Abstract
Introduction: Burkholderia pseudomallei is the bacterium that causes melioidosis. It is mostly a tropical disease, and particularly common in Southeast Asia and northern Australia. The intensive intravenous phase and the oral prolonged eradication phase are the two phases of melioidosis treatment. The current recommended treatment for melioidosis eradication is oral co-trimoxazole (TMP/SMX). Case report: Two patients were diagnosed with B. pseudomallei bacteremia without a focus and were treated with oral TMP/SMX with folic acid during the eradication phase. Both presented with neutropenic sepsis with pneumonia and pyelonephritis at days 48 and 45 following TMP/SMX 320/1600 mg q12h (4 tablets) and in both of them, the folic acid compliance was poor. One patient died and the other survived following intensive treatment for neutropenia. At the presentation following neutropenic sepsis among both patients, the red blood cells and platelets were within normal limits. Both patients were on a high dose of TMP/SMX, as both were within 40–60 kg of body weight the ideal TMP/SMX dose would be 240/1200 mg q12h (3 tablets). Pancytopenia caused by TMP/SMX can frequently develop gradually over time. Alternately, it can develop rapidly and swiftly escalate to fulminant sepsis, disseminated intravascular coagulation, and fast hemolysis. However, the development of isolated neutropenia is rarely described in the literature. Conclusions: Prolonged use of TMP/SMX is important to eradicate B. pseudomallei and always the possibility of rare adverse effects has to be considered. Always weight-based TMP-SMX dosing has to be encouraged with need to ensure the compliance of folic acid. During the eradication phase, continuous monitoring of blood cell lines with weekly full blood count would be essential to identify neutropenia in advance.
Introduction
Whitmore's disease, also known as melioidosis, is an infectious disease that can affect both humans and animals.[1] Burkholderia pseudomallei is the bacterium that causes melioidosis. It is mostly a tropical disease, and it is particularly common in Southeast Asia and northern Australia.[2]
Melioidosis-causing bacteria can be found in contaminated soil and water. Animals and people can contract it by direct contact with a polluted source through ingestion, inhalation, and inoculation.[3] Melioidosis can be deadly if left untreated and some areas in Sri Lanka are currently considered as endemic.[2,3]
Melioidosis requires prolonged antimicrobial therapy and when partially treated or following emergence of resistance to current medications, it can lead to development of recurrences. However, primary causes of the recurrence are believed to be B. pseudomallei's capacity to produce glycocalyx and survive in phagocytes. Long-term oral eradication therapy is the key determinant in lowering the likelihood of recurrences and it is essential for managing the infection.[1,2,3]
The current recommended treatment for melioidosis eradication is oral cotrimoxazole (trimethoprim/sulfamethoxazole, TMP/SMX), while doxycycline or amoxicillin-clavulanate may also be used for at least 12 to 20 weeks. After 1-year follow-up, 6% of patients experienced culture-confirmed recurrences despite TMP/SMX treatment.[4] Once an in vitro investigation showed that doxycycline counteracted the antibacterial activity of TMP/SMX against B. pseudomallei, the effectiveness of TMP/SMX plus doxycycline was questioned.[1,2,3,4,5] Also, following adverse drug reactions 19% of the patients were switched to different treatment regimes. Several less toxic, alternate eradication regimens, such as amoxicillin-clavulanate, quinolone, and a combination of azithromycin and ciprofloxacin, have been tested, however, poor adherence to treatment is linked to the recurrence of melioidosis.[4,6] All of these regimens, meanwhile, were linked to recurrence rates above 15%; hence, another more efficient regimen is required.[6,7,8]
TMP/SMX alone has been successfully used in the Philippines for the treatment of acute melioidosis and is used for eradication therapy in Thailand, Australia and Sri Lanka.[1,3,7] When we use TMP/SMX, a folic acid supplement is always encouraged to counter bone marrow suppression in humans.[8]
Herein, we report two patients with melioidosis who were on TMP/SMX maintenance therapy and developed neutropenic sepsis.
Case report no. 1
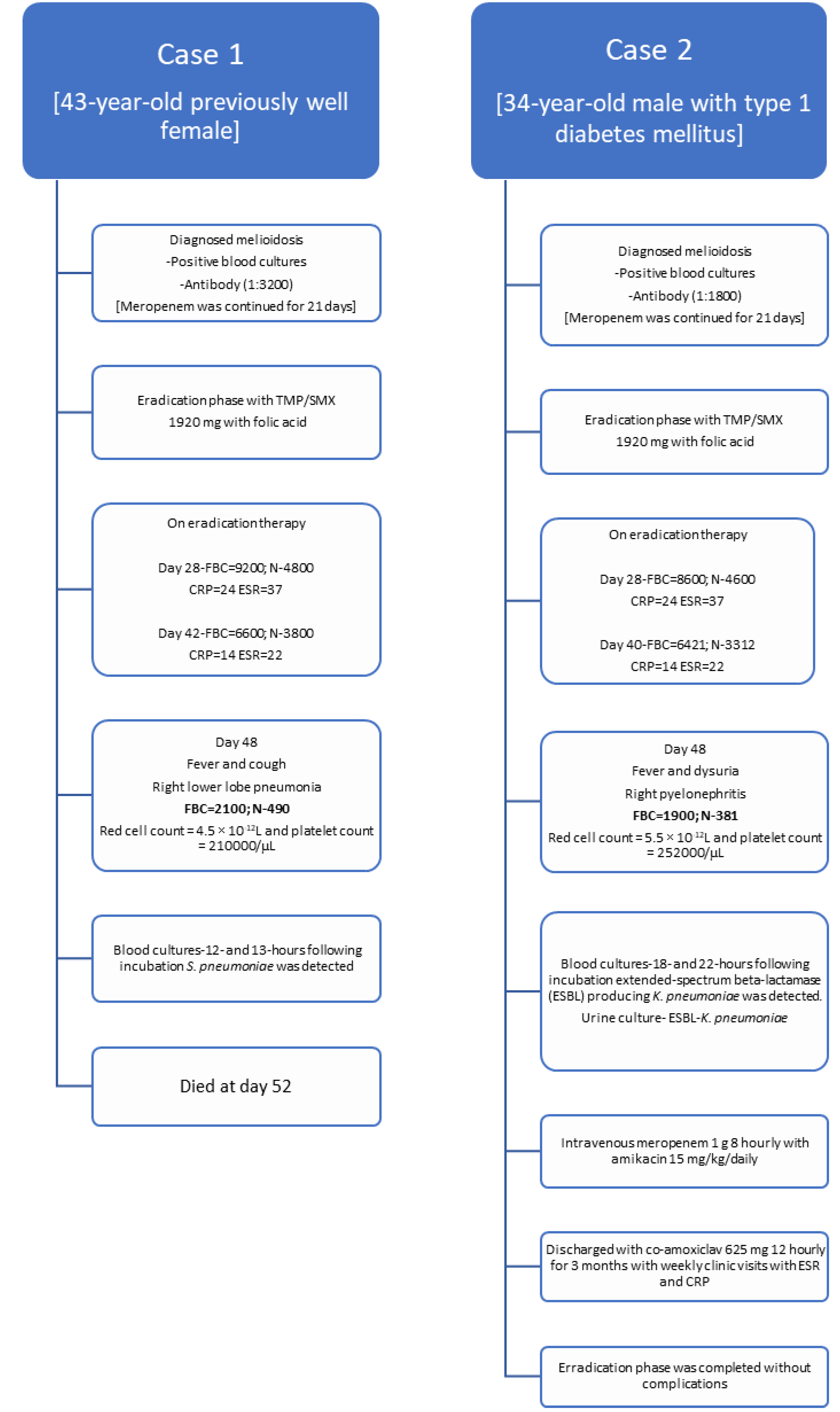
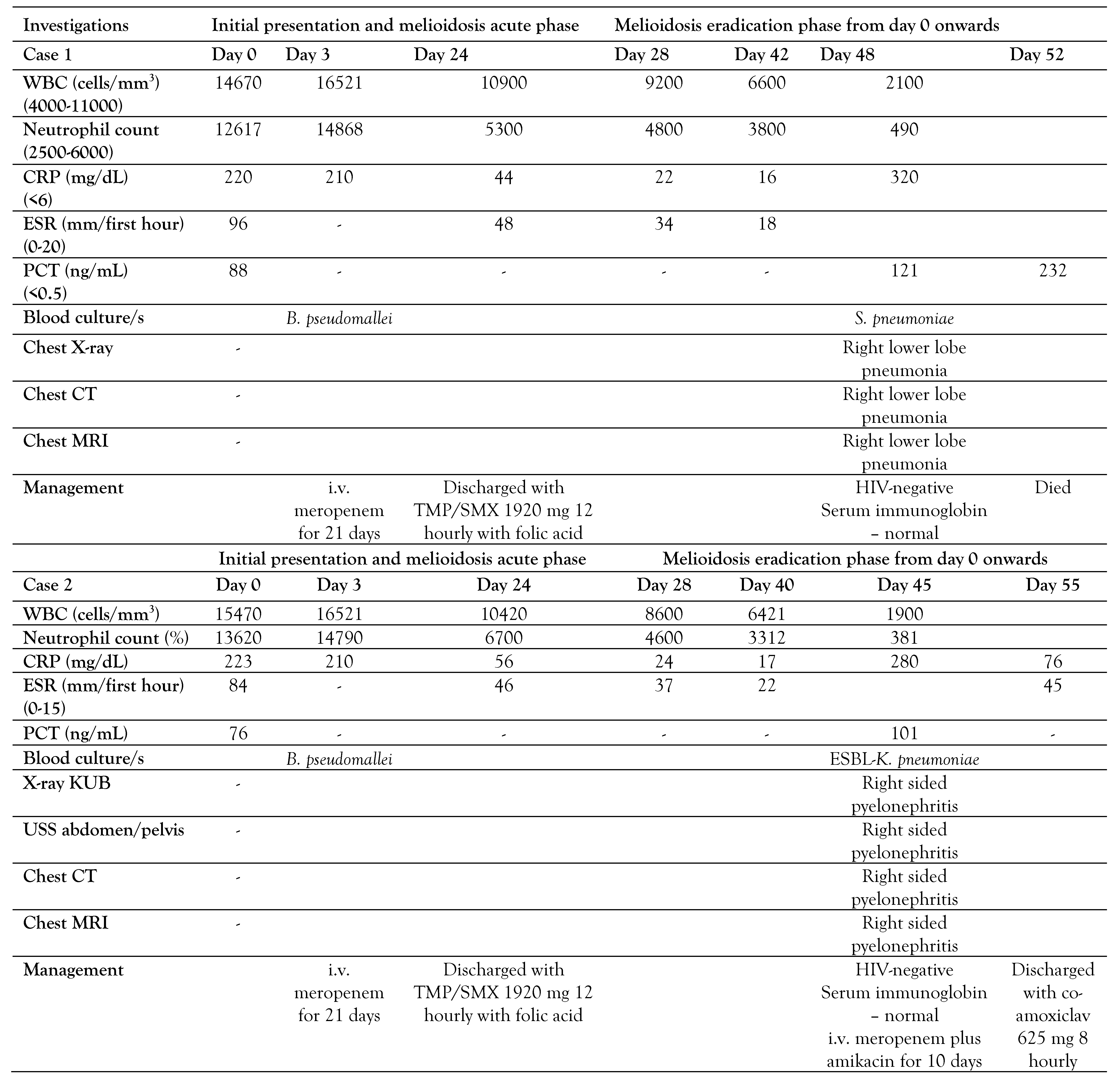
A 43-year-old female farmer presented with high-grade fever and generalized fatigability for 3 days. Her past medical history was not significant. Her body weight was 55.2 kg. She looked ill and on admission blood pressure was 120/70 mmHg and her pulse rate was 104 per minute. The rest of the clinical examination was normal. She was admitted to a high-dependency unit, two blood cultures were taken and empiric ceftriaxone was started. On admission, her white cell count was 14670 cells/mm3 and predominant neutrophil leukocytosis (N=86%) was detected (Table 1). Her C-reactive protein (CRP) was 220 mg/dL, erythrocyte sedimentation rate (ESR) was 96 mm/first hour and procalcitonin (PCT) was 88 ng/mL. Two days later, B. pseudomallei was identified from both blood cultures following 32 and 34 hours of incubation in BD BACTEC™ (Becton Dickinson, USA) automated blood culture system and confirmed by Vitek 2 (bioMérieux, France). B. pseudomallei was susceptible to TMP/SMX, ceftazidime, meropenem, and resistant to aminoglycosides and colistin. Her serum B. pseudomallei antibody titer was 1:3200, measured by enzyme linked immunosorbent assay (ELISA).

Table 1.
Timeline of the two cases.
Ceftriaxone was omitted and intravenous meropenem was started. Ultrasound abdomen and chest X-ray were taken to exclude intra-abdominal abscesses and pneumonia and lung abscess. Meropenem was continued for 21 days and she was discharged with oral TMP/SMX 1920 mg 12 hourly with folic acid. During the maintenance phase, she was reviewed on days 28 and 42 at the medical clinic. Her total white cell count was 9200/mm3 (N=4800/mm3) and 6600/mm3 (N=3800/mm3), respectively. Her CRP and ESR were 22, 16 and 34, and 18 respectively which suggests reduction of inflammatory process, in turn would reflect the process of melioidosis eradication.
On day 48 she presented to the emergency department with a fever and cough for 3 days. She looked ill, and respiratory examination revealed right lower lobe consolidation and chest X-ray was suggestive of right lower lobe pneumonia. She was conscious and rational. Her blood pressure was 100/60 mmHg and her pulse rate was 104/min. Quick sequential organ functional assessment score (qSOFA) was 2 and she was transferred to the intensive care unit. Two blood cultures were taken and intravenous meropenem 1 g 8 hourly with intravenous levofloxacin 750 mg 12 hourly was empirically initiated while oral TMP/SMX 1920 mg 12 hourly was omitted. Her total white cell count was 2100/mm3 and N was 490/mm3 suggestive of neutropenia. Her red cell count was 4.5 × 10-12L and her platelet count was 210,000/µL (Figure 1).
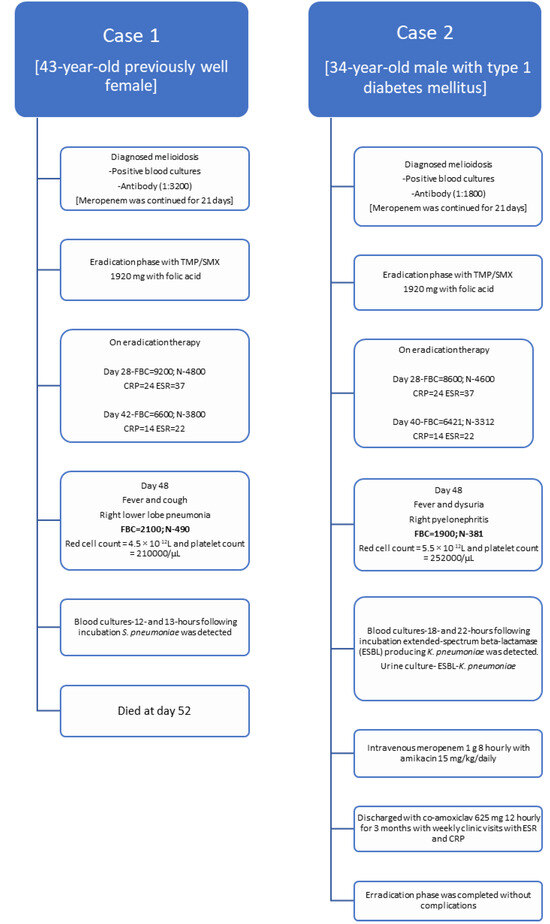
Figure 1.
Timeline of management of melioidosis and development of neutropenic sepsis during the eradication phase following co-trimoxazole.
The following day, her two blood cultures were positive in 12- and 13 hours following incubation, and Streptococcus pneumoniae was detected, which was sensitive to ceftriaxone (penicillin minimal inhibitory concentration 0.12 mg/L). Her blood picture was suggestive of neutropenic sepsis and no hematological malignancies was detected. Full body computed tomography and magnetic resonance imaging were suggestive of having right lower lobe pneumonia and no solid organ malignancies were detected. Her total protein was normal (62 g/L) and immunoglobin concentrations were within the normal range (IgG 12.1 g/L, IgA 2.4 g/L, IgM 2.4 g/L). Human immunodeficiency virus screening was negative. She passed away on day 4 in the intensive care unit.
Case report no 2
A 34-year-old male presented with high-grade fever and generalized fatigability for 4 days. He was a known patient with type 1 diabetes mellitus. His body weight was 58.6 kg. He looked ill and on admission blood pressure was 130/80 mmHg and his pulse rate was 96 per minute. The rest of the clinical examination was normal. He was admitted to the medical ward, two blood cultures were taken and empiric ceftriaxone was started. On admission, his white cell count was 15470 cells/mm3 and predominant neutrophil leukocytosis (N=88%) was detected (Table 1). His CRP was 223 mg/dL, ESR was 84 mm/first hour and PCT was 76 ng/mL. Two days later, B. pseudomallei was identified from both blood cultures following 28 and 33 hours of incubation in BD BACTEC™ (Becton Dickinson, USA) automated blood culture system and confirmed by Vitek 2 (bioMérieux, France). B. pseudomallei was susceptible to TMP/SMX, ceftazidime, meropenem and resistant to aminoglycosides and colistin. His serum B. pseudomallei antibody titer was 1:1800.
Ceftriaxone was omitted and intravenous meropenem was started. Ultrasound abdomen and chest X-ray were taken to exclude intra-abdominal abscesses, pneumonia and lung abscess. Meropenem was continued for 21 days and he was discharged with oral TMP/SMX 1920 mg 12 hourly with folic acid. During the maintenance phase, he was reviewed on days 28 and 40 at the medical clinic. His total white cell count was 8600/mm3 (N=4600/mm3) and 6421/mm3 (N=3312/mm3), respectively. His CRP and ESR were 24, 14 and 37, and 22 respectively, which suggests reduction of the inflammatory process, and in turn would reflect the process of melioidosis eradication.
On day 45 he presented to the emergency department with fever, right loin pain, and dysuria for 2 days. He looked ill, and an abdominal examination revealed right renal angle tenderness and an ultrasound scan of the abdomen was suggestive for right-sided pyelonephritis. He was conscious and rational. His blood pressure was 100/72 mmHg and his pulse rate was 101/min. qSOFA was 2 and he was transferred to the intensive care unit. A urine culture and 2 blood cultures were taken and intravenous meropenem 1 g 8 hourly with amikacin 15 mg/kg/daily was empirically initiated while oral co-trimoxazole 1920 mg 12 hourly was omitted. His total white cell count was 1900/mm3 and N was 381/ mm3 suggestive of neutropenia. His red cell count was 5.5 × 10-12L and his platelet count was 252000/µL.
The following day, his two blood cultures were positive in 18 and 22 hours following incubation and extended-spectrum beta-lactamase (ESBL) producing Klebsiella pneumoniae were detected. Urine culture was also positive for ESBL-Klebsiella pneumoniae. His CRP was 280 mg/L and his PCT was 101 ng/mL.
Histology of his blood smear was suggestive of neutropenic sepsis and no hematological malignancies was detected. Full body computed tomography and magnetic resonance imaging were suggestive of right pyelonephritis with mild hydronephrosis and no solid organ malignancies were detected. His total protein was normal (64 g/L) and immunoglobin concentrations were within the normal range [IgG 13.2 g/L, IgA 2.5 g/L, IgM 2.6 g/L]. Human immunodeficiency virus screening was negative.
Following 10 days of therapy (meropenem and 5 days of amikacin) he improved and was discharged with co-amoxiclav 625 mg 8 hourly for 3 months with weekly clinic visits with ESR and CRP to complete the eradication phase of melioidosis.
Discussion
The intensive intravenous phase and the oral eradication phase are the two phases of melioidosis treatment.[1,2,3,4] The literature does not show uniformity of the therapeutic doses, combinations, or durations appropriate for the eradication phase of melioidosis, although TMP/SMX has been in use for several years.[1,3,8] Neither the effectiveness nor the safety of TMP/SMX have been reported in randomized controlled trials; however, case reports and research studies have shown the effectiveness.[1,7]
Anemia, hyponatremia, hyperkalemia, rarely hypokalemia, severe hyponatremia, gastrointestinal side effects, and rash were reported as common adverse effects of TMP/SMX. However, a few life-threatening side effects have been reported; these can include pancytopenia, hepatitis, Steven-Johnson syndrome, and renal tubular acidosis.[1,3,8]
TMP/SMX-associated pancytopenia is dose-dependent, usually it is not severe, only rarely being of clinical significance; it is generally easily treated or prevented by folate supplementation. TMP/SMX inhibits the tetrahydrofolic acid (THF) synthesis of bacteria following the inhibition of enzyme systems progressively. THF's decreased availability prevents the synthesis of thymidine, which prevents the synthesis of DNA. In both cases, we observed only neutropenia and presented with neutropenic sepsis, and other cell lines, platelets, and red blood cells remained within the normal range at presentation. Only during septic shock, platelets decreased.[8,9] This is a rare occurrence that isolated neutropenia was developed following TMP/SMX use.
Pancytopenia caused by TMP/SMX can frequently develop gradually over time. Alternately, it can develop rapidly and swiftly escalate to fulminant sepsis, disseminated intravascular coagulation, and fast hemolysis. According to a study of the Swedish population, 1 in 18000 people who use TMP/SMX has blood dyscrasias.[9] The low hospitalization rate for blood disorders is associated with the use of TMP/SMX, according to a population-based study in Seattle, Washington.[10]
Herein, both cases were in the maintenance phase of melioidosis at days 48 and 45, with TMP/SMX 1920 mg 12 hourly therapy, and developed neutropenic sepsis, respectively. Based on body weight (both were within 40-60 kg)[1,2,8] the ideal TMP/SMX dose would be 240/1200 mg q12h (3 tablets) but, and for both 4 tablets were prescribed (1920 mg) 12 hourly, which is high. Also, the compliance of folic acid intake was low in both cases as it is important to counter TMP/SMX-associated bone marrow suppression in humans.
Conclusions
Prolonged use of TMP/SMX is important to eradicate B. pseudomallei and always the possibility of rare adverse effects has to be considered. Always weight-based TMP-SMX dosing has to be encouraged while ensuring the compliance to folic acid. Continuous monitoring of blood cell lines with weekly full blood count at the medical clinic would be essential to identify neutropenia in advance.
Author Contributions
JAASJ followed up the patient and drafted the manuscript. GRR has provided the supervision for the case management. Both authors read and approved the final version of the manuscript.
Funding
None to declare.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author, upon reasonable request.
Conflicts of Interest
All authors – none to declare.
Consent
Informed written consent was obtained from next-of-kin in case 1 and from the patient in case 2 for publication of the case reports and any accompanying images.
References
- Chetchotisakd, P.; Chierakul, C.; Chaowagul, W.; et al. Trimethoprim-sulfamethoxazole versus trimethoprimsulfamethoxazole plus doxycycline as oral eradicative treatment for melioidosis (MERTH): a multicentre, double-blind, non-inferiority, randomised controlled trial. Lancet. 2014, 383, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghearachchi, H.S.; Muthugama, T.A.; Masakorala, J.; et al. Burkholderia pseudomallei in soil and natural water bodies in rural Sri Lanka: a hidden threat to public health. Front Vet Sci. 2023, 9, 1045088. [Google Scholar] [CrossRef] [PubMed]
- Wijekoon, S.; Prasath, T.; Corea, E.M.; Elwitigala, J.P. Melioidosis presenting as lymphadenitis: a case report. BMC Res Notes. 2014, 7, 364. [Google Scholar] [CrossRef] [PubMed]
- Currie, B.J.; Mayo, M.; Ward, L.M.; et al. The Darwin Prospective Melioidosis Study: a 30-year prospective, observational investigation. Lancet Infect Dis. 2021, 12, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Virk, H.S.; Torres, A.G.; et al. Melioidosis. Nat Rev Dis Primers. 2018, 4, 17107. [Google Scholar] [CrossRef] [PubMed]
- Abeydeera, W.P.H.; Corea, E.M.; Ranasinghe, G.; et al. Burkholderia pseudomallei: case series from the Provincial General Hospital, Kurunegala. Sri Lankan J Infect Dis. 2017, 7, 41. [Google Scholar] [CrossRef]
- Dance, D. Treatment and prophylaxis of melioidosis. Int J Antimicrob Agents. 2014, 43, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Inglis, T.J.; Rolim, D.B.; Rodriguez, J.L. Clinical guideline for diagnosis and management of melioidosis. Rev Inst Med Trop Sao Paulo. 2006, 48, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Keisu, M.; Wiholm, B.E.; Palmblad, J. Trimethoprim-sulphamethoxazole-associated blood dyscrasias. Ten years' experience of the Swedish spontaneous reporting system. J Intern Med. 1990, 228, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.W.; Jick, H. Hospitalization for serious blood and skin disorders following co-trimoxazole. Br J Clin Pharmacol. 1997, 43, 649–651. [Google Scholar] [CrossRef] [PubMed]
© GERMS 2023.