Infective Endocarditis by Carbapenem-Resistant Gram-Negative Bacteria—A Systematic Review
Abstract
Introduction
Methods
Data search
Study selection
Outcomes of interest
Data extraction and definitions
Statistical analysis
Results
Literature search
Included studies’ characteristics
Characteristics of IE by carbapenemresistant Gram-negative bacteria
Treatment and outcomes of IE by carbapenem-resistant Gram-negative bacteria
Statistical analysis of IE by carbapenemresistant Gram-negative bacteria
Discussion
Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; et al. Infective endocarditis in adults: Diagnosis, antimicrobial therapy, and management of complications: A scientific statement for healthcare professionals from the American Heart Association. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Gaca, J.G.; Chu, V.H. Management considerations in infective endocarditis: A review. JAMA 2018, 320, 72–83. [Google Scholar] [CrossRef]
- Habib, G.; Erba, P.A.; Iung, B.; et al. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: A prospective cohort study. Eur. Heart J. 2019, 40, 3222–3232. [Google Scholar] [CrossRef]
- Shah, A.S.V.; McAllister, D.A.; Gallacher, P.; et al. Incidence, microbiology, and outcomes in patients hospitalized with infective endocarditis. Circulation 2020, 141, 2067–2077. [Google Scholar] [CrossRef]
- Cresti, A.; Chiavarelli, M.; Scalese, M.; et al. Epidemiological and mortality trends in infective endocarditis, a 17-year population-based prospective study. Cardiovasc. Diagn. Ther. 2017, 7, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, P.E.; Samonis, G.; Andrianaki, A.M.; et al. Epidemiology, microbiological and clinical features, treatment, and outcomes of infective endocarditis in Crete, Greece. Infect. Chemother. 2018, 50, 21–28. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Zhou, R.; Fang, X.; Zhang, J.; et al. Impact of carbapenem resistance on mortality in patients infected with Enterobacteriaceae: A systematic review and metaanalysis. BMJ Open. 2021, 11, e054971. [Google Scholar] [CrossRef]
- Doi, Y. Treatment options for carbapenem-resistant Gram-negative bacterial infections. Clin Infect Dis. 2019, 69, S565–S575. [Google Scholar] [CrossRef]
- Kofteridis, D.P.; Andrianaki, A.M.; Maraki, S.; et al. Treatment pattern, prognostic factors, and outcome in patients with infection due to pan-drug-resistant gramnegative bacteria. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Ioannou, P.; Kourtidis, M.; Mytilinis, D.O.; Psyllaki, A.; Baliou, S.; Kofteridis, D. Whipple’s disease-associated infective endocarditis: A systematic review. Infect. Dis. 2023, 55, 447–457. [Google Scholar] [CrossRef]
- Fowler, V.G.; Durack, D.T.; Selton-Suty, C.; et al. The 2023 Duke-ISCVID criteria for infective endocarditis: Updating the modified Duke criteria. Clin Infect Dis. 2023, 77, 518–26. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Aydin, K.; Köksal, I.; Kaygusuz, S.; Kaklikkaya, I.; Caylan, R.; Ozdemir, R. Endocarditis caused by Stenotrophomonas maltophilia. Scand. J. Infect. Dis. 2000, 32, 427–430. [Google Scholar] [CrossRef]
- Olut, A.I.; Erkek, E. Early prosthetic valve endocarditis due to Acinetobacter baumannii: A case report and brief review of the literature. Scand. J. Infect. Dis. 2005, 37, 919–921. [Google Scholar] [CrossRef]
- Bomb, K.; Arora, A.; Trehan, N. Endocarditis due to Chryseobacterium meningosepticum. Indian. J. Med. Microbiol. 2007, 25, 161–162. [Google Scholar] [CrossRef] [PubMed]
- Benenson, S.; Navon-Venezia, S.; Carmeli, Y.; et al. Carbapenem-resistant Klebsiella pneumoniae endocarditis in a young adult. Successful treatment with gentamicin and colistin. Int. J. Infect. Dis. 2009, 13, e295–e298. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, H.; Boroumand, M.A.; Anvari, M.S.; Karimi, A.; Moshtaghi, N. Left-sided endocarditis associated with multi-drug resistance Acinetobacter lwoffii. J. Tehran Heart Cent. 2009, 4, 189–192. [Google Scholar]
- Katayama, T.; Tsuruya, Y.; Ishikawa, S. Stenotrophomonas maltophilia endocarditis of prosthetic mitral valve. Intern. Med. 2010, 49, 1775–1777. [Google Scholar] [CrossRef]
- Raymond, T.; Wiesen, J.; Rehm, S.; Auron, M. Carbapenemresistant Klebsiella pneumoniae prosthetic valve endocarditis: A feared combination of technology and emerging pathogens. Infect. Dis. Clin. Pract. 2014, 22, 1135. [Google Scholar] [CrossRef]
- Naha, S.; Naha, K.; Acharya, V.; Hande, H.M.; Vivek, G. Community-acquired multidrug-resistant Gram-negative bacterial infective endocarditis. BMJ Case Rep. 2014, 2014, bcr2014204176. [Google Scholar] [CrossRef]
- Vergara-López, S.; Domínguez, M.C.; Conejo, M.C.; Pascual, Á.; Rodríguez-Baño, J. Prolonged treatment with large doses of fosfomycin plus vancomycin and amikacin in a case of bacteraemia due to methicillin-resistant Staphylococcus epidermidis and IMP-8 metallo-β-lactamaseproducing Klebsiella oxytoca. J. Antimicrob. Chemother. 2015, 70, 313–315. [Google Scholar] [CrossRef][Green Version]
- Durante-Mangoni, E.; Andini, R.; Agrusta, F.; et al. Infective endocarditis due to multidrug resistant gram-negative bacilli: Single centre experience over 5 years. Eur. J. Intern. Med. 2014, 25, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Chaari, A.; Mnif, B.; Chtara, K.; et al. Efficacy of tigecyclinecolistin combination in the treatment of carbapenemresistant Klebsiella pneumoniae endocarditis. J. Glob. Antimicrob. Resist. 2015, 3, 214–216. [Google Scholar] [CrossRef]
- Patel, G.; Perez, F.; Hujer, A.M.; et al. Fulminant endocarditis and disseminated infection caused by carbapenem-resistant Acinetobacter baumannii in a renalpancreas transplant recipient. Transpl. Infect. Dis. 2015, 17, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Cao, H.; Lu, H.; Qiu, Z.; He, J. Bioprosthetic tricuspid valve endocarditis caused by Acinetobacter baumannii complex, a case report and brief review of the literature. J. Cardiothorac. Surg. 2015, 10, 149. [Google Scholar] [CrossRef]
- Domitrovic, T.N.; Hujer, A.M.; Perez, F.; et al. Multidrug resistant Pseudomonas aeruginosa causing prosthetic valve endocarditis: A genetic-based chronicle of evolving antibiotic resistance. Open Forum Infect Dis. 2016, 3, ofw188. [Google Scholar] [CrossRef]
- Kantarcioglu, B.; Bekoz, H.S.; Olgun, F.E.; et al. Allogeneic stem cell transplantation in a blast-phase chronic myeloid leukemia patient with carbapenem-resistant Klebsiella pneumoniae tricuspid valve endocarditis: A case report. Mol. Clin. Oncol. 2016, 5, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Otto, C.; Zeng, J.; et al. Achromobacter endocarditis in native cardiac valves—An autopsy case report and review of the literature. Cardiovasc. Pathol. 2018, 36, 6–10. [Google Scholar] [CrossRef]
- Gürtler, N.; Osthoff, M.; Rueter, F.; et al. Prosthetic valve endocarditis caused by Pseudomonas aeruginosa with variable antibacterial resistance profiles: A diagnostic challenge. BMC Infect. Dis. 2019, 19, 530. [Google Scholar] [CrossRef]
- Prescott, A.; Kennedy, S.; Howard, P.; et al. Ceftolozanetazobactam in combination with fosfomycin for treatment of MDR/XDR P. aeruginosa infective endocarditis. Clin. Infect. Pract. 2019, 2, 100011. [Google Scholar] [CrossRef]
- Peghin, M.; Maiani, M.; Castaldo, N.; et al. Ceftolozane/tazobactam for the treatment of MDR Pseudomonas aeruginosa left ventricular assist device infection as a bridge to heart transplant. Infection. 2018, 46, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Edgeworth, J.D.; Merante, D.; Patel, S.; et al. Compassionate use of cefiderocol as adjunctive treatment of native aortic valve endocarditis due to extremely drug-resistant Pseudomonas aeruginosa. Clin. Infect. Dis. 2019, 68, 1932–1934. [Google Scholar] [CrossRef]
- Dvoretsky, L.I.; Yakovlev, S.V.; Suvorova, M.P.; Varyasin, V.V.; Stepanchenko, A.P.; Karnaushkina, M.A. A rare case of pure white cell aplasia in a patient with thymoma complicated by infective endocarditis. Arch. Balk. Med. Union. 2021, 56, 257–262. [Google Scholar] [CrossRef]
- Alghoribi, M.F.; Alqurashi, M.; Okdah, L.; et al. Successful treatment of infective endocarditis due to pandrugresistant Klebsiella pneumoniae with ceftazidime-avibactam and aztreonam. Sci. Rep. 2021, 11, 9684. [Google Scholar] [CrossRef]
- Lima, O.; Sousa, A.; Filgueira, A.; et al. Successful ceftazidime-avibactam therapy in a patient with multidrug-resistant Pseudomonas aeruginosa infective endocarditis. Infection 2022, 50, 1039–1041. [Google Scholar] [CrossRef]
- Walczak, A.; McCarthy, K.; Paterson, D.L. A contemporary case series of Pseudomonas aeruginosa infective endocarditis. Medicine 2023, 102, e32662. [Google Scholar] [CrossRef] [PubMed]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; et al. Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef]
- Peri, A.M.; Doi, Y.; Potoski, B.A.; Harris, P.N.A.; Paterson, D.L.; Righi, E. Antimicrobial treatment challenges in the era of carbapenem resistance. Diagn. Microbiol. Infect. Dis. 2019, 94, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Jean, S.S.; Harnod, D.; Hsueh, P.R. Global threat of carbapenem-resistant Gram-negative bacteria. Front. Cell Infect. Microbiol. 2022, 12, 823684. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Carrara, E.; Retamar, P.; et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin. Microbiol. Infect. 2022, 28, 521–547. [Google Scholar] [CrossRef] [PubMed]
- Bouza, E.; Muñoz, P.; Burillo, A. Gram-negative endocarditis: Disease presentation, diagnosis and treatment. Curr. Opin. Infect. Dis. 2021, 34, 672–680. [Google Scholar] [CrossRef]
- Morpeth, S.; Murdoch, D.; Cabell, C.H.; et al. Non-HACEK gram-negative bacillus endocarditis. Ann. Intern. Med. 2007, 147, 829–835. [Google Scholar] [CrossRef]
- Ertugrul Mercan, M.; Arslan, F.; Ozyavuz Alp, S.; et al. NonHACEK Gram-negative bacillus endocarditis. Med. Mal. Infect. 2019, 49, 616–620. [Google Scholar] [CrossRef]
- Sebillotte, M.; Boutoille, D.; Declerck, C.; et al. NonHACEK gram-negative bacilli endocarditis: A multicentre retrospective case-control study. Infect. Dis. 2023, 55, 599–606. [Google Scholar] [CrossRef]
- Ioannou, P.; Mavrikaki, V.; Kofteridis, D.P. Infective endocarditis by Acinetobacter species: A systematic review. J. Chemother. 2021, 33, 203–215. [Google Scholar] [CrossRef]
- Ioannou, P.; Miliara, E.; Baliou, S.; Kofteridis, D.P. Infective endocarditis by Klebsiella species: A systematic review. J. Chemother. 2021, 33, 365–374. [Google Scholar] [CrossRef]
- Loubet, P.; Lescure, F.X.; Lepage, L.; et al. Endocarditis due to gram-negative bacilli at a French teaching hospital over a 6-year period: Clinical characteristics and outcome. Infect. Dis. 2015, 47, 889–895. [Google Scholar] [CrossRef]
- Veve, M.P.; McCurry, E.D.; Cooksey, G.E.; Shorman, M.A. Epidemiology and outcomes of non-HACEK infective endocarditis in the southeast United States. PLoS ONE 2020, 15, e0230199. [Google Scholar] [CrossRef]
- Brink, A.J. Epidemiology of carbapenem-resistant Gramnegative infections globally. Curr. Opin. Infect. Dis. 2019, 32, 609–616. [Google Scholar] [CrossRef]
- Katchanov, J.; Asar, L.; Klupp, E.M.; et al. Carbapenemresistant Gram-negative pathogens in a German university medical center: Prevalence, clinical implications and the role of novel β-lactam/β-lactamase inhibitor combinations. PLoS ONE 2018, 13, e0195757. [Google Scholar] [CrossRef]
- Gales, A.C.; Castanheira, M.; Jones, R.N.; Sader, H.S. Antimicrobial resistance among Gram-negative bacilli isolated from Latin America: Results from SENTRY Antimicrobial Surveillance Program (Latin America, 2008-2010). Diagn Microbiol Infect Dis. 2012, 73, 35460. [Google Scholar] [CrossRef]
- Sader, H.S.; Farrell, D.J.; Flamm, R.K.; Jones, R.N. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalised with pneumonia in US and European hospitals: Results from the SENTRY Antimicrobial Surveillance Program, 2009-2012. Int J Antimicrob Agents. 2014, 43, 328–334. [Google Scholar] [CrossRef]
- Rodvold, K.A.; Gotfried, M.H.; Cwik, M.; Korth-Bradley, J.M.; Dukart, G.; Ellis-Grosse, E.J. Serum, tissue and body fluid concentrations of tigecycline after a single 100 mg dose. J. Antimicrob. Chemother. 2006, 58, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Lawrence, C.K.; Adam, H.; et al. Imipenemrelebactam and meropenem-vaborbactam: Two novel carbapenem-β-lactamase inhibitor combinations. Drugs 2018, 78, 65–98. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Ioannou, P.; Samonis, G.; Kofteridis, D.P. Systematic review of antimicrobial combination options for pandrug-resistant Acinetobacter baumannii. Antibiotics 2021, 10, 1344. [Google Scholar] [CrossRef] [PubMed]
- Kaye, K.S.; Marchaim, D.; Thamlikitkul, V.; et al. Colistin monotherapy versus combination therapy for carbapenem-resistant organisms. NEJM Evid. 2023, 2, 10. [Google Scholar] [CrossRef]
- Katip, W.; Uitrakul, S.; Oberdorfer, P. A comparison of colistin versus colistin plus meropenem for the treatment of carbapenem-resistant Acinetobacter baumannii in critically ill patients: A propensity scorematched analysis. Antibiotics 2020, 9, 647. [Google Scholar] [CrossRef] [PubMed]
- López-Viñau, T.; Peñalva, G.; García-Martínez, L.; et al. Impact of an antimicrobial stewardship program on the incidence of carbapenem resistant Gram-negative bacilli: An interrupted time-series analysis. Antibiotics 2021, 10, 586. [Google Scholar] [CrossRef] [PubMed]
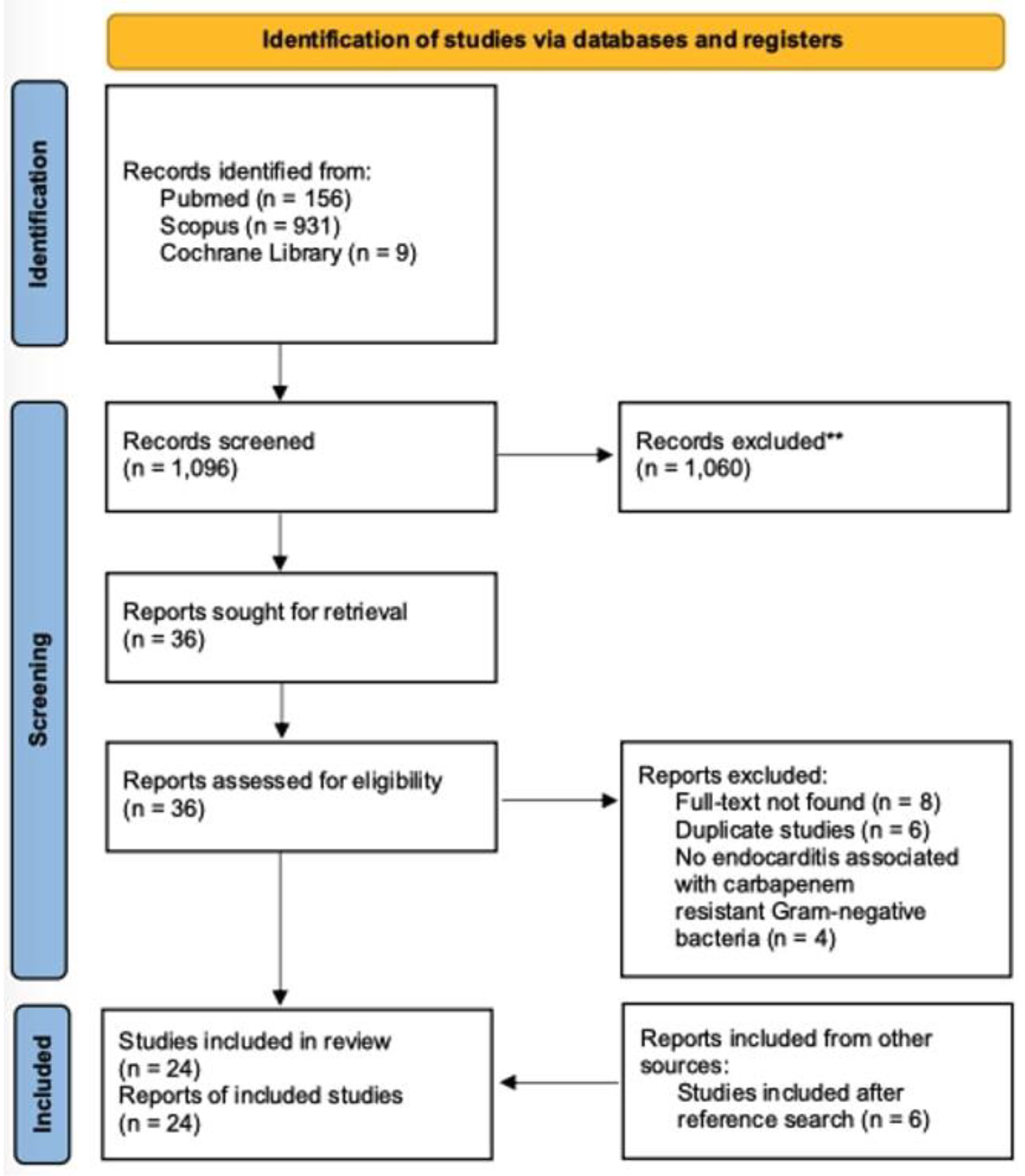
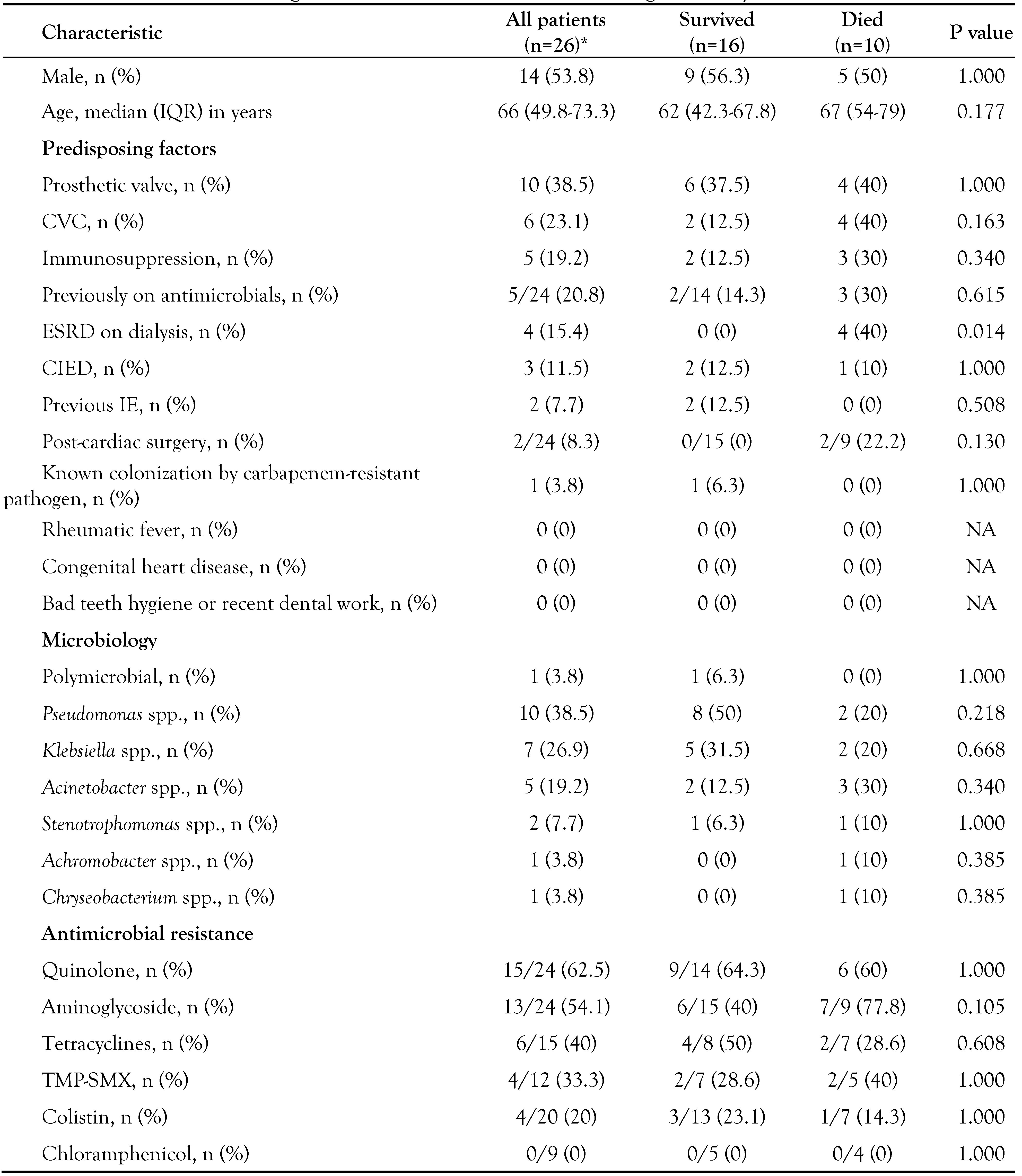 |
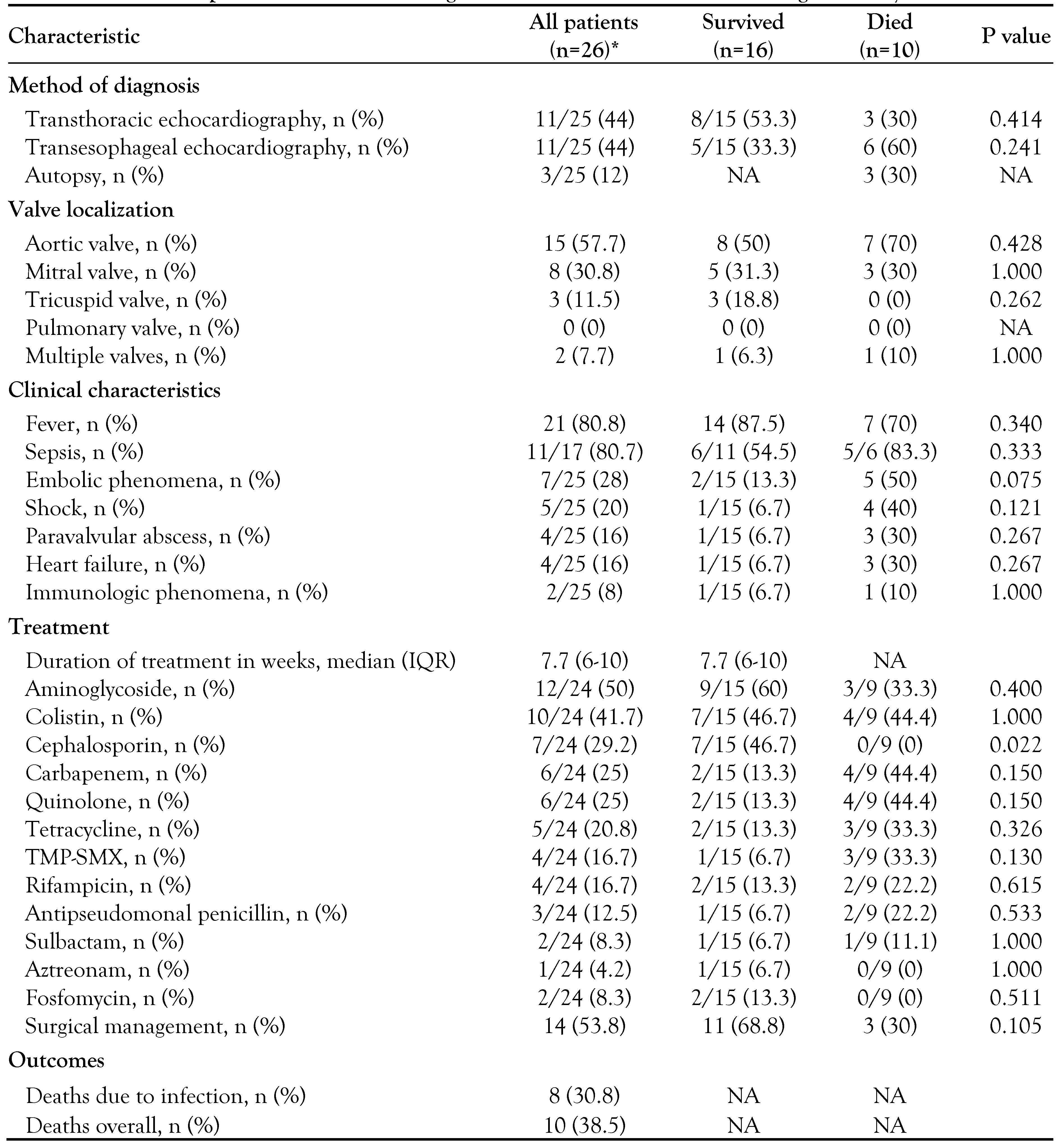 |
© GERMS 2024.
Share and Cite
Pitsikakis, K.; Skandalakis, M.; Fragkiadakis, K.; Baliou, S.; Ioannou, P. Infective Endocarditis by Carbapenem-Resistant Gram-Negative Bacteria—A Systematic Review. Germs 2024, 14, 149-161. https://doi.org/10.18683/germs.2024.1427
Pitsikakis K, Skandalakis M, Fragkiadakis K, Baliou S, Ioannou P. Infective Endocarditis by Carbapenem-Resistant Gram-Negative Bacteria—A Systematic Review. Germs. 2024; 14(2):149-161. https://doi.org/10.18683/germs.2024.1427
Chicago/Turabian StylePitsikakis, Konstantinos, Michail Skandalakis, Konstantinos Fragkiadakis, Stella Baliou, and Petros Ioannou. 2024. "Infective Endocarditis by Carbapenem-Resistant Gram-Negative Bacteria—A Systematic Review" Germs 14, no. 2: 149-161. https://doi.org/10.18683/germs.2024.1427
APA StylePitsikakis, K., Skandalakis, M., Fragkiadakis, K., Baliou, S., & Ioannou, P. (2024). Infective Endocarditis by Carbapenem-Resistant Gram-Negative Bacteria—A Systematic Review. Germs, 14(2), 149-161. https://doi.org/10.18683/germs.2024.1427




