The Emergence of Multidrug-Resistant Gram-Positive Bloodstream Infections in India—A Single Center Prospective Cohort Study
Abstract
Introduction
Methods
Data collection
Identification of bloodstream infections and antimicrobial susceptibility patterns
Statistical analysis
Results
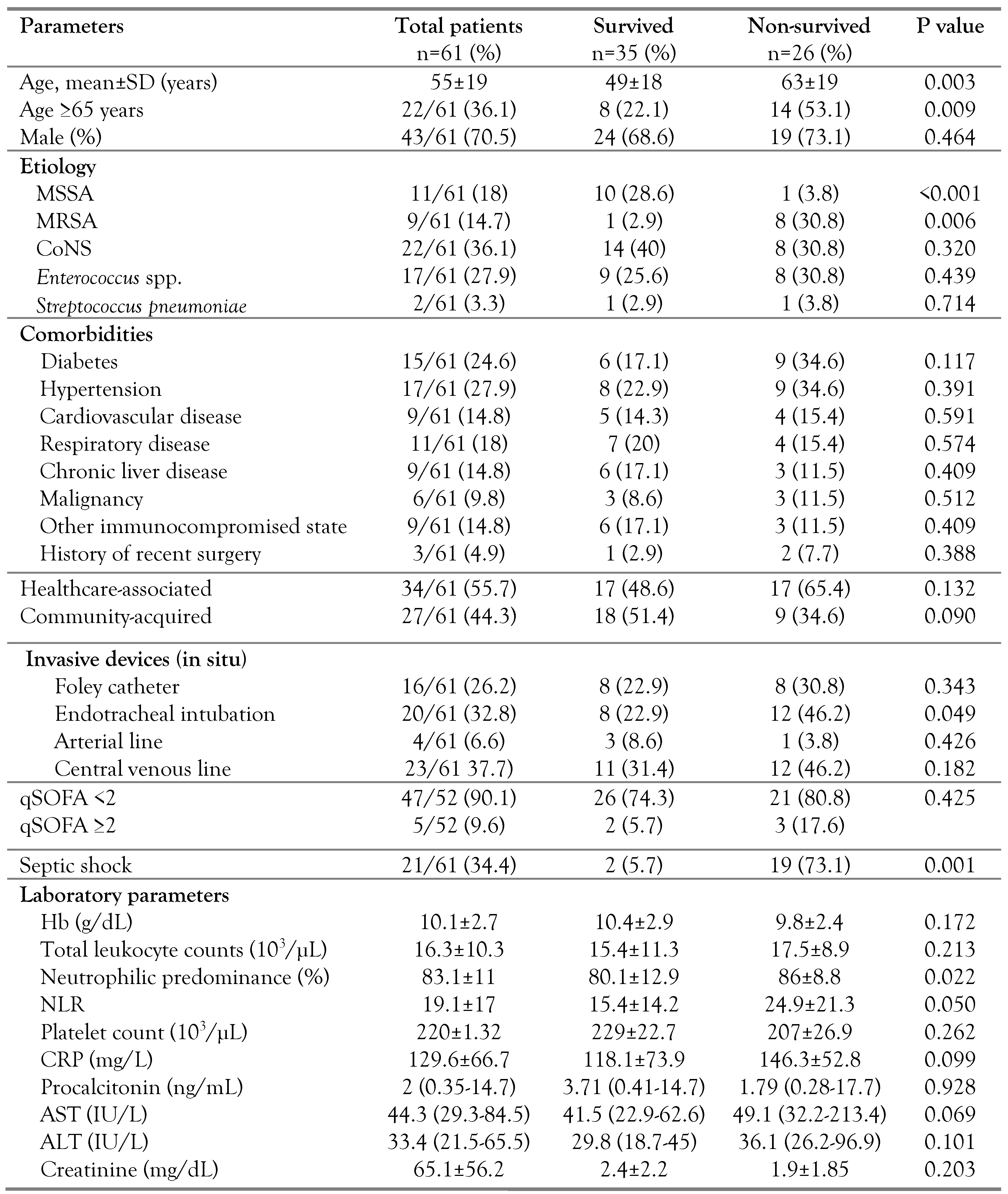
Demographic and clinical characteristics
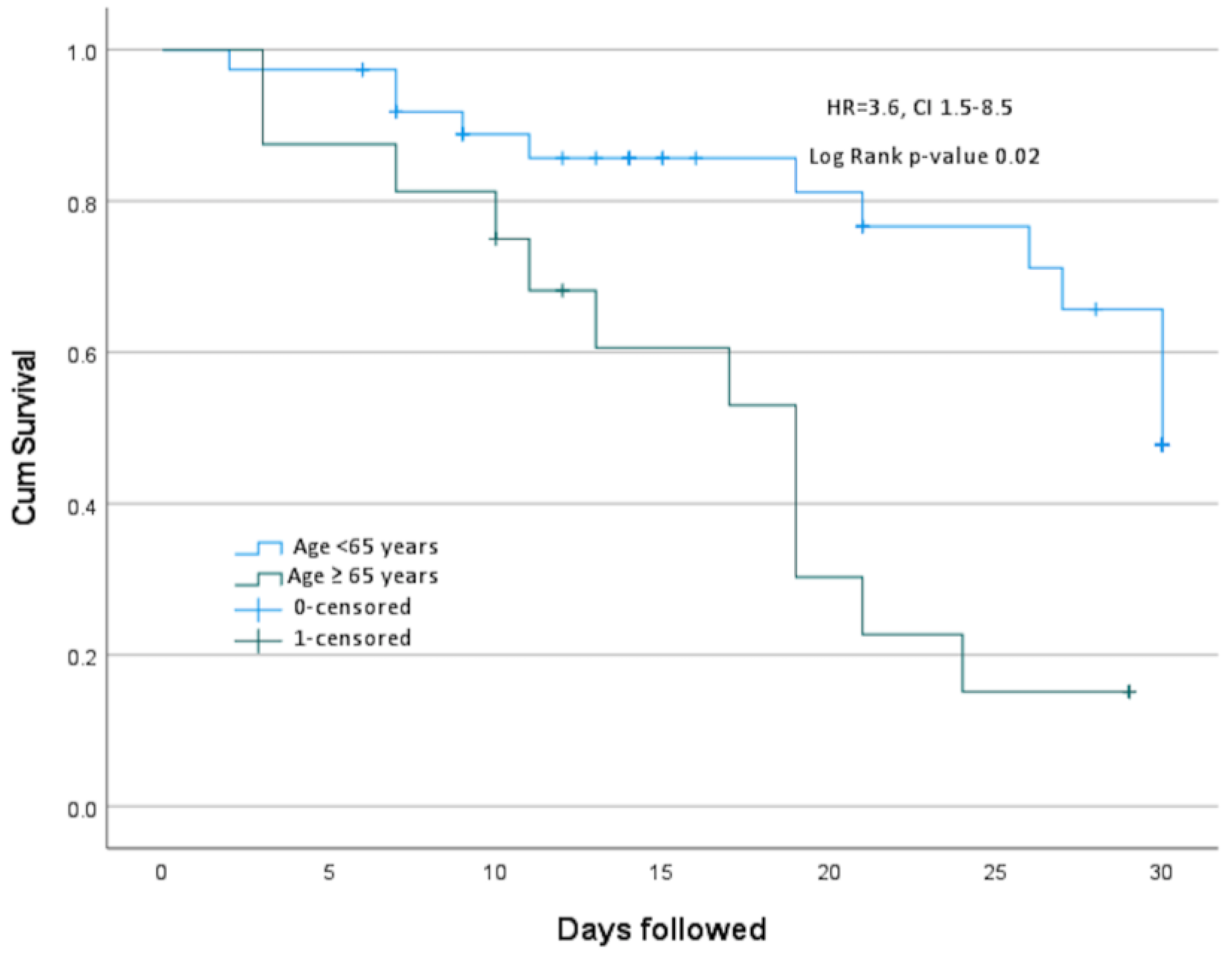
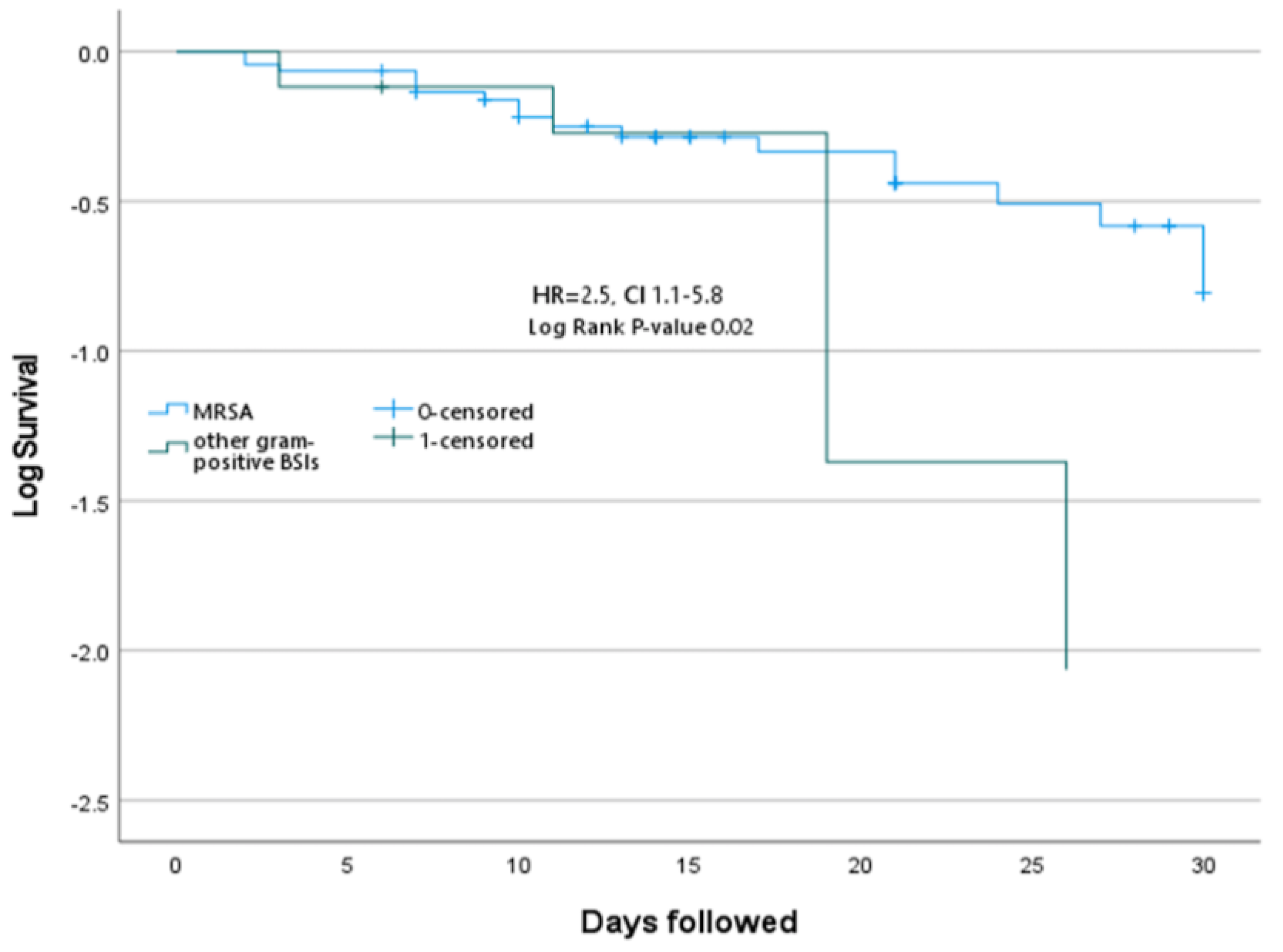
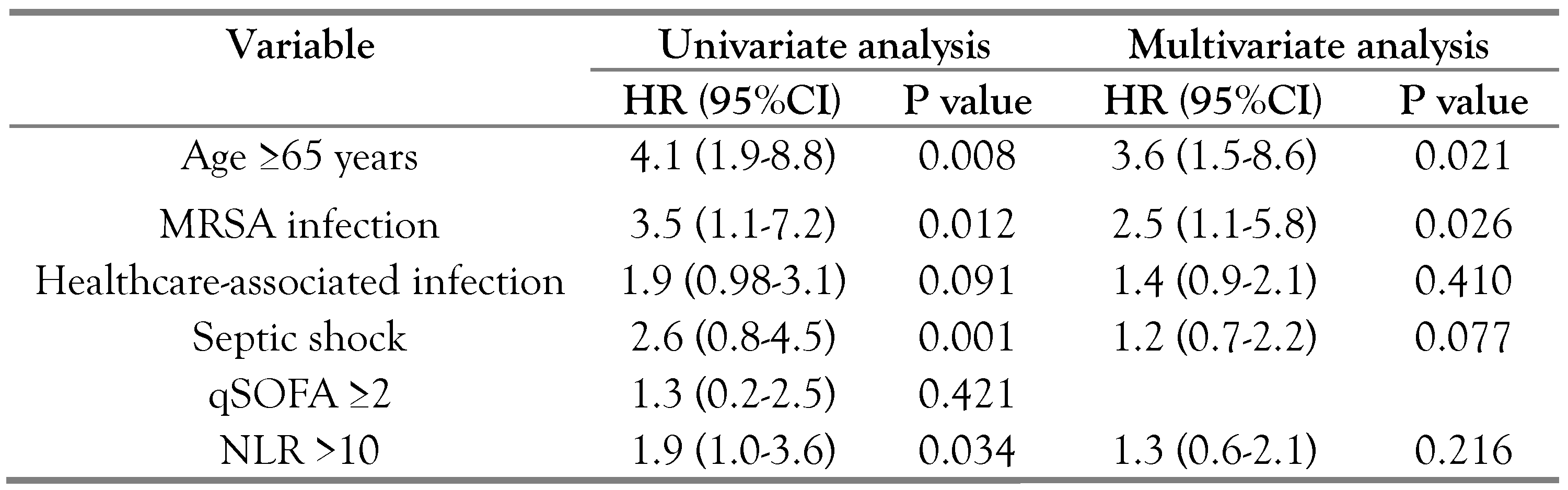
Antimicrobial susceptibility and outcomes
Discussion
Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Viscoli, C. Bloodstream infections: The peak of the iceberg. Virulence 2016, 7, 248–251. [Google Scholar] [CrossRef]
- Laupland, K.B. Defining the epidemiology of bloodstream infections: The ‘gold standard’ of population-based assessment. Epidemiol Infect 2013, 141, 2149–2157. [Google Scholar] [CrossRef]
- Mehl, A.; Åsvold, B.O.; Lydersen, S.; et al. Burden of bloodstream infection in an area of Mid-Norway 2002-2013: A prospective population-based observational study. BMC Infect Dis 2017, 17, 205. [Google Scholar] [CrossRef]
- Verway, M.; Brown, K.A.; Marchand-Austin, A.; et al. Prevalence and mortality associated with bloodstream organisms: A population-wide retrospective cohort study. J Clin Microbiol 2022, 60, e0242921. [Google Scholar] [CrossRef]
- Maki, D.G.; Kluger, D.M.; Crnich, C.J. The risk of bloodstream infection in adults with different intravascular devices: A systematic review of 200 published prospective studies. Mayo Clin Proc 2006, 81, 1159–1171. [Google Scholar] [CrossRef]
- Laupland, K.B.; Lyytikäinen, O.; Søgaard, M.; et al. The changing epidemiology of Staphylococcus aureus bloodstream infection: A multinational population-based surveillance study. Clin Microbiol Infect 2013, 19, 465–471. [Google Scholar] [CrossRef]
- Kulkarni, A.P.; Nagvekar, V.C.; Veeraraghavan, B.; et al. Current perspectives on treatment of gram-positive infections in India: What is the way forward? Interdiscip Perspect Infect Dis 2019, 2019, 7601847. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing, 33rd edition. CLSI Supplement M100; CLSI: Wayne, Pennsylvania, 2023. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Bhardwaj, N.; Kumari, M.; Malhotra, R.; Mathur, P. Prevalence, etiology, and antibiotic resistance profiles of bacterial bloodstream infections in a tertiary care hospital in Northern India: A 4-year study. J Lab Physicians 2018, 10, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Rolston, K.V.; Yadegarynia, D.; Kontoyiannis, D.P.; Raad, I.I.; Ho, D.H. The spectrum of Gram-positive bloodstream infections in patients with hematologic malignancies, and the in vitro activity of various quinolones against Gram-positive bacteria isolated from cancer patients. Int J Infect Dis 2006, 10, 223–230. [Google Scholar] [CrossRef]
- Mendes, R.E.; Sader, H.S.; Castanheira, M.; Flamm, R.K. Distribution of main Gram-positive pathogens causing bloodstream infections in United States and European hospitals during the SENTRY Antimicrobial Surveillance Program (2010-2016): Concomitant analysis of oritavancin in vitro activity. J Chemother 2018, 30, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Thorlacius-Ussing, L.; Sandholdt, H.; Larsen, A.R.; Petersen, A.; Benfield, T. Age-dependent increase in incidence of Staphylococcus aureus bacteremia, Denmark, 2008-2015. Emerg Infect Dis 2019, 25, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Indian Network for Surveillance of Antimicrobial Resistance (INSAR) group, India. Methicillin resistant Staphylococcus aureus (MRSA) in India: Prevalence & susceptibility pattern. Indian J Med Res 2013, 137, 363–369. [Google Scholar]
- Ashagrie, D.; Genet, C.; Abera, B. Vancomycin-resistant enterococci and coagulase-negative staphylococci prevalence among patients attending at Felege Hiwot Comprehensive Specialized Hospital, Bahir Dar, Ethiopia. PLoS ONE 2021, 16, e0249823. [Google Scholar] [CrossRef]
- Mashaly, G.E.; El-Mahdy, R.H. Vancomycin heteroresistance in coagulase negative Staphylococcus blood stream infections from patients of intensive care units in Mansoura University Hospitals, Egypt. Ann Clin Microbiol Antimicrob 2017, 16, 63. [Google Scholar] [CrossRef]
- Sivaradjy, M.; Gunalan, A.; Priyadarshi, K.; Madigubba, H.; Rajshekar, D.; Sastry, A.S. Increasing trend of vancomycin-resistant enterococci bacteremia in a tertiary care hospital of South India: A three-year prospective study. Indian J Crit Care Med 2021, 25, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Bi, R.; Qin, T.; Fan, W.; Ma, P.; Gu, B. The emerging problem of linezolid-resistant enterococci. J Glob Antimicrob Resist 2018, 13, 11–19. [Google Scholar] [CrossRef]
- Zhu, Q.; Yue, Y.; Zhu, L.; et al. Epidemiology and microbiology of Gram-positive bloodstream infections in a tertiary-care hospital in Beijing, China: A 6-year retrospective study. Antimicrob Resist Infect Control 2018, 7, 107. [Google Scholar] [CrossRef]
- Kontula, K.S.K.; Skogberg, K.; Ollgren, J.; Järvinen, A.; Lyytikäinen, O. Population-based study of bloodstream infection incidence and mortality rates, Finland, 2004-2018. Emerg Infect Dis 2021, 27, 2560–2569. [Google Scholar] [CrossRef]
- van Hal, S.J.; Jensen, S.O.; Vaska, V.L.; Espedido, B.A.; Paterson, D.L.; Gosbell, I.B. Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev 2012, 25, 362–386. [Google Scholar] [CrossRef]
- Goto, M.; Jones, M.P.; Schweizer, M.L.; et al. Association of infectious diseases consultation with long-term postdischarge outcomes among patients with Staphylococcus aureus bacteremia. JAMA Netw Open 2020, 3, e1921048. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, N.; Vihari, N.; Meena, D.S.; et al. Clinical profile of bloodstream infections in COVID-19 patients: A retrospective cohort study. BMC Infect Dis 2021, 21, 933. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou-Olivgeris, M.; Kolonitsiou, F.; Karamouzos, V.; et al. Molecular characteristics and predictors of mortality among Gram-positive bacteria isolated from bloodstream infections in critically ill patients during a 5-year period (2012-2016). Eur J Clin Microbiol Infect Dis 2020, 39, 863–869. [Google Scholar] [CrossRef]
- Koizumi, Y.; Sakanashi, D.; Ohno, T.; et al. Plasma procalcitonin levels remain low at the onset of gram-positive bacteremia regardless of severity or the presence of shock: A retrospective analysis of patients with detailed clinical characteristics. J Microbiol Immunol Infect 2021, 54, 1028–1037. [Google Scholar] [CrossRef]
- Sumardi, U.; Prihardianti, D.R.; Sudjana, P. Is neutrophil-lymphocyte count ratio a better indicator of sepsis with Gram-positive bacterial infection? Indian J Crit Care Med 2021, 25, 795–799. [Google Scholar] [CrossRef] [PubMed]
 |
 |
 |
© GERMS 2023.
Share and Cite
Vihari, N.; Bohra, G.K.; Yadev, R.R.; Kumar, D.; Meena, D.S.; Tak, V.; Sharma, A.; Nag, V.; Garg, M.K. The Emergence of Multidrug-Resistant Gram-Positive Bloodstream Infections in India—A Single Center Prospective Cohort Study. Germs 2023, 13, 229-237. https://doi.org/10.18683/germs.2023.1389
Vihari N, Bohra GK, Yadev RR, Kumar D, Meena DS, Tak V, Sharma A, Nag V, Garg MK. The Emergence of Multidrug-Resistant Gram-Positive Bloodstream Infections in India—A Single Center Prospective Cohort Study. Germs. 2023; 13(3):229-237. https://doi.org/10.18683/germs.2023.1389
Chicago/Turabian StyleVihari, Nakka, Gopal Krishana Bohra, Ram Ratan Yadev, Deepak Kumar, Durga Shankar Meena, Vibhor Tak, Ankur Sharma, Vijaylaxmi Nag, and Mahendra Kumar Garg. 2023. "The Emergence of Multidrug-Resistant Gram-Positive Bloodstream Infections in India—A Single Center Prospective Cohort Study" Germs 13, no. 3: 229-237. https://doi.org/10.18683/germs.2023.1389
APA StyleVihari, N., Bohra, G. K., Yadev, R. R., Kumar, D., Meena, D. S., Tak, V., Sharma, A., Nag, V., & Garg, M. K. (2023). The Emergence of Multidrug-Resistant Gram-Positive Bloodstream Infections in India—A Single Center Prospective Cohort Study. Germs, 13(3), 229-237. https://doi.org/10.18683/germs.2023.1389




