Abstract
Introduction: Antimicrobial resistance (AMR) is among the greatest threats to global healthcare. The World Health Organization (WHO) estimates that by 2050 ten million deaths will be attributed to AMR annually. In response, the WHO has implemented antibiotic stewardship programs which focus on optimizing antibiotic use and raise, amongst others, the issue of the preferred method of intravenous antibiotic administration. Various studies have attempted to answer this question with conflicting results. Review: This review examined several studies assessing extended/continuous infusion compared to intermittent infusion of three beta-lactams: piperacillin-tazobactam, cefepime, and meropenem. The findings and conclusions of each study were summarized and compared to one another to provide a general overview of the current evidence. Conclusions: We conclude that continuous/extended infusion showed a greater clinical benefit in highly critical cases, namely sepsis and febrile neutropenia, compared to intermittent infusion. Additionally, in cases where a pathogen was identified, continuous/extended infusion showed superiority. Nonetheless, high-quality studies with larger samples are needed to demonstrate the difference between these two modes of infusion in a way that would better inform guidelines and policies, aiding in the fight against AMR.
Introduction
Antibiotics have laid the foundations for modern medicine and have been fundamental to the success of highly invasive surgeries, immunomodulatory treatments, and various other medical procedures [1]. Unfortunately, this success is threatened by the rise of antimicrobial resistance (AMR). According to the World Health Organization (WHO), AMR is among the greatest threats to global healthcare and human health. In 2019, it was estimated that AMR was directly responsible for 1.27 million deaths globally. Moreover, it is estimated that by 2050, AMR will be responsible for 10 million deaths per year. These numbers reflect the immediate threat of this global healthcare crisis [2]. In addition, based on estimates by the Center for Disease Control and Prevention (CDC), the cost of treating multidrug resistant (MDR) infections is over $4.6 billion, annually. Furthermore, this crisis was further exacerbated by inadequate research and development of new antibiotics to face the increasing prevalence of MDR pathogens [1].
The WHO has implemented multiple strategies to combat the threat of AMR; one such strategy is that of antimicrobial stewardship programmes (ASPs), which focus on optimizing the use of antibiotics, among other antimicrobials. Specifically for beta-lactams, changing the administration strategy of antibiotics from the standard intermittent infusion to extended or continuous infusion has been proposed to improve efficacy and reduce bacterial resistance. This is because beta-lactams exhibit time-dependent bacterial killing in which the time (T) for free antibiotic concentration is maintained above the organisms’ minimum inhibitory concentration (MIC). In other words, the longer the beta-lactam concentration remains above the MIC, the greater the antimicrobial effect [3]. Current guidelines recommend intermittent (II) or extended/continuous infusion (EI/CI) based on this pharmacodynamic principle and observational studies. However, whether the difference in the type of infusion is reflected in improved clinical outcomes is yet to be established. Various studies have compared II with EI/CI yielding mixed results; however, as of now, there have been no high-quality randomized control trials comparing the different infusion types.
This narrative review discusses the multiple studies comparing the different types of infusion involving piperacillin-tazobactam, cefepime, and meropenem. These drugs were chosen based on the relative abundance of data in the literature examining their different modes of infusion, and on top of that, in order to examine a modality that might further optimize the use of older antibiotics as this would allow us to spare the newer broad-spectrum antibiotics, serving as an antibiotic stewardship strategy. More specifically, this review examines the use of these antibiotics in the following patient populations: critically ill, febrile neutropenia, and pediatric patients. Moreover, in this review we defined CI as constant intravenous administration throughout a 24-h period, EI as intravenous administration over 2-4 h, and II as intravenous administration for less than or equal to 30 min.
Severe sepsis in critically ill patients remains challenging to treat, and mortality rates remain high [3]. This is mainly due to several pathophysiological changes such as fluid shift phenomena and augmented renal clearance (ARC) that alter antibiotic pharmacokinetics [4]. Similar to the critically ill, patients with cancer have altered PK/PD parameters due to pathophysiological changes that accompany malignant disease, particularly hematological malignancies [5]. These alterations in pharmacokinetics complicate the administration of antibiotics and increase the likelihood of achieving sub-therapeutic doses and subsequent therapeutic failure [6]. Furthermore, in comparison to adults, children have an expanded volume of distribution, rapid renal clearance, and other variations in drug metabolism and pharmacokinetics. These differences highlight the need for specific dosing regimens for pediatric populations to achieve targeted therapeutic goals [7,8].
Numerous studies in the critically ill and populations of patients with cancer have shown that EI/CI results in longer period in which betalactam dose concentration is higher than MIC compared to II; however, whether this is associated with improved clinical outcomes is still unclear [9]. On the other hand, few studies have focused on the effectiveness of EI/CI regimens compared to II regimens in pediatric populations [10]. Thus, we examined the current evidence regarding the clinical outcomes of the patients belonging to the above-mentioned populations according to the mode of administration of piperacillin-tazobactam, cefepime and meropenem.
Methods
Search criteria
A literature search of PubMed using the keywords “piperacillin-tazobactam”, “cefepime”, “meropenem”, “infusion”, “intermittent”, “extended”, and “continuous” was performed, within a defined timeline (2002-2023). Only English language publications were included. The search was done by the two authors in a period of 2 months, from October to November 2023. The search yielded 109 articles, of which 10 were duplicates, and 56 were selected for this review. Articles that included any of the antibiotics piperacillin-tazobactam, cefepime, or meropenem were included. In addition, articles were selected based on having a clinical outcome measure that included but were not limited to: mortality rate, achievement of clinical cure, intensive care unit (ICU) length of stay, and hospital length of stay. Furthermore, articles with patient populations that included patients with febrile neutropenia, critically ill patients, or pediatric patients were selected. On the other hand, studies with a primary focus on pharmacokinetics and pharmacodynamics of beta-lactams were excluded. The types of studies included were randomized clinical trials, cohort studies, and systematic reviews. The search did yield crosssectional studies, but those were excluded. Data from the WHO, CDC, and the Institute for Health Metrics and Evaluation (IHME) were also used in this review.
Results
Piperacillin-tazobactam
Critically ill patients
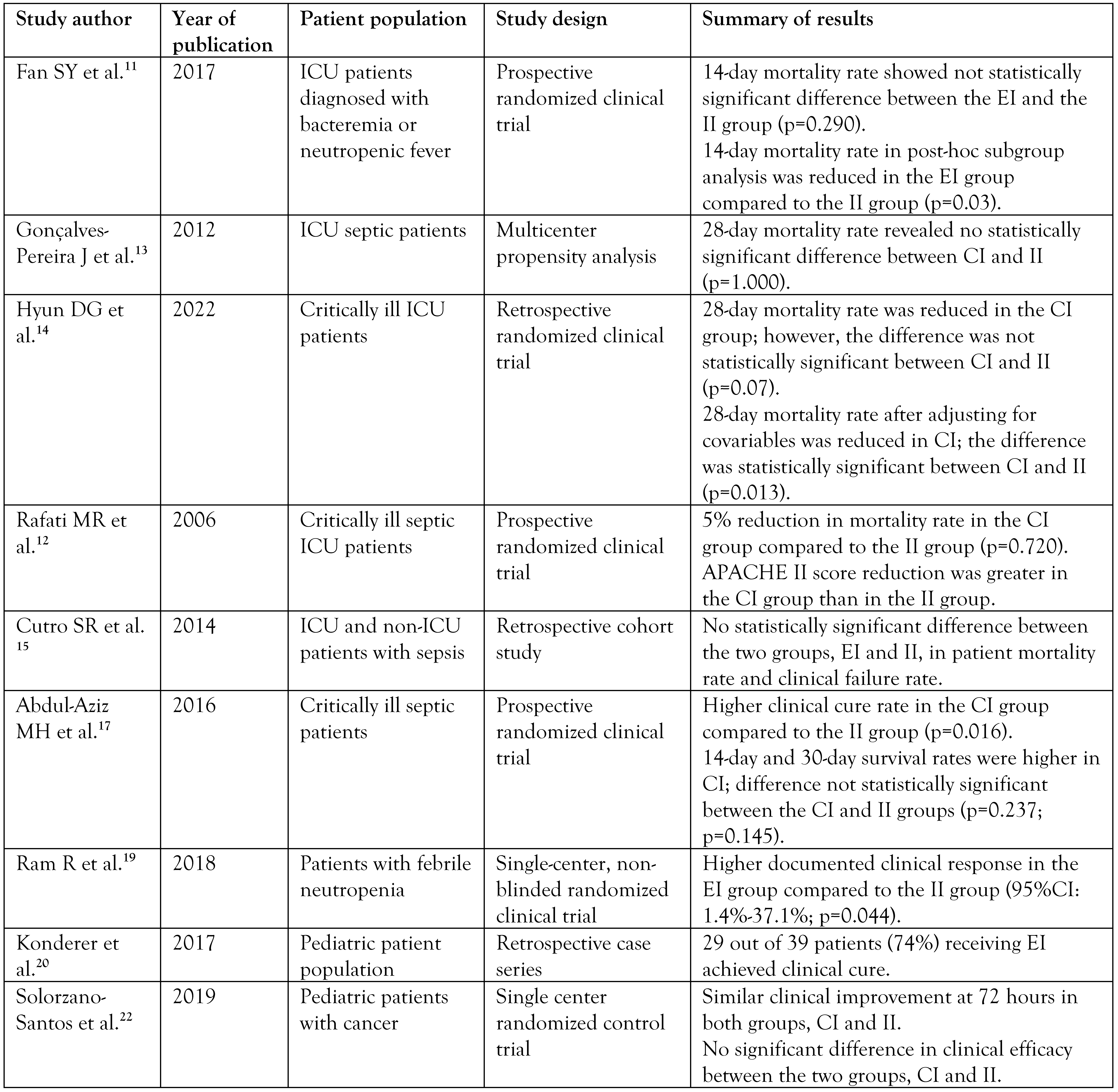
A single-center, prospective clinical trial performed by Fan et al. [11] (Table 1) involving patients from Hong Kong aimed to determine whether critically ill patients receiving EI would have better clinical outcomes than those receiving II. The patient population included 367 ICU patients diagnosed with either bacteremia or neutropenic fever and all patients received piperacillin-tazobactam regardless of the method of infusion. The study’s primary outcome was the 14-day mortality rate, which was similar in both EI and II, 11.5% and 15.7% respectively; however, it was not statistically significant. However, time to defervescence, a secondary outcome, was significantly reduced by two days in the EI group (p=0.01). Other secondary outcomes, such as length of ventilatory support and length of ICU stay, did not differ significantly between the two groups. Moreover, a post hoc subgroup analysis of the primary outcome revealed that the 14-day mortality rate in patients for whom an infectious agent was identified was reduced in the EI group (9.3%) compared to the II group (22.2%), with a relative risk reduction of 58.5% (p=0.03). Additionally, after patients were stratified based on the source of infection, patients with respiratory infection showed a reduction in the 14-day mortality rate in EI compared to the II group (8.9% vs. 18.7%). This stratification revealed a statistically significant difference between EI and II (p=0.02) [11]. A prospective clinical trial by Rafati et al. [12] (Table 1) that included septic ICU patients with a microbiologically documented infection showed a 5% reduction in mortality rates in the CI group compared to the II group; however, this was not statistically significant. Despite that, these results follow the same conclusion drawn from the findings of the previously mentioned post hoc subgroup analysis and highlight the possible clinical benefit of CI when a pathogen is identified. On the other hand, findings from a multicenter propensity analysis performed in 7 adult ICUs in Portugal by Gonçalves-Pereira et al. [13] (Table 1) contradict this conclusion. The analysis included 346 septic ICU patients who received piperacillin-tazobactam to treat microbiologically documented infections. The study’s primary outcome was 28-day all-cause mortality, which was not significantly different between the groups. A total of 98 patients died in the first 28 days, with 49 deaths in each group (p=1.00). The conclusions drawn from this analysis differ from the previous two studies. This might be partially explained by some of the study’s limitations, e.g., the fact that patients who did not attain the pharmacodynamic target were not identified, which might have resulted in subtherapeutic doses and subsequent treatment failure. Also, differences in diagnoses between the two patient populations, despite being all ICU patients, might have led to further biases [13].

Table 1.
Summary of studies involving piperacillin-tazobactam.
A further 2022 single-center retrospective study conducted in South Korea by Hyun DG et al. (Table 1) aimed to evaluate the clinical efficacy of CI of piperacillin-tazobactam in a patient population and setting similar to those of the previous studies [14]. In the CI group the 28-day mortality rate was 12.8%. In contrast, the 28-day mortality rate in the II group was 27.3% (p=0.07). After adjusting for potential covariables such as, among others, congestive heart failure, renal replacement therapy, and primary site of infection, patients that received CI infusion of piperacillin-tazobactam had a 69% lower likelihood of mortality at 28 days than patients who received II (95%CI: 0.12-0.78; p=0.013). Secondary outcomes included length of ICU stay, freedom from ventilation at 14 and 28 days, and normalization of C-reactive protein (CRP) on day 7. Similar to previous studies, there was no significant difference between both groups in ICU length of stay. However, patients in the CI group had a higher probability of being free from ventilation on day 14 (hazard ratio of 1.77, 95%CI: 1.10-2.84; p=0.018) but there was no difference between the two groups on day 28 (hazard ratio of 1.25, 95%CI: 0.76-2.06; p=0.376). In addition, patients in the CI group had higher rates of normalization of CRP at day 7 (9.3% vs. 1.9%; p=0.030). Overall, there was a significant difference between the continuous and intermittent groups concerning mortality rate at 28 days, freedom from ventilation at day 14, and normalization rates of CRP. Notably, differences in CRP normalization rates between the two groups illustrate the possible effectiveness of continuous infusion in the early inflammatory phase in critically ill patients with sepsis [14]. Furthermore, Rafati MR et al. documented a faster reduction rate in the APACHE II score in the CI group compared to the II group [12]. The reduction rate went from 2.6 on day 1 to 6.2 on day 6 for the CI group, while for the II group, it went from 1.1 on day 1 to 4.2 on day 6. This further suggests the possible role of CI in reducing inflammation in septic patients and remains consistent with the conclusions drawn by Hyun DG et al. [14]
A large retrospective cohort study by Cutro et al. (Table 1) of patients with sepsis who received either II or EI of piperacillin-tazobactam conducted in New York in 2014 aimed to analyze the clinical outcomes in both patient groups [15]. The study included ICU and non-ICU patients. However, all patients had presumed sepsis syndrome. The study’s primary outcomes were inpatient mortality, hospital length of stay, and ICU length of stay. The rate of clinical failure was included in the study as a secondary outcome. A total of 662 and 181 patients were assigned to the EI and II groups, respectively. The study did not find a statistically significant difference among the primary outcomes. In-patient mortality rate was 10.9% in the EI group and 13.8% in the II group (p=0.282). Moreover, the average hospital length of stay was also similar (10 days in the EI group and 12 days in the II group, p=0.171). Regarding the secondary outcomes, clinical failure rates were nearly identical in the two groups as well (18.4% and 19.9%, respectively; p=0.756). Furthermore, a subset analysis was performed on ICU-only patients, who were further stratified based on their clinical disease, that suggested patients with urinary tract infections (2.5% versus 16.7%; p=0.016) and intra-abdominal infections (7.3% versus 18.8%; p=0.086) improved mortality rates with EI [15]. A systematic review and meta-analysis by Yang H et al. [16] included studies that enrolled both ICU and non-ICU patients and showed findings that were consistent with the results of the cohort study by Cutro et al. [15], concluding that EI did not result in a significant difference in mortality rate. Comparing the results of the cohort study by Cutro et al. [15] with the results of the systematic review by Yang H et al. [16], it seems that the most significant benefit from CI is in high dependency units and ICUs; however, this would require further investigations involving ICU-only patients and heterogenous patient populations of both ICU and non-ICU patients.
All studies mentioned were done in developed countries. Data from other regions of the world, notably those afflicted with more resistant bacteria and different patient populations, are limited. The BLISS study (Table 1) was one of the few studies that focused entirely on a population of a developing country [17]. It was a prospective, two-center, open-label randomized controlled trial that compared CI and II of betalactams in critically ill patients with sepsis who were not receiving renal replacement therapy. Piperacillin-tazobactam was one of the three betalactams included in this study, along with cefepime and meropenem. Seventy patients were allocated to the CI group, of which 54% received piperacillin-tazobactam. Similarly, seventy patients were assigned to the II group, with 67% receiving piperacillin-tazobactam. For the patients that received piperacillin-tazobactam, the study’s primary outcome was clinical cure at 14 days after antibiotic cessation, with clinical cure defined as either resolution of the clinical signs of infection or marked reduction in the severity of the illness. Secondary endpoints included median ICU stay and survival at day 14 and day 30. Regarding the clinical cure rate, 58% in the CI group achieved clinical cure, while only 32% in the II group had a similar outcome. These results were statistically significant with a p value of 0.016. The CI group’s 14-day and 30-day survival rates were 80% and 74%, respectively. In contrast, the 14day and 30-day survival rates in the II group were 71% and 63%, respectively. However, these results were statistically not significant. Overall, continuous infusion of beta-lactams in critically ill patients with sepsis demonstrated higher clinical cure rates and better survival rates than intermittent infusion[17] These results are consistent with previously mentioned studies involving similar patient populations despite the geographical variation.
Febrile neutropenia
Beta-lactams, specifically piperacillintazobactam, are the most commonly used drugs in treating febrile neutropenia initially. However, only a handful of studies included a cohort of febrile neutropenic patients. None of these studies focused on piperacillin-tazobactam only, usually including several of the most commonly used beta-lactams in this patient setting [18]. Nevertheless, these studies yielded noteworthy results. One such study was a single-center, nonblinded, randomized clinical trial that assessed the clinical efficacy of EI versus II of either piperacillin-tazobactam or ceftazidime in patients with febrile neutropenia, conducted by Ram et al. [19] The study’s primary outcome was to determine whether EI showed clinical superiority compared to II. Clinical response was defined as one of the following: resolution of fever for at least 24 h, sterile cultures on days 3 and 4, and resolution of clinical signs and symptoms. A total of 105 patients were enrolled, with 47 in the EI group and 58 in the II group. Of note, 96 patients (91.4%) were treated with piperacillintazobactam. Clinical response was documented in 35 patients (74.4%) in the EI group and 32 patients (55.1%) in the II group (95%CI: 1.4%37.1%; p=0.044). Moreover, further stratification revealed that microbiologically documented infections, bloodstream infections, and pneumonia had significantly higher clinical response in the EI group compared to the II group. For instance, patients with a microbiologically documented infection had a clinical response rate of 71.7% in the EI group and 30% in the II group (p=0.0013), while patients with bloodstream infections had a clinical response rate of 25% in the EI and 10.7% in the II group (p=0.0011). Overall, EI resulted in a higher clinical response rate than II, which was more prominent when patients were stratified. The small difference between the groups before stratification can be attributed to the fact that only a fraction of the patients with febrile neutropenia had an underlying infection, which would render antibiotics ineffective in such cases. Additionally, the resolution and recurrence of fever might have been affected by multiple factors such as mucositis, reaction to blood products and cytotoxic drugs, and neoplastic fever, which might have led to the interpretation of the lack of defervescence as therapy failure. It is therefore essential to consider these variables when interpreting the study results, as they dilute the antibiotic effect. This is further reinforced by the significant difference in clinical response rates between the CI and II groups observed in the subgroup analysis, where patients with documented infections benefited greatly from CI. Overall, it is evident from this study that CI achieves significantly higher clinical response rates than II in patients with febrile neutropenia that have documented infection.
Pediatric patients
A 2017 retrospective case series study conducted by Knoderer et al. [20] included 39 patients aged one month through 17 years with a documented Gram-negative infection, all receiving piperacillin-tazobactam for at least 48 h [20]. The study’s primary outcome was the clinical cure rate, defined as experiencing complete resolution of symptoms, having normal white blood cell (WBC) count, or having negative follow-up cultures after 21 days of therapy. Secondary outcomes included 30-day mortality. Overall, 74% (n=29) of patients met the predefined criteria for clinical cure. No deaths were reported in this cohort. In conclusion, the majority of children who received EI of piperacillin-tazobactam achieved clinical cure, with those not achieving clinical cure being younger and more critically ill. Although these differences were not statistically significant, comparative studies with larger cohorts are required to illustrate these differences better or nullify them.
In addition to the natural complexity of treating critically ill pediatric patients, as mentioned above, pediatric cancer patients pose an even greater challenge, as they can manifest severe and life-threatening complications. With the rise of AMR, treatment failure of these complications, particularly febrile neutropenia, has become more common, prompting a search for optimal use of antibiotics [21]. A 2019 single center randomized controlled trial was conducted in Mexico by Solórzano-Santos et al. [22] to evaluate the clinical efficacy of CI vs. II of piperacillintazobactam in pediatric cancer patients with febrile neutropenia. A total of 176 patients were randomized. Clinical cure, defined as either defervescence within 96 h of treatment initiation or resolution of clinical signs of infection, was the study’s primary outcome. Clinical improvement at 72 h was similar in the two groups (80% in the II group and 73% in the CI group, respectively). Given these treatment failure rates, the absolute risk reduction mounted to 0.08% when comparing CI with II. There was one death in each group. Overall, there was no significant difference in the clinical efficacy between CI and II in febrile neutropenic children. Interestingly, fever decreased in the first 48 h in 45% of the cases in this study, while it has been proposed that defervescence should only be attributed to antibiotic therapy if it occurs after 72 h. Additionally, only 8% of the cases had proven bacteremia, which limited the validity of the study results.
Cefepime
Critically ill patients
Cefepime, like other beta-lactams, exhibits time dependent activity and EI/CI could hence potentiate its bactericidal activity due to its wide Gram-negative coverage which also includes betalactamase producing bacteria. Such pathogens have high MICs and EI/CI can increase the probability of target attainment (PTA) of cefepime without dose increases, thus improving the clinical efficacy of the drug. The pharmacokinetic profile of cefepime is suitable for CI due to its high stability. This was described by an experimental study which used high performance liquid chromatography to study the stability and bactericidal activity of cefepime during CI. It was found that cefepime was highly stable for up to 24 h and hence was suitable for CI, especially if stored at colder temperatures and administered via an infusion pump [23].
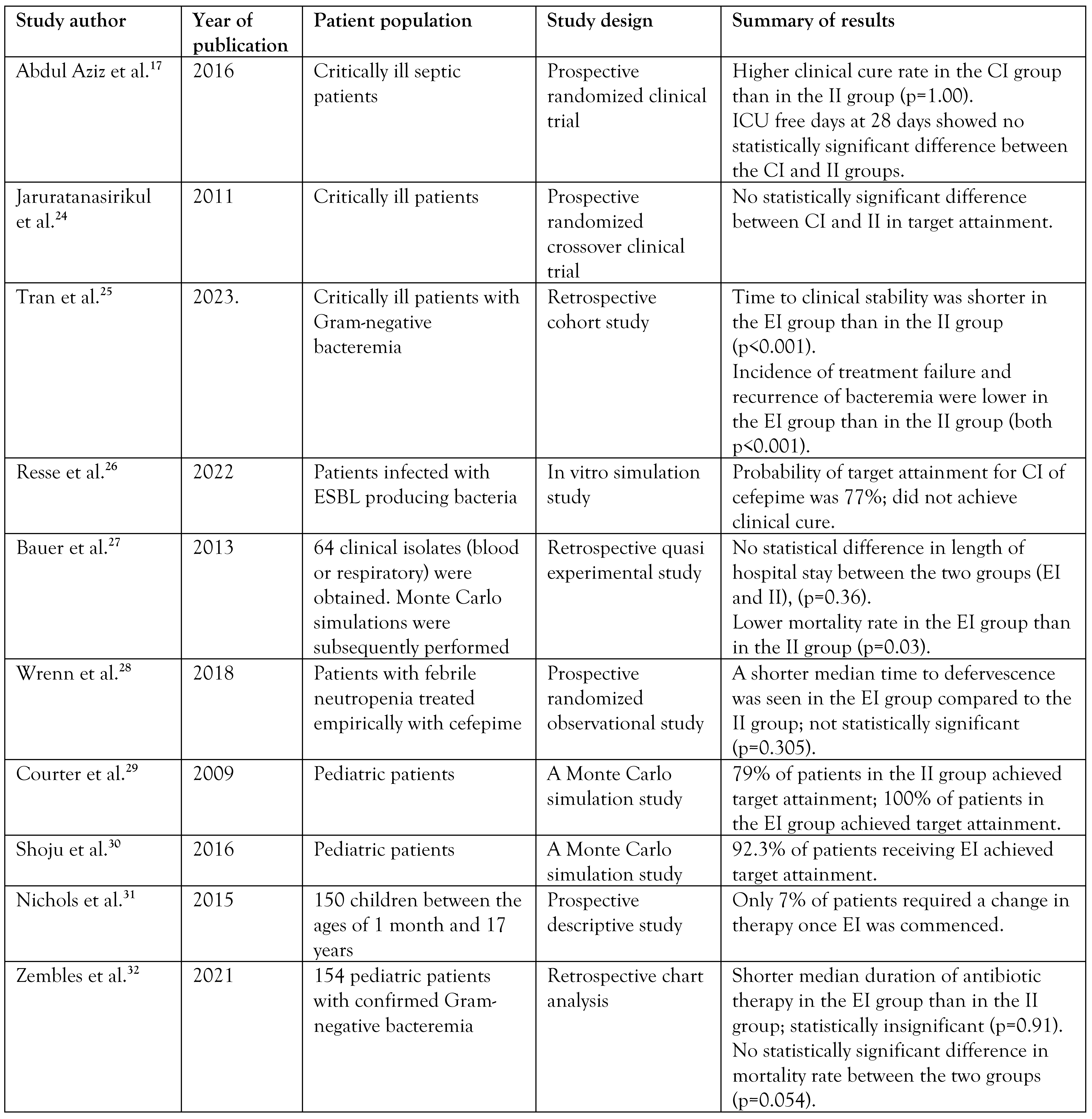
Current clinical evidence is, however, conflicting, failing to show a clear superiority of EI/CI cefepime over II. In the BLISS study, Abdul-Aziz et al. [17] (Table 2) examined the pharmacokinetic profile and clinical effect of CI piperacillin-tazobactam, cefepime and meropenem in patients with severe sepsis. Although this study showed overall favorable results in support of CI, this was not representative for cefepime as patients receiving this antibiotic comprised only 9.3% of the cohort. Within the CI cefepime group, only 3 of 11 patients achieved clinical cure (95%CI: -0.3 to 0.7; p=1.000). Similar findings were described in a prospective crossover trial performed by Jaruratanasirikul et al. [24], which demonstrated no statistically significant difference between target attainments of CI and II cefepime and no clear effect of CI cefepime on bactericidal activity. However, due to the small study population in both these studies, the results cannot be used to disregard the clinical utility of CI cefepime.

Table 2.
Summary of studies involving cefepime.
In contrast, the retrospective cohort study performed by Tran et al. [25] (Table 2), which also examined the clinical effect of EI piperacillintazobactam, cefepime and meropenem, was of a larger scale, with the cefepime group comprising the largest proportion of the cohort (59%). Two hundred-sixty-eight patients with Gram-negative bacteremia were included, and EI resulted in faster achievement of clinical stability, defervescence and WBC normalization. Furthermore, there were lower infection-related mortality and no recurrences in the EI group. Within the cefepime subgroup, these trends remained intact. There were no cases of treatment failure (p<0.001) and recurrence of bloodstream infections (p<0.001) amongst the 79 patients receiving EI cefepime. Hence, the results of this study were supportive of EI cefepime use in critically ill patients with Gram-negative bloodstream infections.
However, when treating infections due to ESBL-producing bacteria, CI cefepime may not result in successful outcomes. In a simulation study of 10,000 patients conducted by Reese et al. [26], 4 g of cefepime CI demonstrated a 77% MIC target attainment, which was not translated into clinical cure. A further clinical trial is required to challenge this finding and conclusively determine the role of CI cefepime in the treatment of these infections.
This contrasts, however, with what has been observed for Pseudomonas aeruginosa infections. These infections pose a significant threat to antibiotic stewardship efforts, due to rapid emergence of resistant strains with high MICs. Cefepime is routinely used for these hard-to-treat infections and EI cefepime was indeed found to further optimize clinical outcomes in patients with respiratory infections and bacteremia caused by P. aeruginosa. A retrospective quasiexperimental study conducted in Ohio by Bauer et al. [27] (Table 2), included 592 patients of whom 202 received EI cefepime. Compared to the 20% mortality rate recorded in the II group, mortality in the EI group was reduced to 3% (p=0.03). EI implementation also resulted in a 3-day decrease in median ICU stay (p=0.04), a 4-day decrease in duration of mechanical ventilation (p=0.42), and a substantial decrease in median hospital cost (p=0.13). Hence, EI cefepime administration for Pseudomonas infections might be associated with considerable therapeutic and economic advantages.
Febrile neutropenia
There is a lack of large-scale trials examining the use of EI cefepime in patients with febrile neutropenia. A pilot study conducted by Wrenn et al. in adult patients with febrile neutropenia showed clinical feasibility, suggesting that EI/CI cefepime might have a role in this patient population [28]. In this small scale prospective randomized observational study, cefepime was administered as empiric treatment in the setting of febrile neutropenia. The first dose was administered as a standard thirty-minute infusion followed by randomization into two groups, with one group receiving thirty-minute infusions while the other group receiving EI of four-hour duration. A shorter median time to defervescence was recorded in the EI group, not reaching statistical significance. Although it was concluded that EI cefepime was clinically feasible, the study had several limitations e.g., its small sample size and the inclusion of pathogens resistant to cefepime. Hence, larger scale clinical trials are required to determine if EI/CI cefepime are indeed useful as empirical therapy in patients with febrile neutropenia [28].
Pediatric patients
The role of EI/CI cefepime in pediatric populations remains unclear due to a lack of clinical, comparative trials. However, simulation studies and pharmacokinetic analyses have shown that EI/CI of cefepime might improve clinical outcomes and encourage further investigation. A Monte Carlo simulation study conducted by Courter et al. showed that when cefepime was administered for a total duration of 30 min, the percentage target attainment for an MIC of 8 mg/L was 79% [29]. When the infusion time was increased to 3 h, this increased dramatically to 100%. This is in line with findings from another Monte Carlo simulation study performed by Shoju et al., in which a significant increase in target attainment was observed when the infusion time was increased to three hours [30]. This shows that, in theory, CI cefepime is pharmacokinetically beneficial and can potentially improve clinical outcomes in pediatric populations.
Furthermore, EI cefepime is clinically feasible within a pediatric setting. A 2013 prospective descriptive study performed by Nichols et al. [31] in a tertiary pediatric hospital, demonstrated that the main challenges were obtaining and maintaining IV access. Despite these challenges regarding EI/CI, changes in treatment were rarely required and 93% of patients receiving EI were able to successfully complete their course. An improvement in clinical outcomes in the EI group was also reported, however data were lacking.
There is a need for well-defined clinical trials which would examine the effect of CI cefepime on clinical outcomes and assess its safety in the pediatric population in general and in specific subpopulations such as neonates, in particular. Although a retrospective chart analysis conducted by Zembles et al. did show a positive effect of EI on clinical outcomes such as total antibiotic therapy duration, this study included 3 antibiotics, namely piperacillin-tazobactam, cefepime, and meropenem [32]. The data provided were not specific for cefepime and no conclusions about the clinical utility of CI cefepime in pediatric populations can be inferred. Although there is an abundance of pharmacokinetic evidence supporting the efficacy of EI/CI cefepime, this does not necessarily result in favorable clinical outcomes and there is a need for large-scale clinical trials.
Meropenem
Critically ill patients
Meropenem, one of the antibiotics with the broadest spectrum in our arsenal, is usually reserved for serious infections. Like other betalactam antibiotics, it also shows time dependent action [33].
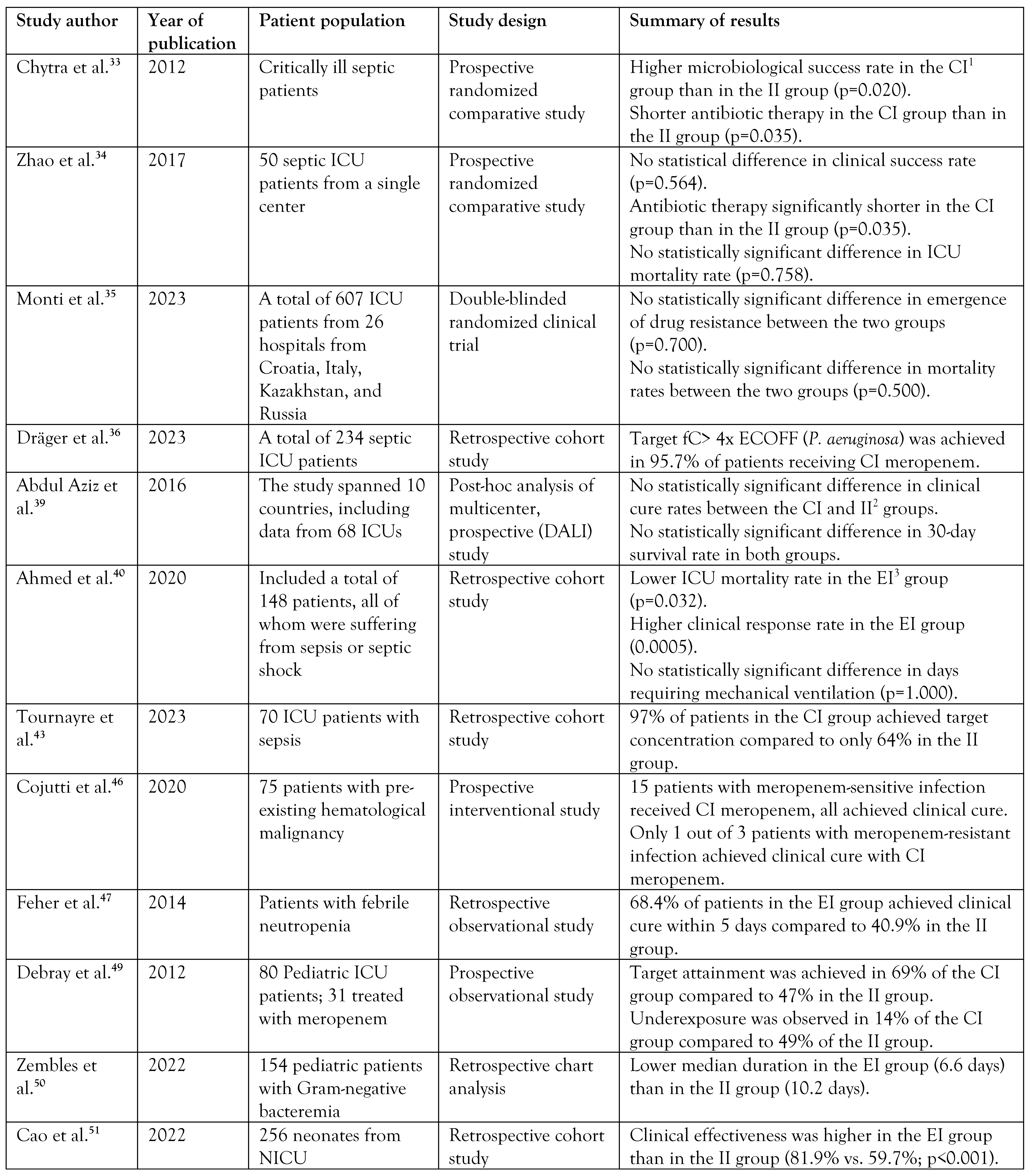
In a prospective randomized comparative study conducted by Chytra et al. [33] (Table 3) including 240 critically ill patients admitted to the ICU, CI of meropenem resulted in greater bacterial eradication (p=0.020), reduced ICU length of stay (p=0.042) and need for mechanical ventilation (p=0.058). Although the rates of clinical success did not differ significantly between the CI and II groups, CI also decreased the duration and total dose of meropenem therapy [33]. Similar results were reported in yet another prospective comparative study performed by Zhao et al. [34] (Table 3) involving 50 patients with severe sepsis and septic shock. The study reported a decreased duration of therapy in the CI group (p=0.035) but failed to demonstrate superiority over II in terms of overall clinical success (p=0.564). In both studies, the lack of improved overall clinical benefit might have been theoretically attributed to the low MIC of the infections included. Furthermore, clinical benefits were not demonstrated in the MERCY trial conducted by Monti et al. [35] (Table 3). This randomized controlled trial included 607 critically ill patients, a subset of whom were administered CI meropenem. At day 28, there was no benefit observed in preventing the emergence of resistance (p=0.700) or reduction of mortality (p=0.500). Although this was a largescale trial and hence, holds more merit, detailed data regarding the cure of the initial infection and therapeutic drug monitoring (TDM) were not provided [35].

Table 3.
Summary of studies involving meropenem.
The superiority of CI of meropenem is more apparent in the treatment of infections with higher MICs and requiring higher concentration targets. This was demonstrated in a single center retrospective cohort study conducted by Dräger et al. [36] (Table 3) where CI of meropenem was found to be significantly more effective than II. The study involved 234 patients and 95.7% of the patients receiving CI of meropenem met the target concentration and were able to maintain it throughout the course of treatment. Unlike II, CI ensures that once target concentrations are reached, trough concentrations are maintained for a longer duration, hence enhancing the drug’s effectivity against more resistant pathogens. Interestingly, it was also hypothesized by two other studies that EI/CI might also enable the use of meropenem to treat infections which are deemed less susceptible based on the antibiogram or even those involving resistant pathogens such as carbapenemase-producing Enterobacterales as CI enabled attainment of very high PTAs in both these studies; however, further trials are required to confirm this [37,38].
Another clinical scenario where EI/CI of meropenem might display an advantage over II is the treatment of pneumonia. Abdul Aziz et al. [39] (Table 3) demonstrated in their post hoc analysis of the DALI trial that EI and CI fared better than II overall and in multiple clinical outcomes, particularly PTA and clinical cure rate, while a significant survival benefit was seen in a subset of patients with primary respiratory infections. Ahmed et al. (Table 3) also found EI to be superior in this subset of patients [40]. Attaining high drug concentrations in the epithelial lining fluid was the most crucial factor in determining good clinical outcomes in these patients. This is enabled by greater PTA in the plasma, which is potentiated by EI/CI of meropenem. Hence, prolonged infusions of meropenem might confer a significant clinical advantage over II in critically ill patients with primary respiratory tract infections due to greater tissue drug penetration. More trials are required to determine if this benefit can also be seen in infections involving other sites.
In addition to its time-dependent nature and similarly to other beta-lactams, meropenem exhibits lower protein binding capacity and hence higher concentrations of unbound active drug. This translates into improved clinical outcomes for CI meropenem. As shown by Dräger et al. [41], 95.7% of patients receiving CI meropenem achieved target unbound plasma drug concentrations, while only 58.3% of patients in the piperacillin-tazobactam group achieved the same. Furthermore, in the study performed by Wong et al. [42], it was shown that in comparison to other beta-lactams included in this analysis, meropenem reached higher unbound concentrations and there was no change in treatment duration required. Eventually, the positive clinical outcome also allowed for deescalation of antibiotic therapy, and there was no increase in the incidence of adverse events.
This increase in unbound concentration supports the use of meropenem CI from a pharmacokinetic standpoint, however it is imperative to consider whether TDM within the clinical setting would be required to avoid overdosing and toxicity. This is supported by the single-center retrospective observational study conducted by Tournayre et al. [43], where patients receiving CI were at a higher risk of overdosing. This study included 70 patients with sepsis who were admitted to the ICU. Although 97% of patients in the CI group achieved the target concentration, which was over 5 times the MIC, these patients were also at a greater risk of overdosing compared to the II group. Nine out of 11 patients whose meropenem concentration levels were above the toxicity threshold were in the CI group. TDM is also advocated in the study by Dräger et al. [41] since it was found that a decrease in dosing was required in 50% of recruited patients. Hence, CI of meropenem might indeed have a clinical advantage over II, however TDM should be considered.
The benefit of EI/CI meropenem is further supported in two systematic reviews which corroborate the clinical benefits of this treatment strategy in critically ill patients. In the metaanalysis conducted by Yu et al., it was shown that meropenem EIs and CIs had statistically significant advantages over II in mortality, clinical cure rates and microbiological eradication [44]. These findings were replicated in the systematic review by Chen et al. [45], adding to the growing body of evidence in favor of prolonged meropenem infusions in critically ill patients.
Febrile neutropenia
CI meropenem has shown promising results in studies conducted so far involving febrile neutropenic patients. In a single-center prospective interventional study conducted by Cojutti et al., target attainment and clinical cure rates were assessed in 75 onco-hematological patients presenting with an acute episode of febrile neutropenia (FN) and treated with meropenem [46]. Of these, 15 were administered meropenem by CI. In the CI group, all patients whose infections were meropenem-susceptible achieved clinical cure. Conversely, in the 3 patients with meropenem-resistant pathogens, only 1 achieved clinical cure with CI meropenem. Hence, it was concluded that in patients with meropenem-susceptible infections, CI meropenem when combined with TDM was highly successful in reaching pharmacokinetic targets, which also translated into improved clinical outcomes.
Another important finding uncovered in this study which makes CI meropenem highly favorable in patients with FN was that among all the patients who were re-hospitalized within three months, there were no rectal swabs positive for carbapenem-resistant Enterobacterales (CRE). As CRE colonization is associated with carbapenem treatment, it was suggested that CI meropenem might have a role in preventing emergence of resistance, an important consideration in this patient population. However, more studies are required to confirm this and to determine if CI administration is independently associated with prevention of resistance [46].
A retrospective observational study by Feher et al. [47] also provided insight into how a 4-h EI of meropenem could fare for patients with FN. Seventy-six patients with FN were administered EI meropenem at 1g q8h for a total duration of 4 h and were assessed for multiple clinical outcomes, e.g., clinical cure, need for addition of other antibiotics, time to defervescence, and decrease in CRP. Compared to 40.9% of patients in the II group, 68.4% of patients in the EI group achieved clinical cure within 5 days. The remaining clinical outcomes had more favorable results in the EI group, apart from length of hospital stay and mortality. Hence, in this study, it was found that EI meropenem administered over 4 h was associated with faster clinical response and decreases in CRP levels. Although no serious adverse effects were reported, more clinical trials are required to assess the safety of a 4-h EI meropenem and conclusively determine the ideal dose and duration for CI meropenem administration.
Pediatric patients
As discussed above regarding beta-lactam pharmacokinetics, infants and children exhibit increased renal clearance compared to adults whereas neonates have decreased clearance due to immaturity of the renal system. Although this would imply that children could benefit highly from EI/CI of antibiotics, this is not apparent clinically. The application of pharmacokinetic data is not straightforward in pediatric populations and there is little clinical evidence supporting the use of EI/CIs in infants and children [48].
In a single-center prospective observational study by Debray et al. [49], 31 out of 80 pediatric intensive care unit patients were administered meropenem with a target concentration 4 times that of the ECOFF or MIC for each pathogen. Underdosing was more frequent in the II group. Conversely, optimal exposure was more likely to be attained in the CI group. However, these patients were at higher risk of overexposure. Although no serious adverse events were reported in the CI group, it was concluded that CI should be considered on a ‘case-to-case’ basis along with TDM, particularly in those with more serious infections and with higher MICs.
Similar findings were found in other studies. A large-scale retrospective chart analysis by Zembles et al. [50] demonstrated that CI was associated with a higher percentage of target attainment, however this did not confer any benefit over II in mortality, readmission rates or hospital stay. Improved clinical outcome was only observed in one subset in this study, namely bone marrow transplant recipients, whose hospital readmission rates were significantly decreased.
An extension of this study was published in 2022 by Zembles et al., focusing on patients with Gram-negative infections. One-hundred and twenty-four children with confirmed bacteremia were included and the study period was extended to a total of 8 years. The results were consistent with the original trial, whereby EI did not display a significant advantage over II in terms of mortality, readmission rates or length of hospital stay. However, EI did shorten the duration of therapy by an average of 4 days compared to the II group. This can be beneficial clinically as it decreases the propensity for adverse effects as well as emergence of resistance [50].
Meropenem is widely used in the treatment of neonatal sepsis. Although based on decreased renal clearance in neonates, one might expect that EI in neonates would not retain an advantage over II, clinical data suggest otherwise. A historical cohort study by Cao et al. [51] published in 2022 which analyzed records from 256 neonates in Peking University neonatal intensive care unit, found that meropenem EI of 2-3 h improved clinical outcomes. The 3-day clinical effectiveness rate in the EI group was 81.9% compared to 59.7% in the II group (p<0.001). Day 3 microbial clearance in the CI group was 94.5%, in comparison with 85.7% in the II group (p=0.015). Furthermore, there were no serious adverse events reported in the II group and no statistically significant difference was found in the levels of CRP, WBC, BUN, creatinine and AST between the groups. It was concluded that meropenem EI is clinically beneficial and, if implemented, it can significantly improve clinical outcomes, without increasing the risk of toxicity. Another study finding relates to neonates with a very low birth weight. Although contrary to pharmacokinetic predictions, this subgroup benefited significantly from meropenem EI. The day 3 effectiveness in this subgroup was 75.6% in the EI and 56.6% in the II group (p=0.007). However, day 3 microbial clearance did not differ significantly between the two groups (p=0.041). Irrespectively, in neonates, meropenem EI seems to be a promising strategy to increase pharmacokinetic and pharmacodynamic target attainment. Since prolonging infusions is safer than increasing the frequency or the dosage in this group to reach optimum concentrations, meropenem EI can be of clinical use when treating MDR pathogens.
The percentage target attainment of meropenem can be further increased in neonates by using a loading dose prior to EI/CI administration. In a simulation cohort study which included 30,000 neonates, it was shown that the addition of a loading dose prior to EI and CI increased target attainment by 51.9%. This translated into a 28% increase in clinical improvement, a 25.2% increase in microbial clearance, a 17% decrease in morality and 17.5% decrease in the incidence of acute kidney injury. Hence, meropenem EI/CI might potentially improve clinical outcomes in neonatal sepsis if implemented [52].
Discussion
For piperacillin-tazobactam, EI and CI can result in improved clinical outcomes, including a reduction in the mortality rate, especially when the pathogen is identified and adequate pharmacodynamic targets are attained. Subset analyses have also revealed that patients with specific infections, such as urinary tract infections and intra-abdominal infections, benefit more from EI. However, more studies in these groups of patients are required to confirm this. CI piperacillin-tazobactam is found to be particularly beneficial in the initial treatment of critically ill patients. In this phase, it results in a greater reduction of inflammatory markers such as CRP compared to II. CI of piperacillin-tazobactam has also proved beneficial in patients with febrile neutropenia, especially those suffering from bloodstream infections and pneumonia. In the pediatric population, EI and CI of piperacillintazobactam show promise in the treatment of critically ill children, however, more studies are required to ascertain their clinical value.
The evidence has been more conflicting for cefepime. Although cefepime is stable as evidenced by the high-performance liquid chromatography study performed by Sprauten et al. [23], the International Consensus [53] states that there isn’t enough evidence to ascertain the stability of cefepime in comparison to other betalactam antibiotics. Studies which examined the effect of CI cefepime have failed to consistently demonstrate its clinical utility, especially in infections caused by ESBL producing bacteria. However, implementation of EI cefepime in the treatment of Gram-negative bloodstream infections and Pseudomonas aeruginosa infection does result in improved clinical outcomes, such as lower mortality, decreased ICU stay, and duration of mechanical ventilation. In patients with febrile neutropenia and in children, there are promising pilot studies which prompt the need for large scale clinical trials to determine any possible advantages of using EI/CI cefepime.
According to the International Consensus recommendations [53] for the use of prolonged infusion of beta-lactam antibiotics, required targets for bactericidal activity were lowest with carbapenems, followed by penicillins, and were higher for cephalosporins. Of all three antibiotics included in this study, the results for EI/CI meropenem have been the most encouraging and its clinical use in critically ill patients has also been supported by systematic reviews. This is more apparent in infections associated with higher MICs and those where higher concentrations are required for tissue penetration, such as pneumonia, where meropenem confers a greater survival benefit and is also successful in reducing the emergence of some resistant strains of bacteria. Similarly, for patients with febrile neutropenia, CI meropenem is successful at achieving clinical cure in patients with meropenem-susceptible infections and is also able to reduce the incidence of resistance emergence. This might be related to the lower protein binding capacity of meropenem; however, it is for this reason that TDM should be considered. According to Grupper et al. [55], TDM allows several limitations posed by altered pharmacokinetics in critically ill patients, such as decreased/augmented renal clearance and third space sequestration, to be overcome and this is imperative for treatment optimization. This is reflected in the International Consensus recommendation [53], which suggests that TDM should be considered on a case-by-case basis for prolonged infusions of all beta-lactam antibiotics. The effect of meropenem’s pharmacokinetics on clinical outcomes is, however, not as straightforward in pediatric patients and should be considered on a case-by-case basis. In contrast, meropenem was far more useful in the treatment of neonatal sepsis, especially when caused by MDR pathogens. The addition of a loading dose before CI was also found to increase clinical efficiency, without increasing the risk of adverse effects.
Conclusions
Collectively, the findings of this review are in accordance with recommendations of studies [53,54,55,56] which stated that EI/CI do confer a benefit in critically ill patients, particularly those with Gram-negative infections, by increasing clinical cure and reducing mortality. However, there is not enough evidence to support their use in other patient groups, such as pediatric patients and those with febrile neutropenia. Lastly, more studies are required to determine the optimum target concentrations and the safety index of prolonged infusions.
Funding
All authors—none to declare.
Conflicts of Interest
None to declare.
References
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistenceImplications for human health and treatment perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Brunetti, L.; Poustchi, S.; Cunningham, D.; et al. Clinical and economic impact of empirical extended-infusion piperacillin-tazobactam in a community medical center. Ann. Pharmacother. 2015, 49, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.N.; Mynatt, R.P.; Kaye, K.S.; Zhao, J.J.; Pogue, J.M. Clinical outcomes with extended versus intermittent infusion of anti-pseudomonal beta-lactams in patients with gram-negative bacteremia. Open Forum Infect Dis. 2023, 10, ofad170. [Google Scholar] [CrossRef]
- Keng, M.K.; Sekeres, M.A. Febrile neutropenia in hematologic malignancies. Curr. Hematol. Malig. Rep. 2013, 8, 370–378. [Google Scholar] [CrossRef]
- Cotner, S.E.; Rutter, W.C.; Burgess, D.R.; Wallace, K.L.; Martin, C.A.; Burgess, D.S. Influence of β-lactam infusion strategy on acute kidney injury. Antimicrob. Agents Chemother. 2017, 61, e00871–17. [Google Scholar] [CrossRef] [PubMed]
- Costenaro, P.; Minotti, C.; Cuppini, E.; Barbieri, E.; Giaquinto, C.; Donà, D. Optimizing antibiotic treatment strategies for neonates and children: Does implementing extended or prolonged infusion provide any advantage? Antibiotics 2020, 9, 329. [Google Scholar] [CrossRef] [PubMed]
- Debray, A.; Callot, D.; Hirt, D.; et al. Beta-lactam exposure and safety in intermittent or continuous infusion in critically ill children: An observational monocenter study. Eur. J. Pediatr. 2023, 182, 965–973. [Google Scholar] [CrossRef]
- Dulhunty, J.M.; Roberts, J.A.; Davis, J.S.; et al. A multicenter randomized trial of continuous versus intermittent βlactam infusion in severe sepsis. Am. J. Respir. Crit. Care Med. 2015, 192, 1298–1305. [Google Scholar] [CrossRef]
- Imburgia, T.A.; Kussin, M.L. A Review of extended and continuous infusion beta-lactams in pediatric patients. J. Pediatr. Pharmacol. Ther. 2022, 27, 214–227. [Google Scholar] [CrossRef]
- Fan, S.Y.; Shum, H.P.; Cheng, W.Y.; Chan, Y.H.; Leung, S.M.; Yan, W.W. Clinical outcomes of extended versus intermittent infusion of piperacillin/tazobactam in critically ill patients: A prospective clinical trial. Pharmacotherapy 2017, 37, 109–119. [Google Scholar] [CrossRef]
- Rafati, M.R.; Rouini, M.R.; Mojtahedzadeh, M.; et al. Clinical efficacy of continuous infusion of piperacillin compared with intermittent dosing in septic critically ill patients. Int. J. Antimicrob. Agents. 2006, 28, 122–127. [Google Scholar] [CrossRef]
- Gonçalves-Pereira, J.; Oliveira, B.S.; Janeiro, S.; et al. Continuous infusion of piperacillin/tazobactam in septic critically ill patients--a multicenter propensity matched analysis. PLoS ONE 2012, 7, e49845. [Google Scholar] [CrossRef]
- Hyun, D.G.; Seo, J.; Lee, S.Y.; et al. Continuous piperacillintazobactam infusion improves clinical outcomes in critically ill patients with sepsis: A retrospective, singlecentre study. Antibiotics 2022, 11, 1508. [Google Scholar] [CrossRef] [PubMed]
- Cutro, S.R.; Holzman, R.; Dubrovskaya, Y.; et al. Extendedinfusion versus standard-infusion piperacillin-tazobactam for sepsis syndromes at a tertiary medical center. Antimicrob. Agents Chemother. 2014, 58, 4470–4475. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Cui, X.; Ma, Z.; Liu, L. Evaluation outcomes associated with alternative dosing strategies for piperacillin/tazobactam: A systematic review and metaanalysis. J. Pharm. Pharm. Sci. 2016, 19, 274–289. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Sulaiman, H.; Mat-Nor, M.B.; et al. Betalactam infusion in severe sepsis (BLISS): A prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med. 2016, 42, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Jaruratanasirikul, S.; Limapichat, T.; Jullangkoon, M.; Aeinlang, N.; Ingviya, N.; Wongpoowarak, W. Pharmacodynamics of meropenem in critically ill patients with febrile neutropenia and bacteraemia. Int. J. Antimicrob. Agents. 2011, 38, 231–236. [Google Scholar] [CrossRef]
- Ram, R.; Halavy, Y.; Amit, O.; et al. Extended vs. bolus infusion of broad-spectrum β- lactams for febrile neutropenia: An unblinded, randomized trial. Clin. Infect. Dis. 2018, 67, 1153–1160. [Google Scholar] [CrossRef]
- Knoderer, C.A.; Karmire, L.C.; Andricopulos, K.L.; Nichols, K.R. Extended infusion of piperacillin/tazobactam in children. J. Pediatr. Pharmacol. Ther. 2017, 22, 212–217. [Google Scholar] [CrossRef]
- Kebudi, R.; Kizilocak, H. Febrile neutropenia in children with cancer: Approach to diagnosis and treatment. Curr. Pediatr. Rev. 2018, 14, 204–209. [Google Scholar] [CrossRef]
- Solórzano-Santos, F.; Quezada-Herrera, A.; FuentesPacheco, Y.; et al. Piperacillin/tazobactam in continuous infusion versus intermittent infusion in children with febrile neutropenia. Rev. Invest. Clin. 2019, 71, 283–290. [Google Scholar] [CrossRef]
- Sprauten, P.F.; Beringer, P.M.; Louie, S.G.; Synold, T.W.; Gill, M.A. Stability and antibacterial activity of cefepime during continuous infusion. Antimicrob. Agents Chemother. 2003, 47, 1991–1994. [Google Scholar] [CrossRef]
- Jaruratanasirikul, S.; Limapichat, T.; Jullangkoon, M.; Aeinlang, N.; Ingviya, N.; Wongpoowarak, W. Pharmacodynamics of meropenem in critically ill patients with febrile neutropenia and bacteraemia. Int. J. Antimicrob. Agents. 2011, 38, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.N.; Mynatt, R.P.; Kaye, K.S.; Zhao, J.J.; Pogue, J.M. Clinical outcomes with extended versus intermittent infusion of anti-pseudomonal beta-lactams in patients with Gram-negative bacteremia. Open Forum Infect Dis. 2023, 10, ofad170. [Google Scholar] [CrossRef] [PubMed]
- Imburgia, T.A.; Kussin, M.L. A Review of extended and continuous infusion beta-lactams in pediatric patients. J. Pediatr. Pharmacol. Ther. 2022, 27, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.A.; West, J.E.; O’Brien, J.M.; Goff, D.A. Extendedinfusion cefepime reduces mortality in patients with Pseudomonas aeruginosa infections. Antimicrob. Agents Chemother. 2013, 57, 2907–2912. [Google Scholar] [CrossRef]
- Wrenn, R.H.; Cluck, D.; Kennedy, L.; Ohl, C.; Williamson, J.C. Extended infusion compared to standard infusion cefepime as empiric treatment of febrile neutropenia. J. Oncol. Pharm. Pract. 2018, 24, 170–175. [Google Scholar] [CrossRef]
- Courter, J.D.; Kuti, J.L.; Girotto, J.E.; Nicolau, D.P. Optimizing bactericidal exposure for beta-lactams using prolonged and continuous infusions in the pediatric population. Pediatr. Blood Cancer. 2009, 53, 379–385. [Google Scholar] [CrossRef]
- Shoji, K.; Bradley, J.S.; Reed, M.D.; van den Anker, J.N.; Domonoske, C.; Capparelli, E.V. Population pharmacokinetic assessment and pharmacodynamic implications of pediatric cefepime dosing for susceptibledose-dependent organisms. Antimicrob. Agents Chemother. 2016, 60, 2150–2156. [Google Scholar] [CrossRef]
- Nichols, K.R.; Karmire, L.C.; Cox, E.G.; Kays, M.B.; Knoderer, C.A. Implementing extended-infusion cefepime as standard of care in a children’s hospital: A prospective descriptive study. Ann. Pharmacother. 2015, 49, 419–426. [Google Scholar] [CrossRef]
- Zembles, T.N.; Schortemeyer, R.; Kuhn, E.M.; Bushee, G.; Thompson, N.E.; Mitchell, M.L. Extended infusion of betalactams is associated with improved outcomes in pediatric patients. J. Pediatr. Pharmacol. Ther. 2021, 26, 187–193. [Google Scholar] [CrossRef]
- Chytra, I.; Stepan, M.; Benes, J.; et al. Clinical and microbiological efficacy of continuous versus intermittent application of meropenem in critically ill patients: A randomized open-label controlled trial. Crit Care. 2012, 6, R113. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Gu, J.; Lyu, J.; et al. Pharmacokinetic and pharmacodynamic efficacies of continuous versus intermittent administration of meropenem in patients with severe sepsis and septic shock: A prospective randomized pilot study. Chin. Med. J. 2017, 130, 1139–1145. [Google Scholar] [CrossRef]
- Monti, G.; Bradic, N.; Marzaroli, M.; et al. Continuous vs. intermittent meropenem administration in critically ill patients with sepsis: The MERCY randomized clinical trial. JAMA 2023, 330, 141–151. [Google Scholar] [CrossRef]
- Dräger, S.; von Rotz, M.; Labhardt, N.D.; et al. Early target attainment with continuous infusion meropenem and piperacillin/tazobactam and utilization of therapeutic drug monitoring in critically ill patients: A retrospective cohort study from 2017 to 2020. Open Forum Infect Dis. 2023, 10, ofad143. [Google Scholar] [CrossRef]
- Tournayre, S.; Mathieu, O.; Villiet, M.; et al. Factors associated with meropenem pharmacokinetic/pharmacodynamic target attainment in septic critically ill patients treated with extended intermittent infusion or continuous infusion. Int. J. Antimicrob. Agents. 2023, 62, 106868. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.P.; Cojutti, P.; Pea, F. Pharmacokinetics and pharmacodynamics of continuous infusion meropenem in overweight, obese, and morbidly obese patients with stable and unstable kidney function: A step toward dose optimization for the treatment of severe gram-negative bacterial infections. Clin. Pharmacokinet. 2015, 54, 93341. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Lipman, J.; Akova, M.; et al. Is prolonged infusion of piperacillin/tazobactam and meropenem in critically ill patients associated with improved pharmacokinetic/pharmacodynamic and patient outcomes? An observation from the Defining Antibiotic Levels in Intensive care unit patients (DALI) cohort. J. Antimicrob. Chemother. 2016, 71, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Jen, S.P.; Altshuler, D.; Papadopoulos, J.; Pham, V.P.; Dubrovskaya, Y. Evaluation of meropenem extended versus intermittent infusion dosing protocol in critically ill patients. J. Intensive Care Med. 2020, 35, 763–771. [Google Scholar] [CrossRef]
- Dräger, S.; von Rotz, M.; Labhardt, N.D.; et al. Early target attainment with continuous infusion meropenem and piperacillin/tazobactam and utilization of therapeutic drug monitoring in critically ill patients: A retrospective cohort study from 2017 to 2020. Open Forum Infect Dis. 2023, 10, ofad143. [Google Scholar] [CrossRef]
- Wong, G.; Taccone, F.; Villois, P.; et al. β-lactam pharmacodynamics in Gram-negative bloodstream infections in the critically ill. J. Antimicrob. Chemother. 2020, 75, 429–433. [Google Scholar] [CrossRef]
- Tournayre, S.; Mathieu, O.; Villiet, M.; et al. Factors associated with meropenem pharmacokinetic/pharmacodynamic target attainment in septic critically ill patients treated with extended intermittent infusion or continuous infusion. Int. J. Antimicrob. Agents. 2023, 62, 106868. [Google Scholar] [CrossRef]
- Yu, Z.; Pang, X.; Wu, X.; Shan, C.; Jiang, S. Clinical outcomes of prolonged infusion (extended infusion or continuous infusion) versus intermittent bolus of meropenem in severe infection: A meta-analysis. PLoS ONE 2018, 13, e0201667. [Google Scholar] [CrossRef]
- Chen, P.; Chen, F.; Lei, J.; Zhou, B. Clinical outcomes of continuous vs. intermittent meropenem infusion for the treatment of sepsis: A systematic review and metaanalysis. Adv. Clin. Exp. Med. 2020, 29, 993–1000. [Google Scholar] [CrossRef]
- Cojutti, P.G.; Lazzarotto, D.; Candoni, A.; et al. Real-time TDM-based optimization of continuous-infusion meropenem for improving treatment outcome of febrile neutropenia in oncohaematological patients: Results from a prospective, monocentric, interventional study. J. Antimicrob. Chemother. 2020, 75, 3029–3037. [Google Scholar] [CrossRef]
- Fehér, C.; Rovira, M.; Soriano, A.; et al. Effect of meropenem administration in extended infusion on the clinical outcome of febrile neutropenia: A retrospective observational study. J. Antimicrob. Chemother. 2014, 69, 2556–2562. [Google Scholar] [CrossRef]
- Gonçalves-Pereira, J.; Oliveira, B.S.; Janeiro, S.; et al. Continuous infusion of piperacillin/tazobactam in septic critically ill patients--a multicenter propensity matched analysis. PLoS ONE 2012, 7, e49845. [Google Scholar] [CrossRef]
- Debray, A.; Callot, D.; Hirt, D.; et al. Beta-lactam exposure and safety in intermittent or continuous infusion in critically ill children: An observational monocenter study. Eur. J. Pediatr. 2023, 182, 965–973. [Google Scholar] [CrossRef]
- Zembles, T.N.; Kuhn, E.M.; Thompson, N.E.; Mitchell, M.L. Extended infusion β-lactams for the treatment of gramnegative bacteremia in children. J. Pediatr. Pharmacol. Ther. 2022, 27, 677–681. [Google Scholar] [CrossRef]
- Cao, G.; Zhou, P.; Zhang, H.; Sun, B.; Tong, X.; Xing, Y. Extended infusion of meropenem in neonatal sepsis: A historical cohort study. Antibiotics 2022, 11, 341. [Google Scholar] [CrossRef]
- Leegwater, E.; Wewerinke, L.; de Grauw, A.M.; van Veen, M.; Storm, B.N.; Kruizinga, M.D. Optimization of β-lactam dosing regimens in neonatal infections: Continuous and extended administration versus intermittent administration. Clin. Pharmacokinet. 2023, 62, 715–724. [Google Scholar] [CrossRef]
- Hong, L.T.; Downes, K.J.; FakhriRavari, A.; et al. International consensus recommendations for the use of prolonged-infusion beta-lactam antibiotics: Endorsed by the American College of Clinical Pharmacy, British Society for Antimicrobial Chemotherapy, Cystic Fibrosis Foundation, European Society of Clinical Microbiology and Infectious Diseases, Infectious Diseases Society of America, Society of Critical Care Medicine, and Society of Infectious Diseases Pharmacists. Pharmacotherapy 2023, 43, 740–777. [Google Scholar] [CrossRef]
- Chen, M.; Buurma, V.; Shah, M.; Fahim, G. Evaluation of studies on extended versus standard infusion of betalactam antibiotics. Am. J. Health Syst. Pharm. 2019, 76, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Grupper, M.; Kuti, J.L.; Nicolau, D.P. Continuous and prolonged intravenous β-lactam dosing: Implications for the clinical laboratory. Clin. Microbiol. Rev. 2016, 29, 75972. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Dulhunty, J.M.; Bellomo, R.; Lipman, J.; Roberts, J.A. Continuous beta-lactam infusion in critically ill patients: The clinical evidence. Ann. Intensive Care. 2012, 2, 37. [Google Scholar] [CrossRef] [PubMed]
© GERMS 2025.