The Infectious Disease Burden Among War Related Internally Displaced People in the Lviv Region of Ukraine
Abstract
Introduction
Methods
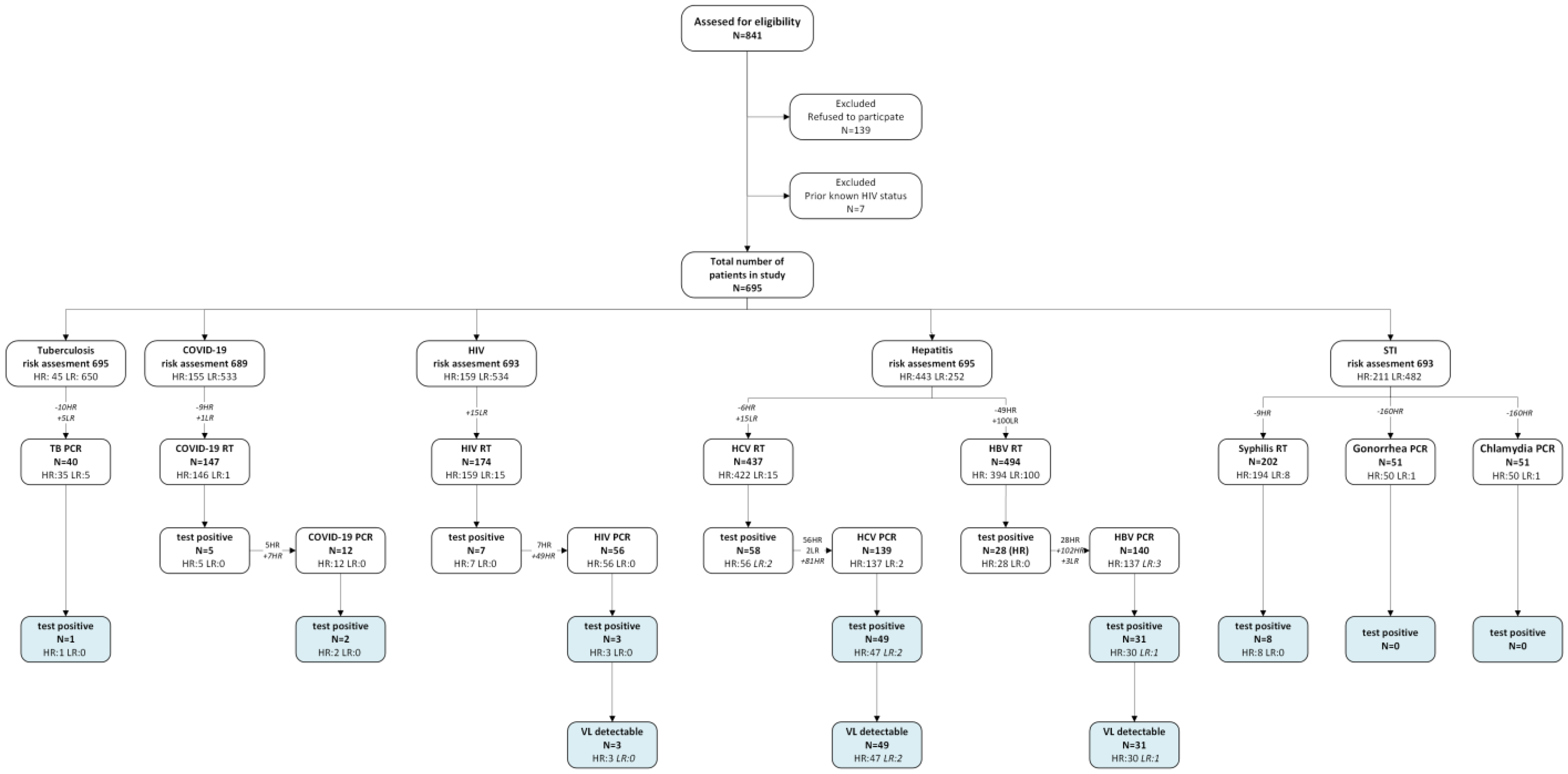
Study design
Study population
Study procedures
Endpoints
Statistical analysis
Results
Baseline characteristics
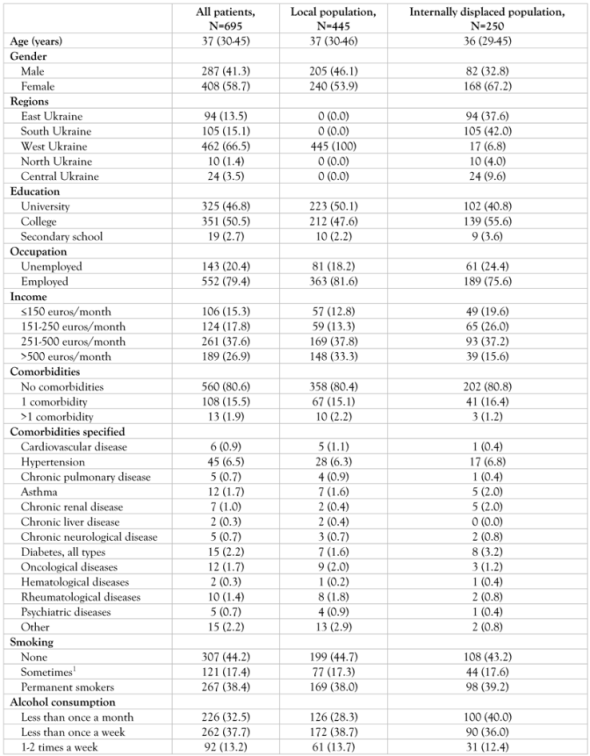 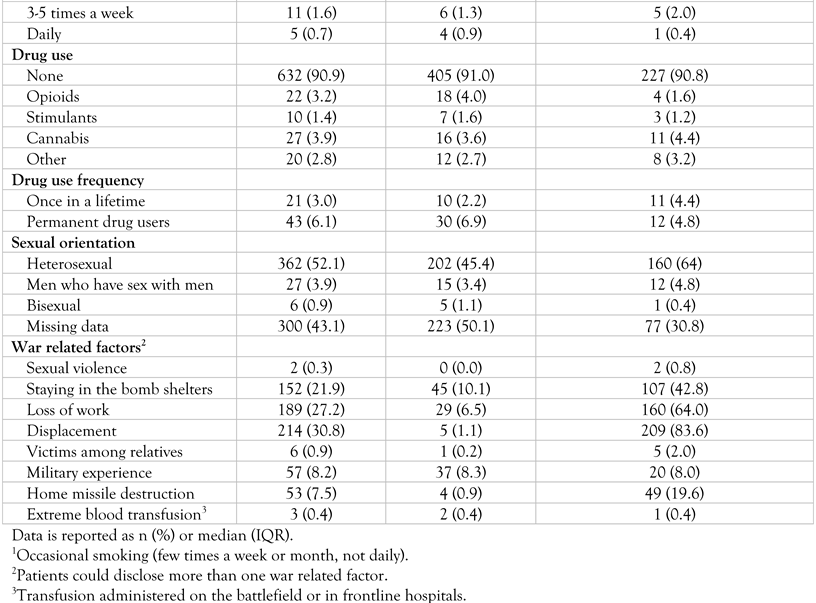 |
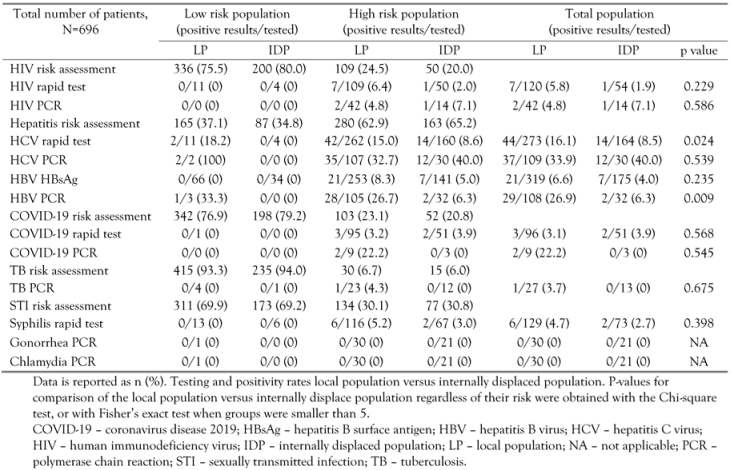 |
Case positivity rate
Discussion
Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of interest
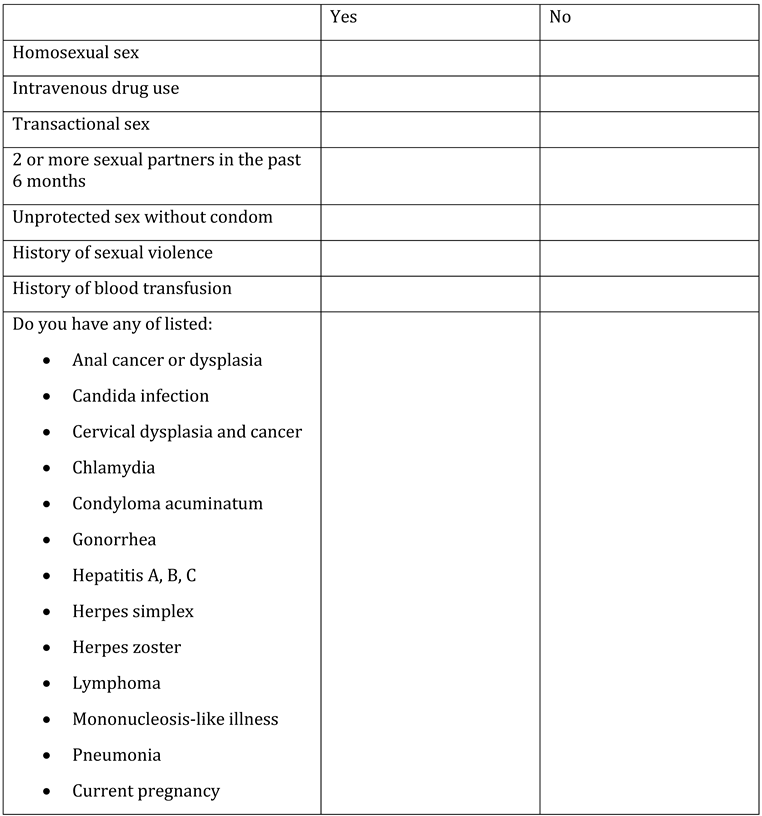
Appendix A. Questionnaire
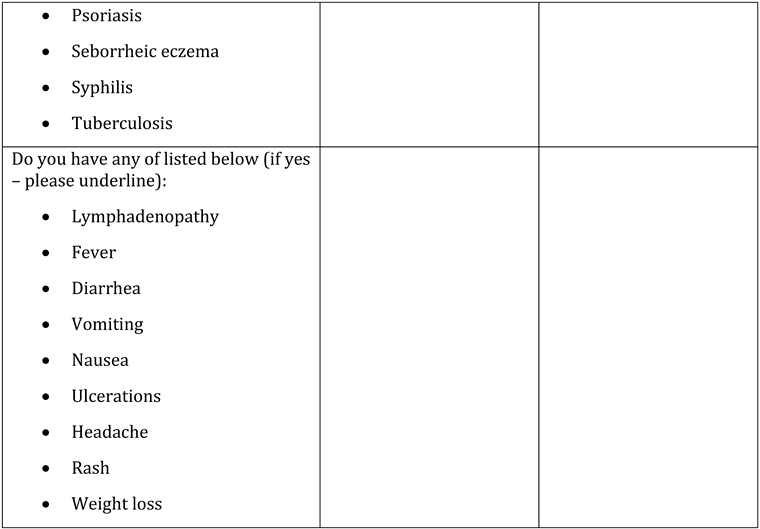
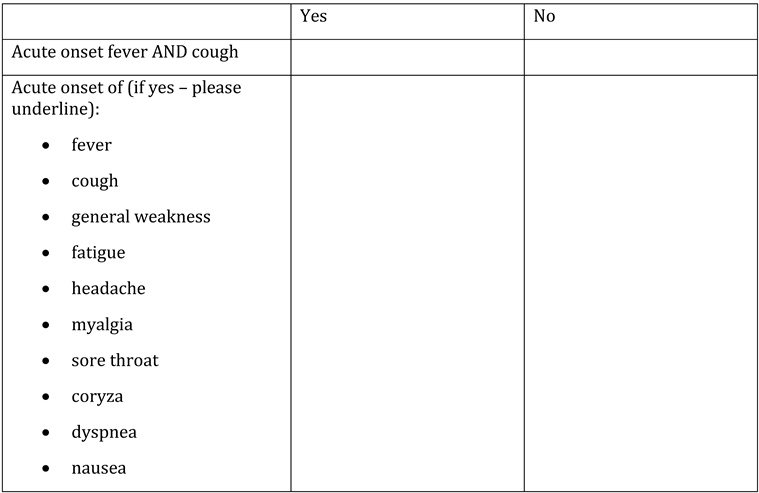

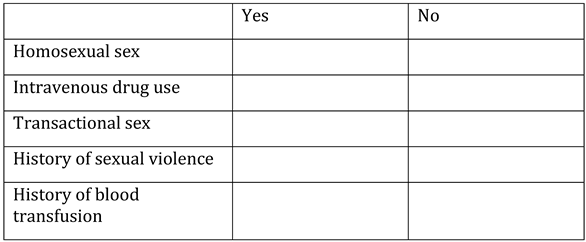
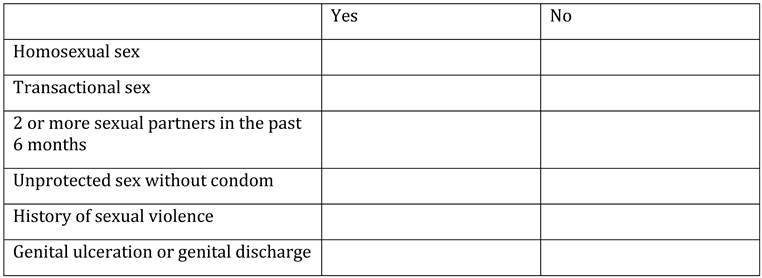
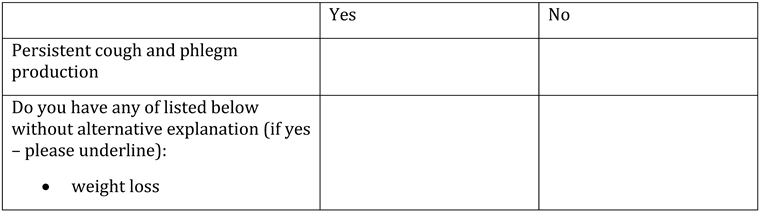


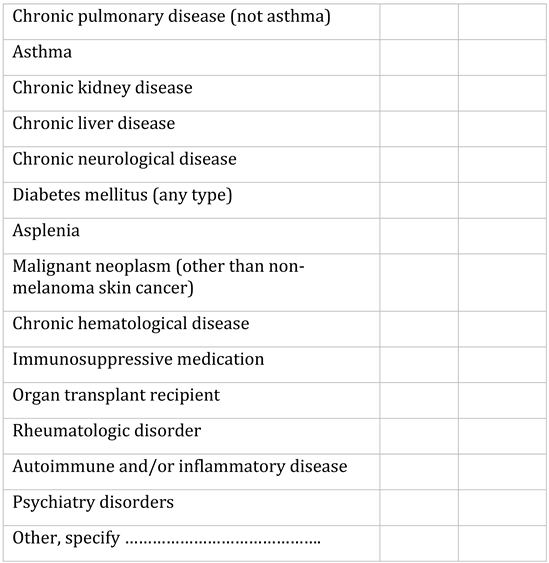
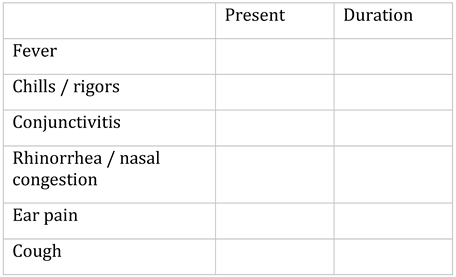

Appendix B. Testing algorithm for HIV, HBV, HCV, COVID-19, tuberculosis and sexually transmitted diseases
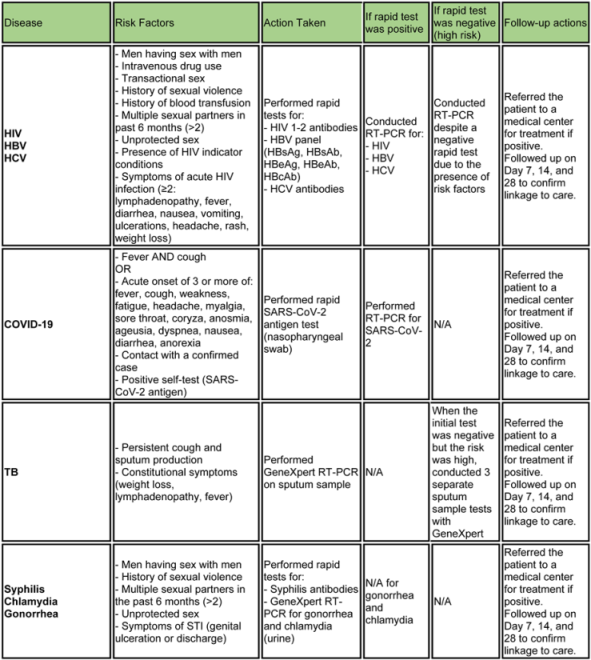
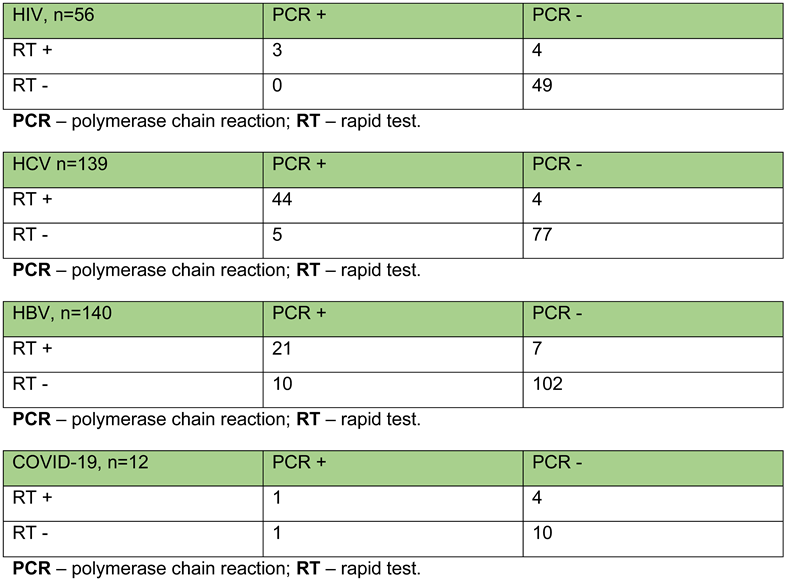
Appendix C. Outcomes of rapid tests and PCR in patients with dual test administration
References
- The Lancet Infectious Diseases. War and infectious diseases: brothers in arms. Lancet Infect Dis. 2022, 22, 563. [Google Scholar] [CrossRef] [PubMed]
- Parczewski, M.; Jabłonowska, E.; Wójcik-Cichy, K.; et al. Clinical perspective on human immunodeficiency virus care of Ukrainian war refugees in Poland. Clin Infect Dis. 2023, 76, 1708–1715. [Google Scholar] [CrossRef] [PubMed]
- Topluoglu, S.; Taylan-Ozkan, A.; Alp, E. Impact of wars and natural disasters on emerging and re-emerging infectious diseases. Front Public Health. 2023, 11, 1215929. [Google Scholar] [CrossRef] [PubMed]
- Goniewicz, K.; Burkle, F.M.; Horne, S.; Borowska-Stefańska, M.; Wiśniewski, S.; Khorram-Manesh, A. The influence of war and conflict on infectious disease: a rapid review of historical lessons we have yet to learn. Sustainability. 2021, 13, 10783. [Google Scholar] [CrossRef]
- Kerridge, B.T.; Saha, T.D.; Hasin, D.S. Armed conflict, substance use and HIV: a global analysis. AIDS Behav. 2016, 20, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Essar, M.Y.; Matiashova, L.; Tsagkaris, C.; Vladychuk, V.; Head, M. Infectious diseases amidst a humanitarian crisis in Ukraine: a rising concern. Ann Med Surg (Lond). 2022, 78, 103950. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control/WHO Regional Office for Europe. HIV/AIDS surveillance in Europe 2023 – 2022 data; ECDC: Stockholm, 2023. [Google Scholar]
- Massmann, R.; Groh, T.; Jilich, D.; et al. HIV-positive Ukrainian refugees in the Czech Republic. AIDS. 2023, 37, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Vasylyeva, T.I.; Liulchuk, M.; Friedman, S.R.; et al. Molecular epidemiology reveals the role of war in the spread of HIV in Ukraine. Proc Natl Acad Sci U S A. 2018, 115, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver, WHO Regional Office for Europe, European Centre for Disease Prevention and Control. Ensuring high-quality viral hepatitis care for refugees from Ukraine; 2022. Available online: https://easl.eu/wp-content/uploads/2022/05/WHO-ECDC-EASL-statement-FINAL_2May2022.pdf. (accessed on 22 July 2022).
- Dohál, M.; Dvořáková, V.; Šperková, M.; et al. Tuberculosis in Ukrainian war refugees and migrants in the Czech Republic and Slovakia: a molecular epidemiological study. J Epidemiol Glob Health. 2024, 14, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Kvasnevska, Y.; Faustova, M.; Voronova, K.; Basarab, Y.; Lopatina, Y. Impact of war-associated factors on spread of sexually transmitted infections: a systemic review. Front Public Health. 2024, 12, 1366600. [Google Scholar] [CrossRef] [PubMed]
- Vasylyev, M.; Skrzat-Klapaczyńska, A.; Bernardino, J.I.; et al. Unified European support framework to sustain the HIV cascade of care for people living with HIV including in displaced populations of war-struck Ukraine. Lancet HIV. 2022, 9, e438–e448. [Google Scholar] [CrossRef] [PubMed]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002, 52, 69–77. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. COVID-19 Case definition. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance_Case_Definition-2022.1 (accessed on 22 July 2022).
© GERMS 2024.
Share and Cite
Vasylyev, M.; Lamberink, H.; Svyst, I.; Khlypnyach, O.; Sluzhynska, O.; Sluzhynska, M.; Shtoiko, I.; Hrushynska, O.; Demianenko, D.; Rokx, C. The Infectious Disease Burden Among War Related Internally Displaced People in the Lviv Region of Ukraine. GERMS 2024, 14, 322-343. https://doi.org/10.18683/germs.2024.1443
Vasylyev M, Lamberink H, Svyst I, Khlypnyach O, Sluzhynska O, Sluzhynska M, Shtoiko I, Hrushynska O, Demianenko D, Rokx C. The Infectious Disease Burden Among War Related Internally Displaced People in the Lviv Region of Ukraine. GERMS. 2024; 14(4):322-343. https://doi.org/10.18683/germs.2024.1443
Chicago/Turabian StyleVasylyev, Marta, Hanne Lamberink, Ivanna Svyst, Oksana Khlypnyach, Oleksandra Sluzhynska, Maryana Sluzhynska, Iryna Shtoiko, Oleksandra Hrushynska, Dmytro Demianenko, and Casper Rokx. 2024. "The Infectious Disease Burden Among War Related Internally Displaced People in the Lviv Region of Ukraine" GERMS 14, no. 4: 322-343. https://doi.org/10.18683/germs.2024.1443
APA StyleVasylyev, M., Lamberink, H., Svyst, I., Khlypnyach, O., Sluzhynska, O., Sluzhynska, M., Shtoiko, I., Hrushynska, O., Demianenko, D., & Rokx, C. (2024). The Infectious Disease Burden Among War Related Internally Displaced People in the Lviv Region of Ukraine. GERMS, 14(4), 322-343. https://doi.org/10.18683/germs.2024.1443




