Abstract
Introduction: Febrile urinary tract infections (UTIs) in children are among the most serious bacterial infections. Inadequate treatment can lead to kidney scarring and permanent kidney damage. Eight to ten percent of children with UTIs could have concomitant bacteremia. The study aimed to estimate the prevalence of UTI-associated bacteremia and identify common organisms causing UTIs and their antimicrobial susceptibility patterns to help guide empiric antimicrobial therapy. Methods: The current study was conducted over a 6-month period on children admitted with febrile UTIs at Alexandria University Children’s Hospital. Blood and urine samples were collected for culture and antimicrobial susceptibility. Results: A total of 103 children with a median age of 12 months (IQR 6.0–24.0) were included in the study. Concomitant bacteremia was present in 63.1% (n = 65). The median temperature of 38.40 °C (IQR 38.15–38.60) and the median creatinine level of 0.18 mg/dL (IQR 0.14–0.25) were significantly higher in the bacteremic group compared to the non-bacteremic group (p = 0.005, p = 0.034, respectively). E. coli (n = 51; 49.5%) and Klebsiella pneumoniae (n = 30; 29.1%) were the most common isolated organisms. Most (n = 68; 66%) of the isolated organisms were multidrug-resistant (MDR), followed by extensively drug-resistant (XDR) (n = 16; 15.5%), and pan-drug-resistant (PDR) organisms (n = 1; 1%). E. coli showed lower resistance to gentamicin and ceftriaxone (9.8 % and 13.7%, respectively). Conclusions: E. coli remains the most important UTI pathogen. Ceftriaxone and gentamicin are good empiric options for febrile UTIs in our hospital.
Introduction
Concurrent bacteremia is estimated to affect 8-10% of infants with febrile urinary tract infections (UTIs) [1]. It is challenging to clinically differentiate between bacteremic and non-bacteremic UTIs due to conflicting data regarding symptoms and signs between both groups [2,3]. Young ages have consistently been identified as a risk factor for bacteremia and UTI-associated morbidity, including kidney damage [4,5]. The majority of pediatric UTIs are caused by Gram-negative bacteria that enter the urinary tract via the ascending route [6]. E. coli is the most common uropathogen, accounting for approximately 80% to 90% of pediatric UTIs. Klebsiella, Proteus, Enterobacter, and Enterococcus are common uropathogens [4].
Early and appropriate treatment of febrile UTI, especially with concomitant bacteremia, will lead to decreased morbidity and better outcomes. Unfortunately, rising antibiotic resistance makes empiric prescriptions for febrile UTIs challenging. This study aimed to estimate the prevalence of concomitant bacteremia among children with febrile UTI and to identify common bacterial pathogens and their antibiotic susceptibility patterns at Alexandria University Children’s Hospital to guide antimicrobial therapy.
Methods
Study design
The current case-control study was conducted over six months at Alexandria University Children’s Hospital, a tertiary care university pediatric hospital in Alexandria, Egypt. Children ≥1 month with a temperature of ≥38 °C and symptoms suggestive of UTI were included in the current study. Neonates, children with immunodeficiency, children who were already previously diagnosed with urinary tract abnormalities, and those without withdrawn blood cultures were excluded from the study. A total of 103 children with microbiologically confirmed UTIs were enrolled for further analysis. The diagnosis of concomitant bacteremia is based on the isolation of the same organism in the patient’s urine and blood culture within 48-hour intervals [7].
Collection of data
All relevant demographic, clinical, and laboratory data were collected.
Microbiological investigations
Sample collection
All samples were collected before the start of antibiotic therapy. Two to five mL of blood for blood culture and urine samples (by clean catch midstream urine or straight catheterization) for urine culture were collected using standard collection procedures after following a strict aseptic technique. The collected samples were immediately transferred to the microbiology lab of Alexandria Children’s Hospital for processing [8].
Processing of samples
All collected samples were processed according to standard microbiological procedures [8].
1. Culture procedure
1.1. Inoculated blood culture bottles were incubated for 5 days in an automated BACT/ALERT blood culture system (bioMérieux, USA). Positive signal bottle contents were inoculated onto the surface of chocolate blood agar, blood agar, and MacConkey’s agar and streaked for isolation.
For blood culture, any recognized blood pathogen isolated from a single blood culture, or an original contaminant pathogen isolated from two blood cultures confirmed bloodstream infection.
1.2. Urine samples were cultured semi-quantitatively using a 1-µL calibrated loop. Cultures were performed using blood agar and MacConkey’s agar. Cultures were examined for colony counts and morphological types of organisms. The bacterial count in colony-forming units/mL was calculated according to the following formula: number of colonies × 1000. Definitive identification of isolates and antimicrobial susceptibility testing was done for the significant growth of the following:
- -
- Single organism with colony count ≥105 colony forming units (CFU)/mL
- -
- Two organisms ≥105 CFU/mL for each with significant pyuria (≥10 WBC/high power field).
- -
- The growth of two isolates with ≥105 CFU/mL for one isolate and <104 CFU/mL for the other one with significant pyuria; identification was done only for the first isolate.
The growth of two isolates with colony count <105 CFU/mL for each or the growth of ≥three isolates was not processed.
2. Identification of isolated organisms
Full microbiological identification was based on Gram’s-stained film, morphological colonial characters (hemolytic or non-hemolytic colonies on blood agar, lactose, or non-lactose colonies on MacConkey’s agar), and standard biochemical tests (catalase, coagulase, oxidase, bile esculin, triple sugar iron, urease, citrate, motility, and indole tests) [8].
3. Antimicrobial susceptibility testing
Antimicrobial susceptibility was determined using the Kirby-Bauer disc diffusion method according to Clinical Laboratory Standards Institute (CLSI) guidelines (2021) [9]. E. coli (ATCC 25923) and Staphylococcus aureus (ATCC 25922) were used as controls for the test. Phenotypic detection of extended-spectrum beta-lactamases (ESBL) in Gram-negative bacilli was based on using cefoxitin (30 μg disc diffusion) and confirmed by a combined disc diffusion test [9]. Multidrug-resistant (MDR), extensively drug-resistant (XDR), and pan-drug-resistant (PDR) organisms were defined according to standard international terminology presented by ECDC (European Centre for Disease Prevention and Control) and CDC (Centre for Disease Prevention and Control) [10].
Statistical analysis
The data were analyzed using IBM SPSS software version 20.0 (IBM Corp., USA) Qualitative data were described using numbers and percentages. The Kolmogorov-Smirnov test was used to verify the normality of the distribution. Quantitative data were described using range (minimum and maximum), mean, standard deviation, median, and interquartile range (IQR). The significance of the obtained results was judged at the 5% level. A Chi-square test was performed to study the significant association between different categorical variables and the occurrence of bacteremia. Fischer-Exact and Monte-Carlo significance were employed if more than 20% of the total expected cell counts <5 at the 0.05 level of significance. The Mann-Whitney test detected a significant difference in the median quantitative variables between negative and positive bacteremia based on the variables’ distribution by the Kolmogorov-Smirnov test.
Results
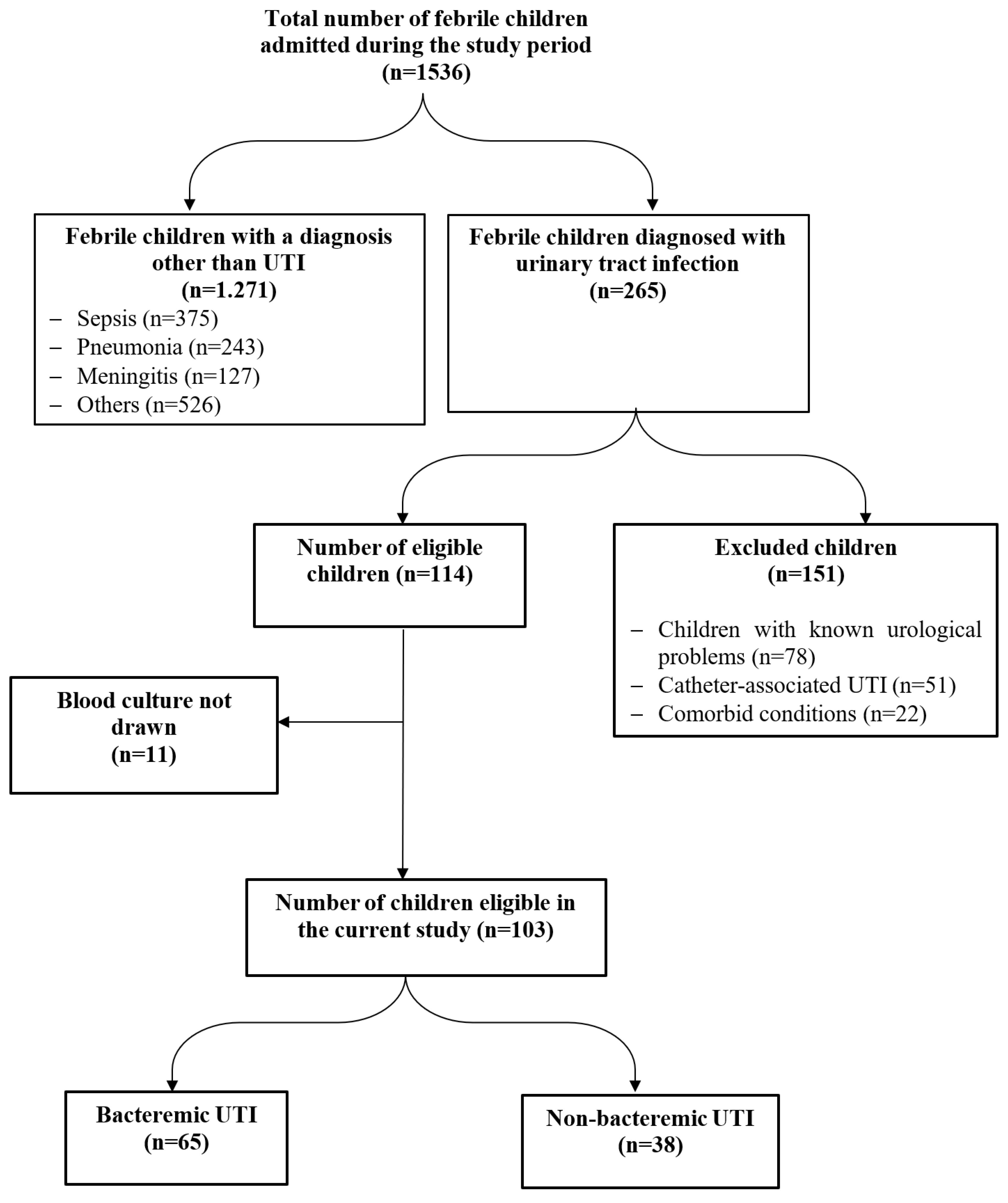
During the study period, 265/1536 (17.3%) febrile children with culture-confirmed UTI were hospitalized, of whom 103 fulfilled the inclusion criteria – Figure 1. The age of the included children ranged from 1 to 72 months with a median of 12.0 (IQR 6.0-24.0) months. Most of the studied children (91.3%) (n=94) were aged less than 3 years, with an equal percentage of infants and children over the age of one year. Females were slightly higher than males (54.4% versus 45.6%).
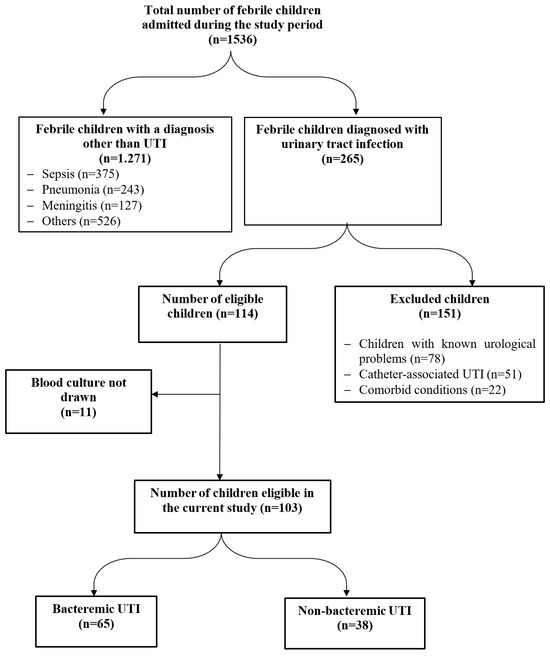
Figure 1.
RECORD flow diagram of studied cases.
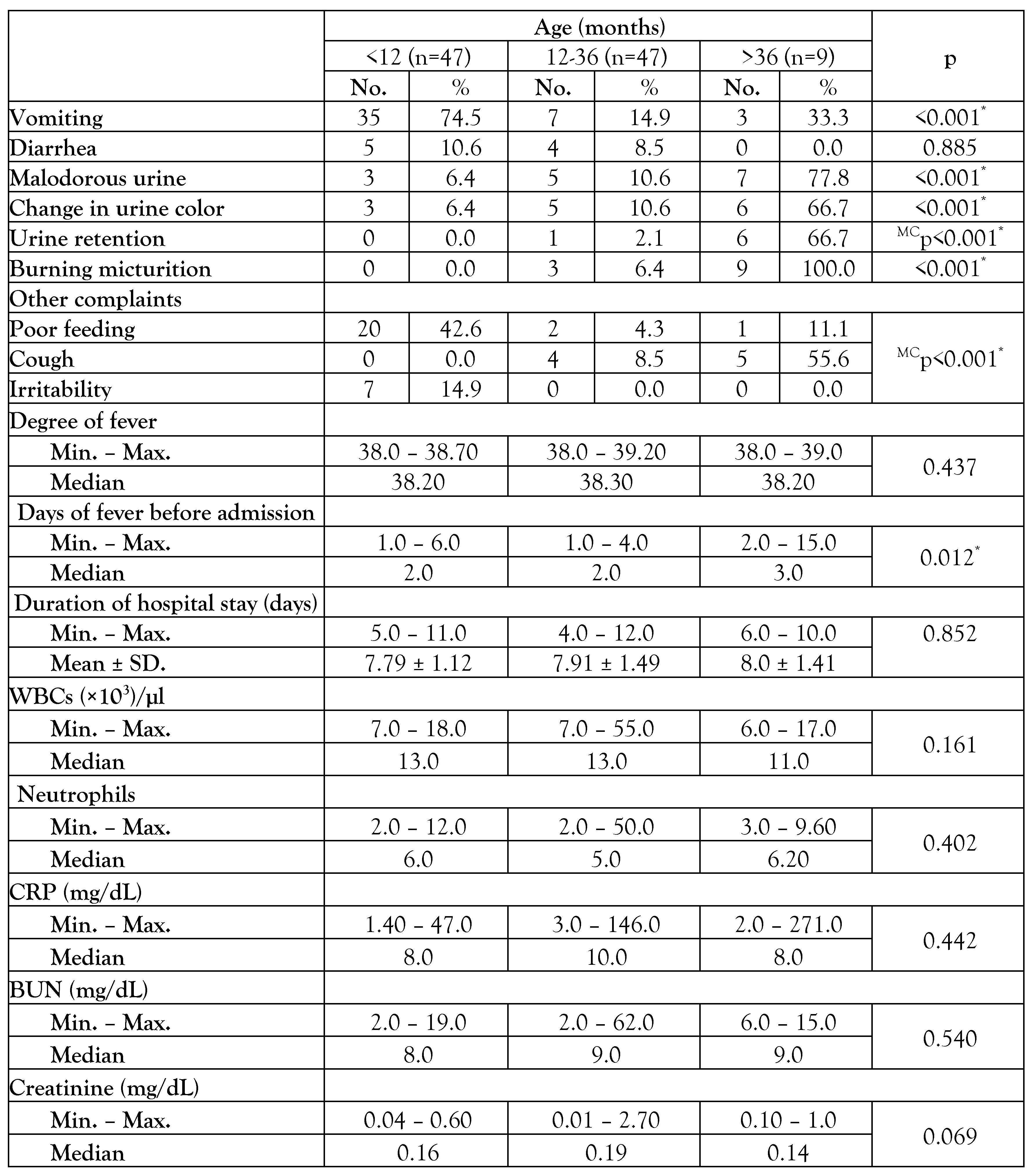
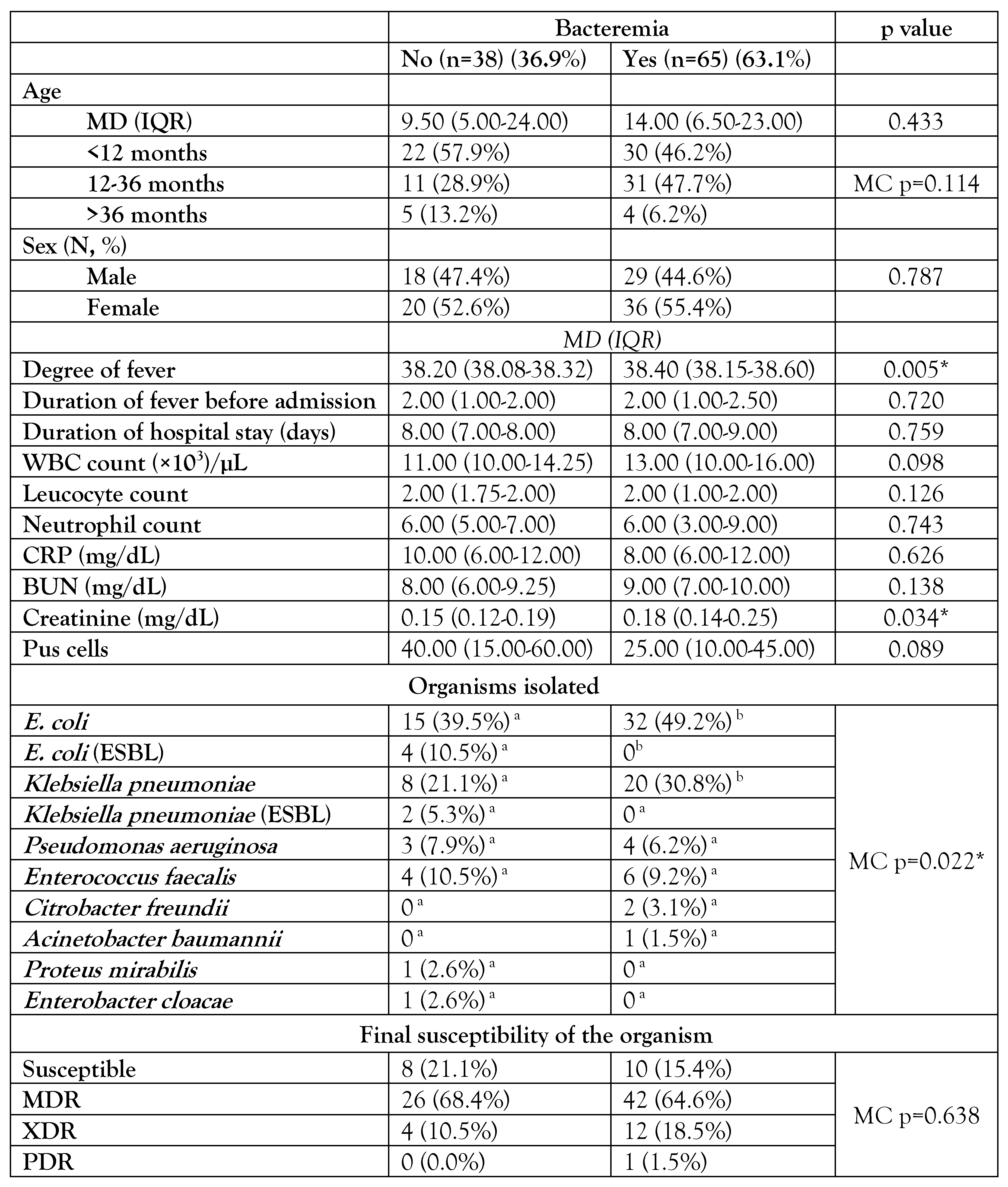
The clinical manifestations differed significantly by age (Table 1), as classical urinary symptoms were more common in children over the age of 36 months (n=9), with burning micturition accounting for 100% (n=9), malodorous urine accounting for 77.8% (n=7), and both changes in urine color and urine retention accounting for 66.7% (n=6). Non-classical symptoms including vomiting (74.5% (n=35)), irritability (14.9% (n=7)), and poor feeding (42.6% (n=20)) were common in children younger than one year. Children older than 36 months significantly (p=0.012) had longer median days of fever before admission [3 (2-15) days]. Concomitant bacteremia among children with febrile UTI was 63.1% (n=65). Most (n=61; 93.9%) of bacteremia occurred in children younger than 36 months. The median temperature (38.4 °C IQR (38.15-38.60)) and median creatinine level (0.18 mg/dL IQR (0.14-0.25)) were significantly higher in the bacteremic group compared to the non-bacteremic group, with p values of 0.005 and 0.034, respectively. In both bacteremic and non-bacteremic groups, E. coli and Klebsiella pneumoniae were the most often isolated species, with 49.5% (n=51) and 29.1% (n=30), respectively – Table 2. ESBL-producing isolates represented 15.8% (n=6) of the non-bacteremic group. Enterococcus faecalis was the only isolated Gram-positive organism, representing 9.2% (n=6) and 10.5% (n=4) in the bacteremic and non-bacteremic groups, respectively.

Table 1.
Comparison between different age groups regarding clinical and laboratory characteristics.

Table 2.
Comparison between bacteremic and non-bacteremic urinary tract infections.
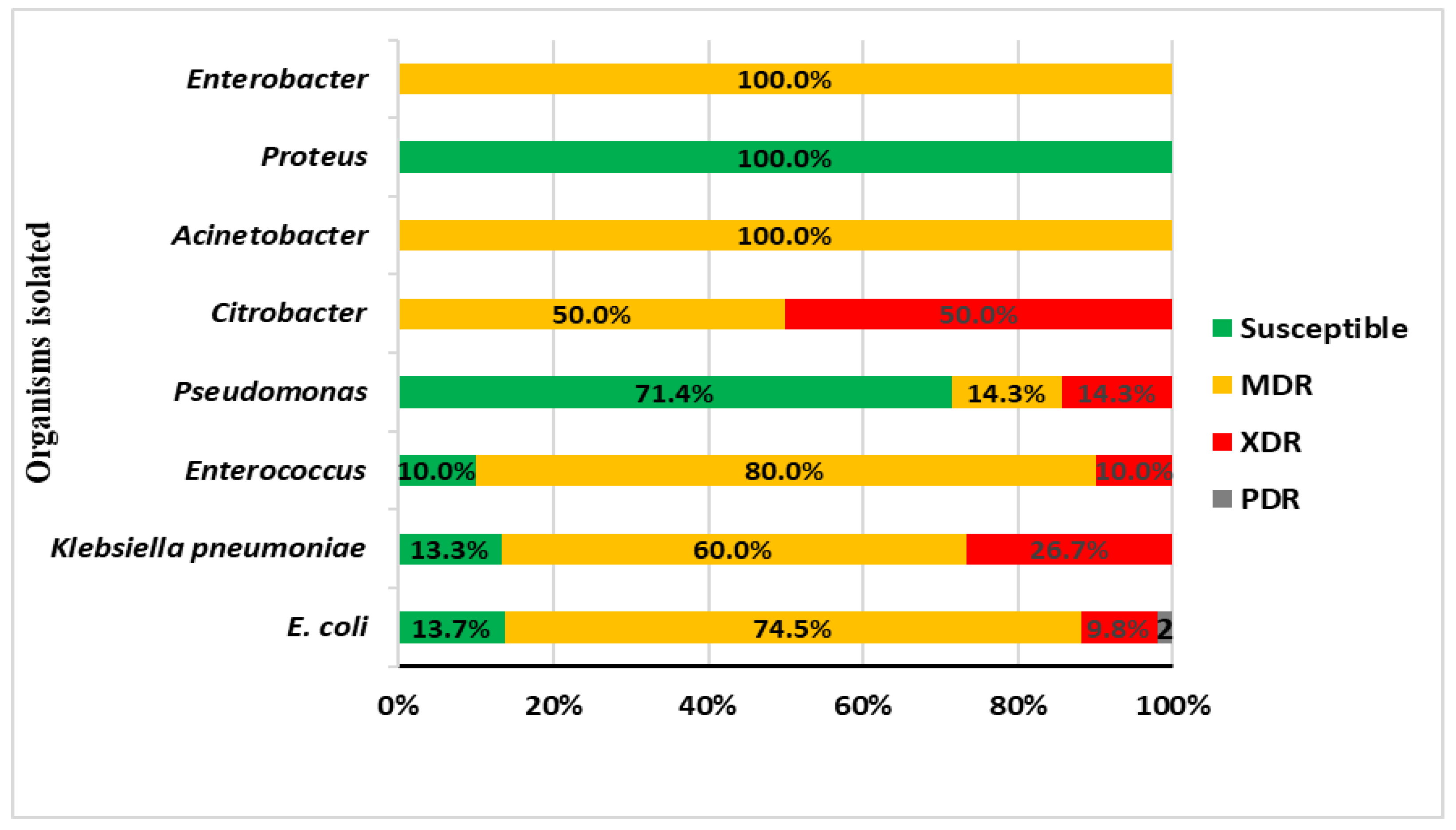
Overall, there was an alarming increase in the resistance patterns of isolated organisms, 82.5% (n=85) were resistant. The MDR organisms were the most prevalent at 66.0% (n=68), followed by XDR at 15.6% (n=16) and PDR organisms at 1.5% (n=1). Although both MDR (64.6%) and XDR (18.5%) were more frequently isolated in the bacteremic group, this was not statistically significant (p=0.638). The MDR organisms were most detected among Enterococcus faecalis (80%), E. coli (74.5%), and Klebsiella pneumoniae (60%) – Figure 2.
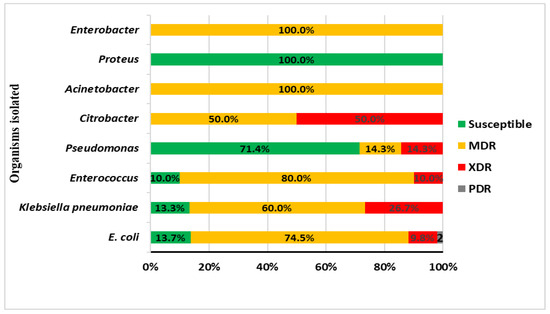
Figure 2.
Antimicrobial susceptibility pattern of isolated organisms.
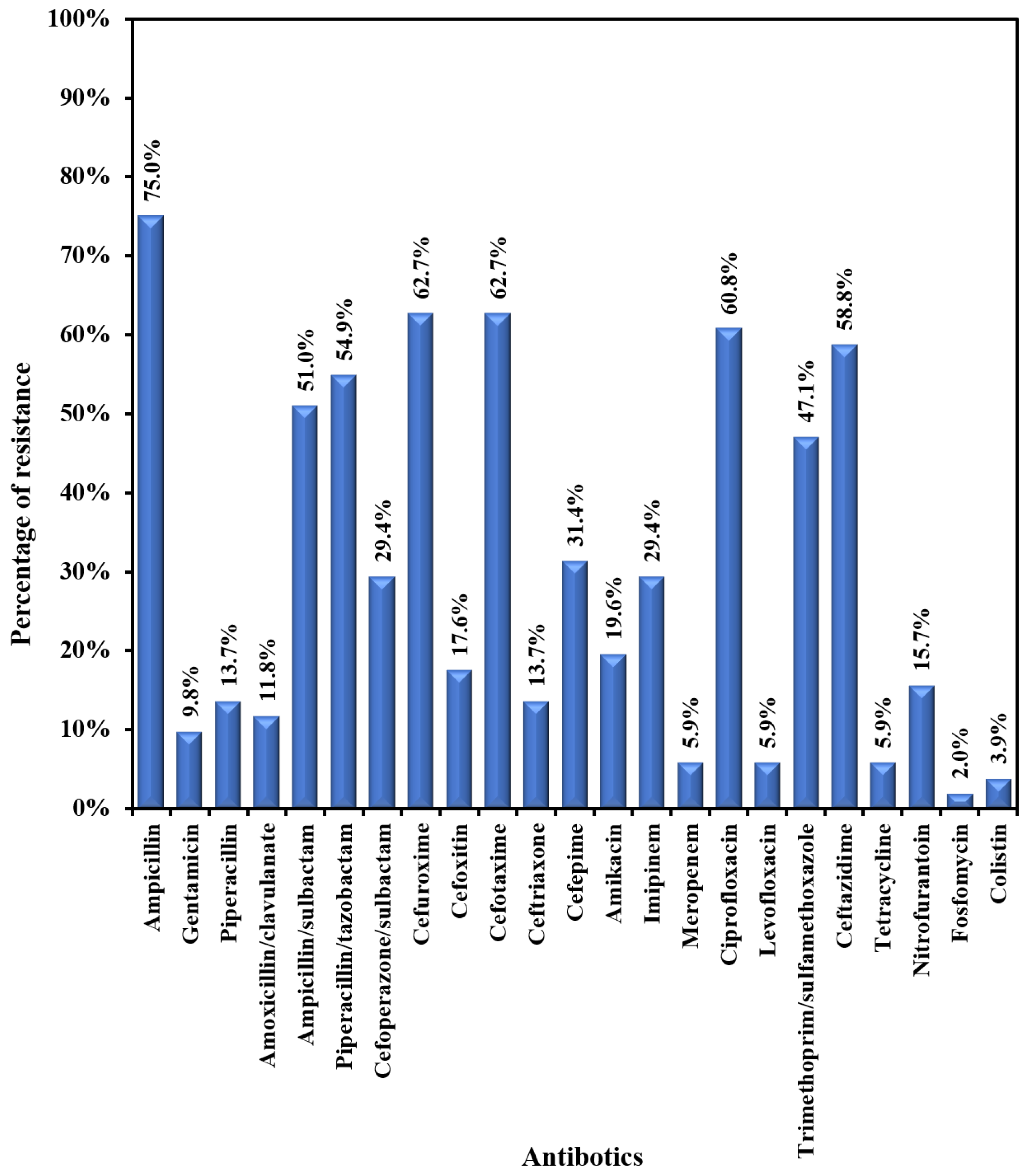
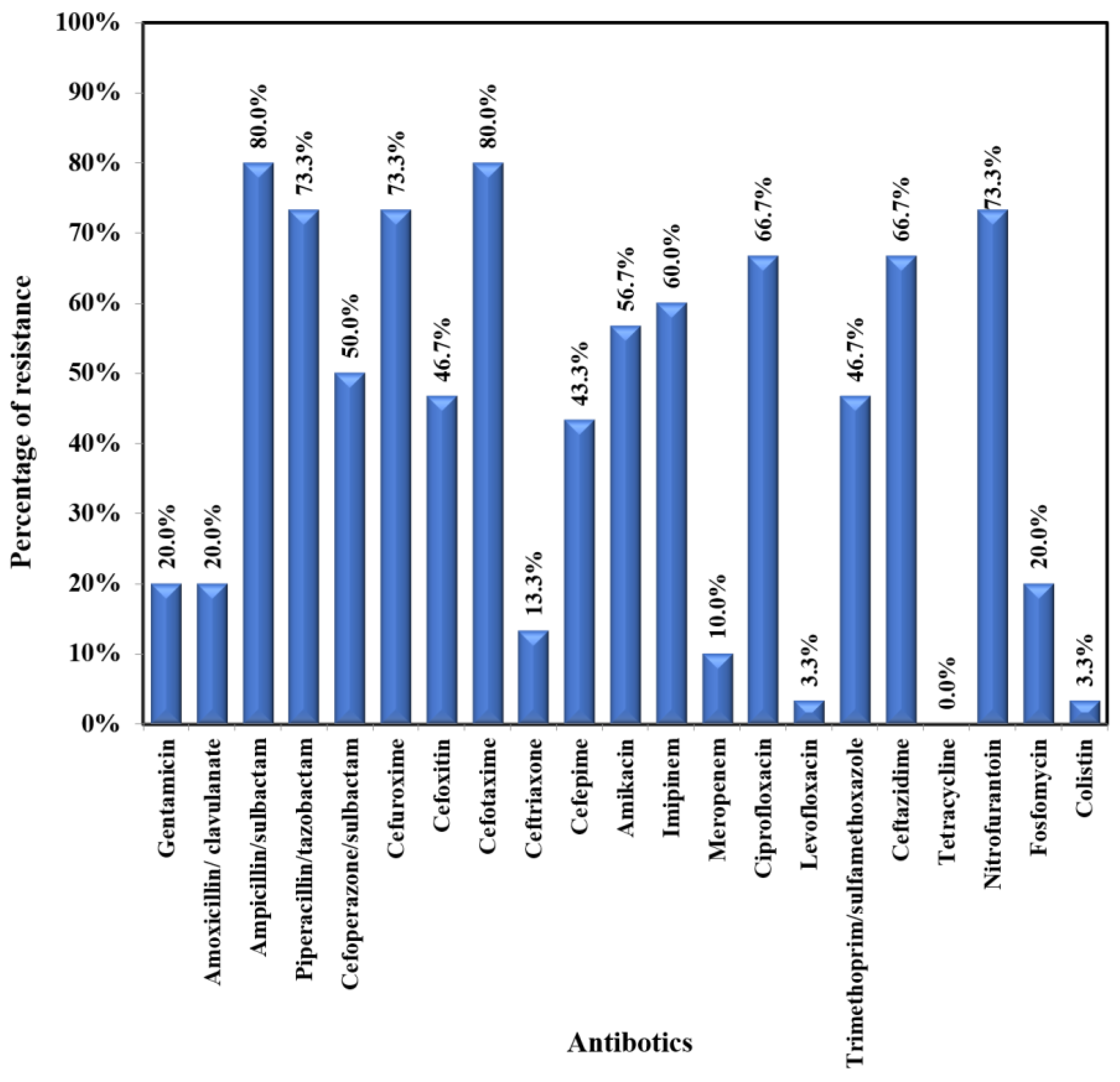
E. coli showed moderate to high resistance to most of the tested antibiotics (47.1-75.5%) – Figure 3A. The lowest resistance was exhibited towards the following antibiotics: fosfomycin (2%); colistin (3.9%); meropenem (5.9%); levofloxacin (5.9%); gentamicin (9.8%); amoxicillin/clavulanic acid (11.8%); ceftriaxone (13.7%); and nitrofurantoin (15.7%). Klebsiella pneumoniae exhibited a very high rate of resistance (43.3%-80%) to most tested antibiotics (Figure 3B). The lowest rates of resistance were recorded for colistin (3.3%), levofloxacin (3.3), ceftriaxone (13.3%), and meropenem (10.0%). Pseudomonas aeruginosa was susceptible to most of the tested antibiotics, with moderate resistance (14.3%-28.6%) to the following antibiotics: imipenem, ciprofloxacin, ceftazidime, aminoglycosides, and cefepime.
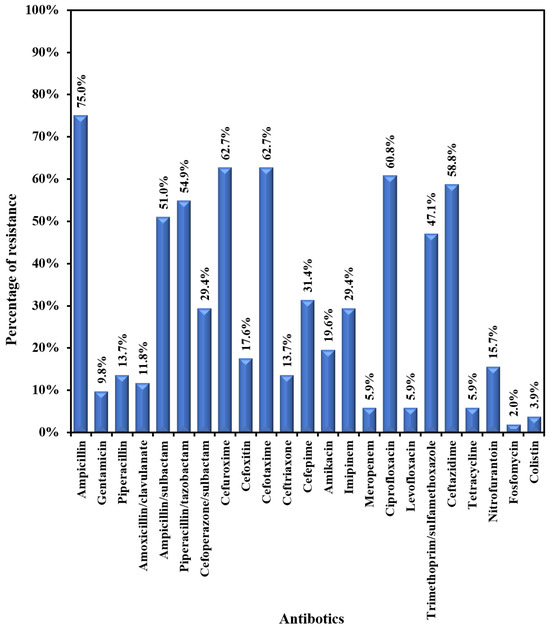
Figure 3A.
Antibiogram of E. coli.
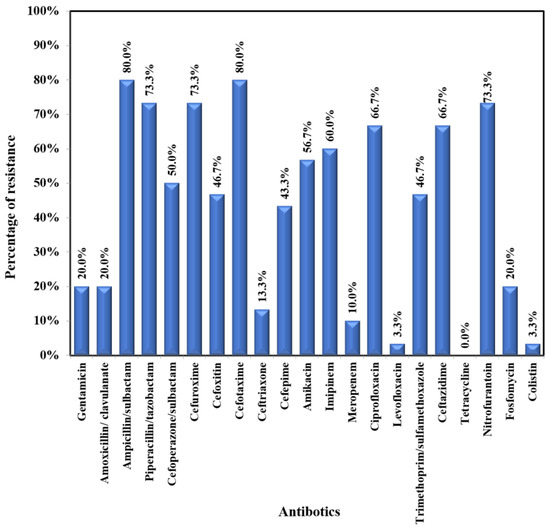
Figure 3B.
Antibiogram of Klebsiella pneumoniae.
Discussion
The current study aimed to estimate the prevalence of bacteremia and common organisms causing febrile urinary tract infections and their resistance patterns to help guide empiric antibiotic treatment. Concomitant bacteremia in UTIs is associated with a longer hospital stay and higher morbidity and mortality rates in adults and children [13]. As a result, many studies have been designed to identify early predictors of concomitant bacteremia. One of the most concerning findings in the current study is the high prevalence of bacteremia (63.0% (n=65)) among children with febrile UTIs. Other studies detected a lower rate of bacteremia (5.7%-17%) [13,15,17]. The current study’s high prevalence of bacteremic UTIs could be explained by the high prevalence of resistant organisms causing bacteremic UTIs; among the bacteremic group, 42.6% (n=42) of organisms were MDR and 18.2% (n=12) were XDR. Another explanation is that the younger the age, the higher the prevalence of bacteremia, as reported by other studies [14,15]. In the current study, 47.7% (n=31) and 46.2% (n=30) of children with bacteremia were aged 12-36 months and younger than 12 months, respectively. The young age implies a hematogenous spread of infection due to the immaturity of the immune system in this age group. Lastly, as we are in a tertiary care referral hospital and most of the referred cases are complicated, after the trial of outpatient treatment.
The median temperature of 38.40 °C (IQR 38.15-38.60) and median creatinine level of 0.18 mg/dL (IQR 0.14-0.25) were significantly higher in bacteremic children, with p values of 0.005 and 0.034, respectively. Although the median WBC count of 13.00 (×103)/µL (IQR 10.00-16.00) was higher among the bacteremic group, this was not statistically significant (p=0.098). Comparable results were reported in several studies. Megged et al. [9] reported significantly higher creatinine in children with bacteremic UTI, and although the mean WBC (16.2±7.2) was higher, this was not statistically significant (p=0.17). Similar findings have been reported in other studies [16,17]. Elevated creatinine levels among children with bacteremia could be explained by renal hypoperfusion and dehydration due to poor oral feeding and vomiting.
In the current study, Gram-negative bacteria were the most isolated uropathogens, in 90.3% (n=93) of the cases. E. coli accounted for 49.5% of UTIs (n=51), Klebsiella pneumoniae 29.1% (n=30), Enterococcus faecalis 9.7% (n=10), Pseudomonas aeruginosa 6.8% (n=7), and Citrobacter 1.9% (n=2). This was consistent with the findings of numerous other studies [18,19,20,21]. The only Gram-positive organism isolated was Enterococcus faecalis (9.7%, n=10). Unlike in other studies, Staphylococcus aureus was identified as the causative agent of UTI in Ethiopia (27.3%) [19] and Nepal (7%) [21]. Uropathogens that cause UTIs vary depending on age, geographic location, and concomitant co-morbidities. Accordingly, conducting an epidemiological study to guide antibiotic therapy based on local antibiograms and prevalent causative agents is crucial.
Antibiotic resistance is an important problem that is acknowledged globally. It is essential to understand how antimicrobial susceptibility patterns change over time in a particular region to control the use of antibiotics, identify and manage antimicrobial resistance, and effectively treat bacterial infections. One of the most striking results in the current study is the alarming increase in the resistant organisms, as 66.0% were MDR, followed by XDR in 15.6%, and PDR organisms in 1% of isolates. Overall, 91.3% of the investigated children were under three years old, which may help to explain the high incidence of resistant organisms. The immaturity of the immune system in young infants makes them vulnerable to multiple febrile viral episodes with subsequent exposure to multiple unnecessary antibiotic courses in primary care. Therefore, the establishment of an antimicrobial stewardship program is crucial. Concordant results were found in Ethiopia [19]. The estimated MDR isolates among Gram-negative bacteria were 61%, while 22.4% and 4% of the Gram-negative organisms were XDR and PDR, respectively. Parajuli et al. [22] and Merga Duffa et al. [23] found MDR organisms in 64.9% and 73.7% of their samples, respectively.
In contrast, with a lower prevalence rate in Nepal [21], MDR and XDR organisms were detected in 32% and 5% respectively. ESBL-producing organisms have emerged as a challenge in treating community-acquired UTIs [24]. In our series, ESBL represented 5.8% (n=6) of isolates in E. coli (7.8% (n=4)) and Klebsiella pneumoniae (6.7% (n=2)). In contrast to the current results, Shrestha et al. (40%) [21], and Parajuliuli et al. (38%) [22], both found a higher rate of ESBL. Differences in the resistance pattern of organisms emphasize the importance of routine surveillance to help establish local guidelines for treating such cases.
Focusing on E. coli and Klebsiella pneumoniae antibiograms, both showed low resistance to fosfomycin (2% and 20%, respectively) and amoxicillin-clavulanate (11.8% and 20%, respectively). Therefore, these antibiotics can be used as an initial treatment for uncomplicated UTIs. Furthermore, ceftriaxone and gentamicin demonstrated lower resistance among E. coli (13.7% and 9.8%) and Klebsiella pneumoniae (13.3% and 20%) isolates, supporting their use as empiric treatment for febrile UTIs in immunocompetent children. Both exhibited low resistance to meropenem (5.9% and 10%, respectively). On the other hand, resistance to nitrofurantoin was higher in K. pneumoniae versus E. coli (73.3% versus 15.7%), discouraging its use as an empiric oral therapy for uncomplicated UTI. Similar outcomes were seen in Tanzania [25] and Turkey [26]. Both exhibited a high rate of resistance to trimethoprim-sulfamethoxazole, so it couldn’t be used as empiric therapy for UTI.
The current study has some limitations: first, it is a single-center experience; additional studies on different cohorts are required to establish a guide for antimicrobial prescription in children with febrile UTI. Second, there is a lack of radiological evaluation of children with concurrent bacteremia to identify any undiagnosed underlying urological problems.
Conclusions
There is an alarming increase in the resistance pattern of organisms causing febrile UTIs therefore regular surveillance should be carried out to guide the management of such cases and establish an antimicrobial stewardship program. Low resistance to ceftriaxone and gentamicin encourages their use as empiric therapy in children with febrile UTIs who have no known co-morbidities. Concomitant bacteremia among children with febrile UTIs was high. Children with concomitant bacteremia had significantly higher creatinine levels and a high degree of fever.
Author Contributions
HH is responsible for the revision of data, its analysis, and the revision of the manuscript. MM was responsible for the laboratory tests, cultures, susceptibility patterns, and revision of the results. AF was responsible for data collection after the consent of the parents and recording, statistical processing of data, and interpretation. EH was responsible for the main idea of the research, following up on the data collection and preparation, revision of statistical results, and drafting and finalization of the manuscript. All authors reviewed and approved the final version of the manuscript.
Funding
None to declare.
Institutional Review Board Statement
The study was approved by the Research Ethics Committee (IRB: 00012098 – FWA: 00018699) at Alexandria University, Faculty of Medicine in August 2021. Informed consent was obtained from all patient caregivers with ascertainment of the confidentiality of personal data.
Data Availability Statement
All data generated during this study are included in this published article (and its tables and figure).
Acknowledgments
The authors acknowledge all children and their parents for their contribution to the current study.
Conflicts of Interest
All authors – none to declare.
References
- Desai, S.; Aronson, P.L.; Shabanova, V.; et al. Parenteral antibiotic therapy duration in young infants with bacteremic urinary tract infections. Pediatrics 2019, 144, e20183844. [Google Scholar] [CrossRef] [PubMed]
- Goeller, C.; Desmarest, M.; Garraffo, A.; Bonacorsi, S.; Gaschignard, J. Management of febrile urinary tract infection with or without bacteraemia in children: A French case-control retrospective study. Front Pediatr 2020, 8, 237. [Google Scholar] [CrossRef]
- Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management; Roberts, K.B. Urinary tract infection: Clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 2011, 128, 595–610. [Google Scholar] [CrossRef]
- Kaufman, J.; Temple-Smith, M.; Sanci, L. Urinary tract infections in children: An overview of diagnosis and management. BMJ Paediatr Open 2019, 3, e000487. [Google Scholar] [CrossRef]
- Tullus, K. Fifteen-minute consultation: Why and how do children get urinary tract infections? Arch Dis Child Educ Pract Ed 2019, 104, 244–247. [Google Scholar] [CrossRef]
- Korbel, L.; Howell, M.; Spencer, J.D. The clinical diagnosis and management of urinary tract infections in children and adolescents. Paediatr Int Child Health 2017, 37, 273–279. [Google Scholar] [CrossRef]
- Megged, O. Bacteremic vs nonbacteremic urinary tract infection in children. Am J Emerg Med 2017, 35, 36–38. [Google Scholar] [CrossRef]
- Tille, P. Bailey and Scott’s Diagnostic Microbiology, 15th ed.; Mosby Elsevier: St Louis, Missouri, 2022. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing, 31st ed.; CLSI supplement M100; CLSI: Wayne, Pennsylvania, USA, 2021. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; et al. Multidrug-resistant, extensively drug-resistant, and pan drug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Schnadower, D.; Kuppermann, N.; Macias, C.G.; et al. Febrile infants with urinary tract infections at very low risk for adverse events and bacteremia. Pediatrics 2010, 126, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, Y.S.; Jeong, R.; Kim, Y.J.; Ahn, S. Predictive factors of bacteremia in patients with febrile urinary tract infection: An experience at a tertiary care center. Infection 2014, 42, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Averbuch, D.; Nir-Paz, R.; Tenenbaum, A.; et al. Factors associated with bacteremia in young infants with urinary tract infection. Pediatr Infect Dis J 2014, 33, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Shin, H.; Lee, K.H.; et al. Predictive factors for bacteremia in febrile infants with urinary tract infection. Sci Rep 2020, 10, 4469. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, T.; Mishima, Y.; Matsuda, N.; et al. Clinical characteristics of pediatric febrile urinary tract infection in Japan. Int J Infect Dis 2021, 104, 97–101. [Google Scholar] [CrossRef]
- Kim, D.; Lee, S.H.; Tchah, H.; Ryoo, E.; Cho, H.K.; Kim, Y.M. Association between elevated alanine aminotransferase and urosepsis in children with acute pyelonephritis. Pediatr Gastroenterol Hepatol Nutr 2016, 19, 54–60. [Google Scholar] [CrossRef]
- Hameed, T.; Al Nafeesah, A.; Chishti, S.; Al Shaalan, M.; Al Fakeeh, K. Community-acquired urinary tract infections in children: Resistance patterns of uropathogens in a tertiary care center in Saudi Arabia. Int J Pediatr Adolesc Med 2019, 6, 51–54. [Google Scholar] [CrossRef]
- Fenta, A.; Dagnew, M.; Eshetie, S.; Belachew, T. Bacterial profile, antibiotic susceptibility pattern and associated risk factors of urinary tract infection among clinically suspected children attending at Felege-Hiwot comprehensive and specialized hospital, Northwest Ethiopia. A prospective study. BMC Infect Dis 2020, 20, 673. [Google Scholar] [CrossRef]
- Demir, M.; Kazanasmaz, H. Uropathogens and antibiotic resistance in the community and hospital-induced urinary tract infected children. J Glob Antimicrob Resist 2020, 20, 68–73. [Google Scholar] [CrossRef]
- Falup-Pecurariu, O.; Leibovitz, E.; Vorovenci, C.; et al. First UTI episode in life in infants <1 year of age: Epidemiologic, clinical, microbiologic and disease recurrence characteristics. Pediatr Neonatol 2020, 61, 613–619. [Google Scholar] [CrossRef]
- Shrestha, L.B.; Baral, R.; Poudel, P.; Khanal, B. Clinical, etiological, and antimicrobial susceptibility profile of pediatric urinary tract infections in a tertiary care hospital of Nepal. BMC Pediatr 2019, 19, 36. [Google Scholar] [CrossRef]
- Parajuli, N.P.; Maharjan, P.; Parajuli, H.; et al. High rates of multidrug resistance among uropathogenic Escherichia coli in children and analyses of ESBL producers from Nepal. Antimicrob Resist Infect Control 2017, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Merga Duffa, Y.; Terfa Kitila, K.; Mamuye Gebretsadik, D.; Bitew, A. Prevalence and antimicrobial susceptibility of bacterial uropathogens isolated from pediatric patients at Yekatit 12 Hospital Medical College, Addis Ababa, Ethiopia. Int J Microbiol 2018, 2018, 8492309. [Google Scholar] [CrossRef]
- Horie, A.; Nariai, A.; Katou, F.; et al. Increased community-acquired upper urinary tract infections caused by extended-spectrum beta-lactamase-producing Escherichia coli in children and the efficacy of flomoxef and cefmetazole. Clin Exp Nephrol 2019, 23, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Sangeda, R.Z.; Paul, F.; Mtweve, D.M. Prevalence of urinary tract infections and antibiogram of uropathogens isolated from children under five attending Bagamoyo District Hospital in Tanzania: A cross-sectional study. F1000Research 2021, 10, 449. [Google Scholar] [CrossRef]
- Gunduz, S.; Uludağ Altun, H. Antibiotic resistance patterns of urinary tract pathogens in Turkish children. Glob Health Res Policy 2018, 3, 10. [Google Scholar] [CrossRef] [PubMed]
© GERMS 2023.