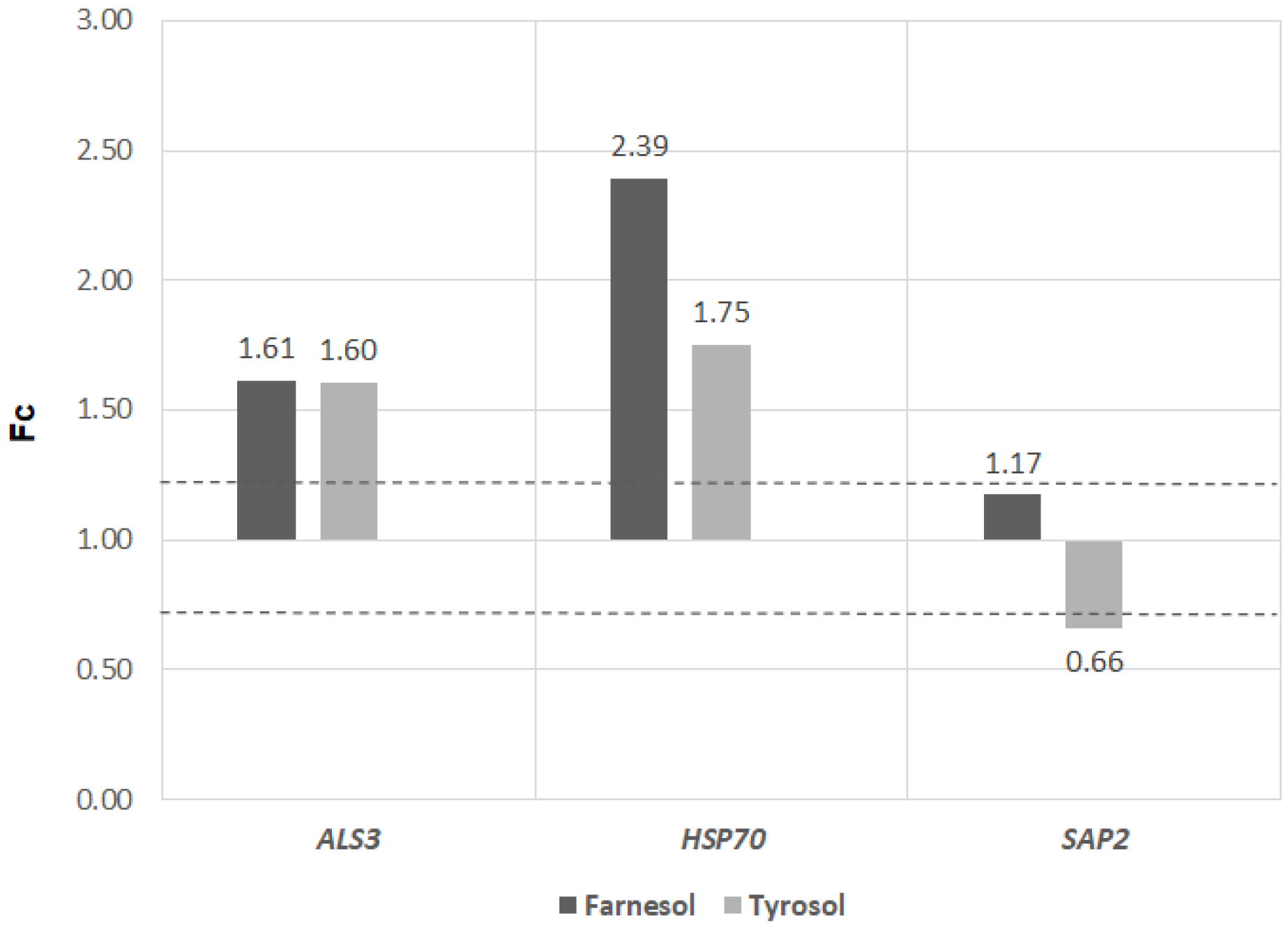
The expression of
ALS3 was enhanced by both farnesol and tyrosol, in an almost equal amount. Both farnesol and tyrosol stimulated the expression of the
HSP70 gene, but the stimulation by farnesol was higher than the effect of tyrosol. The
SAP2 gene was not influenced by farnesol, but it was inhibited by tyrosol (
Figure 5).
Discussion
Since their discovery, QSMs have been seen as promising targets for novel therapies [
8], but the anti-QS therapy still has a series of disadvantages [
9], and understanding the effects of these molecules on the virulence traits of microorganisms is crucial for deciding if quorum-sensing-interfering agents or QSMs in different concentrations can be used as antimicrobials. On average, under physiological conditions,
C. albicans secretes 35.5±16.5 µM of farnesol, while non-
albicans species secrete lower amounts:
C. parapsilosis secretes 0.6±0.3 µM,
C. tropicalis 1.0±0.7,
C. krusei 0.6±0.1 µM, and
C. guilliermondii 0.8±0.8 µM [
10].
C. albicans and
C. auris had a consistent response to farnesol (the growth rates were inhibited on all the measured time points), while other species like
C. krusei,
C. parapsilosis, and
C. guilliermondii had a different response at different time points of incubation. This might be because the response to farnesol is species-dependent, and some species might have adaptive mechanisms. Also, the concentration of farnesol influences the response of the fungal cells. The
Candida species that showed an inconsistent inhibition of the growth rate might respond to higher concentrations of farnesol, as farnesol is known to inhibit the growth rate of the same
Candida spp. at specific concentrations [
11].
The
Candida genus is a paraphyletic genus with an increasing number of new species [
12] and while considering QSMs substances with potential therapeutic applications for
Candida infections, their effect should be studied on multiple (ideally on all) species. Our results highlight the fact that farnesol and tyrosol influence the growth rate, but their effects are species-specific and dependent on the used concentration.
Rossignol et al. showed that farnesol drastically inhibited the growth rate of
C. parapsilosis in the first 2 hours of incubation, but this arrest was not connected with a specific stage of the cell cycle. The cells recovered and after 24 hours there was no significant difference between the yeast cells treated with farnesol and the control samples [
13]. In our study, the same phenomenon was observed: an initial inhibition was followed by no significant changes after 24 hours. Interestingly, after 12 hours, the growth rate of
C. parapsilosis was significantly increased.
The effects of tyrosol on the growth rate are less studied as compared with farnesol. Jakab et al. showed that tyrosol (15 mM) inhibits the growth rate of
C. parapsilosis within 2 hours after addition, without affecting the adhesion of the fungal cells. Cells treated with tyrosol showed a higher production of reactive species [
14]. In our study, after 6 hours, the growth rate of
C. parapsilosis was inhibited, despite using lower concentrations of tyrosol. As farnesol is not the only QSM and interspecies communication is a complex and still incompletely understood subject, the present study fills a gap in the literature and raises questions about how different
Candida species react to QSMs.
The biofilms formed by non-
albicans species have a higher metabolic activity [
15] and different regulatory networks [
16]. The different responses that the studied non-
albicans species had to farnesol and tyrosol met our expectations in terms of variability.
In
C. albicans, the
ALS3 gene encodes a cell surface glycoprotein (Als3, agglutinin-like sequence protein 3) that plays a key role in adherence and biofilm formation. By changing the architecture of the biofilm,
ALS3 is involved in the increased resistance exerted by biofilms to antifungal drugs [
17]. Higher
ALS3 expression correlates with an enhanced ability of the fungal cells to form biofilms [
18]. Both farnesol (100 µM) and tyrosol (100 µM) increase the expression of ALS3. The same concentration of farnesol inhibited the biofilm formation of
C. albicans and the same concentration of tyrosol did not affect the biofilm production ability of
C. albicans.
ALS3 is expressed exclusively on
C. albicans hyphae [
19] and farnesol influences the
C. albicans morphology, by inhibiting adenylate cyclase (Cyr1) and thus, inhibiting the hyphal growth [
20].
ALS3 is only a part of the vast regulatory network of genes involved in the production and maturation of biofilms. Fine alterations in the expression of genes can be triggered by various intrinsic or extrinsic factors [
21]. We showed that farnesol can influence the expression of
ALS3 and it is one of the many factors that influence the complex network of genes that orchestrate the formation of the
C. albicans biofilms.
Conclusions
The study of QSM-induced physiological and molecular events can provide new prevention and treatment alternatives for Candida spp. infections. In terms of growth rate and biofilm formation ability, different Candida spp. have particular responses to farnesol and tyrosol. Generally, farnesol was found to inhibit the growth rate and biofilm formation mostly in non-albicans species, while tyrosol exerted a non-consistent response on the different Candida species.
QSMs influence the virulence traits of different Candida spp. in a species-dependent manner and depending on the concentration. Farnesol and tyrosol have the potential to be used in the future as virulence modulators, but more research is required to properly understand their molecular mechanism of action. Notwithstanding the rarity of some Candida spp. like C. auris and C. guilliermondii, studying their response to QSMs can provide interesting insights about what, when, and how virulence traits are triggered.