Antimicrobials are among the main breakthroughs of contemporary medicine. The advent of antibiotic treatment in the 20th century has saved countless lives and has added over 23 years to the average life expectancy [1].
However, there is a fine line between antimicrobial use and misuse, the latter including both overtreatment and undertreatment. While inappropriate use of any drug may have unintended negative consequences on the patient, inappropriate use of antimicrobials can have a domino effect, affecting both the patient and other people, by transmission of resistant microorganisms either directly or through the environment. For this reason, every effort should be made to optimize the use of antimicrobials.
In dental practice, there are only a few clear indications for prophylactic or therapeutic antibiotic use. The hallmark of antibiotic prophylaxis refers to patients at high risk of infective endocarditis (IE) who undergo specific bleeding-prone oro-dental procedures. This type of prophylactic use is mandated by cardiologists in their specialty-specific guidelines, such as those of the American Heart Association (AHA) [2] as well as their joint statement with the American College of Cardiology (ACC) [3] for the USA. Different other countries have also developed their national guidelines or consensus statements, and such examples include Japan [4], Turkey [5], Croatia [6], to name only a few. In Europe, the best known and most used guidelines are those of the European Society of Cardiology (ESC), recently revised in 2023 to better define which patients are at high risk, and which dental procedures pose higher risk [7].
Treatment, however, is much less standardized, and should be reserved for the few dental conditions that are indeed associated with infection, i.e., abscesses, suppurations, cellulitis, phlegmons, and should be withheld in conditions that are mainly inflammatory in nature. Unfortunately, not much standardization is available and in the absence of a general consensus for each dental condition, the decision to prescribe has to be made for each individual case based on the existence of local and/or systemic signs or symptoms suggestive of infection.
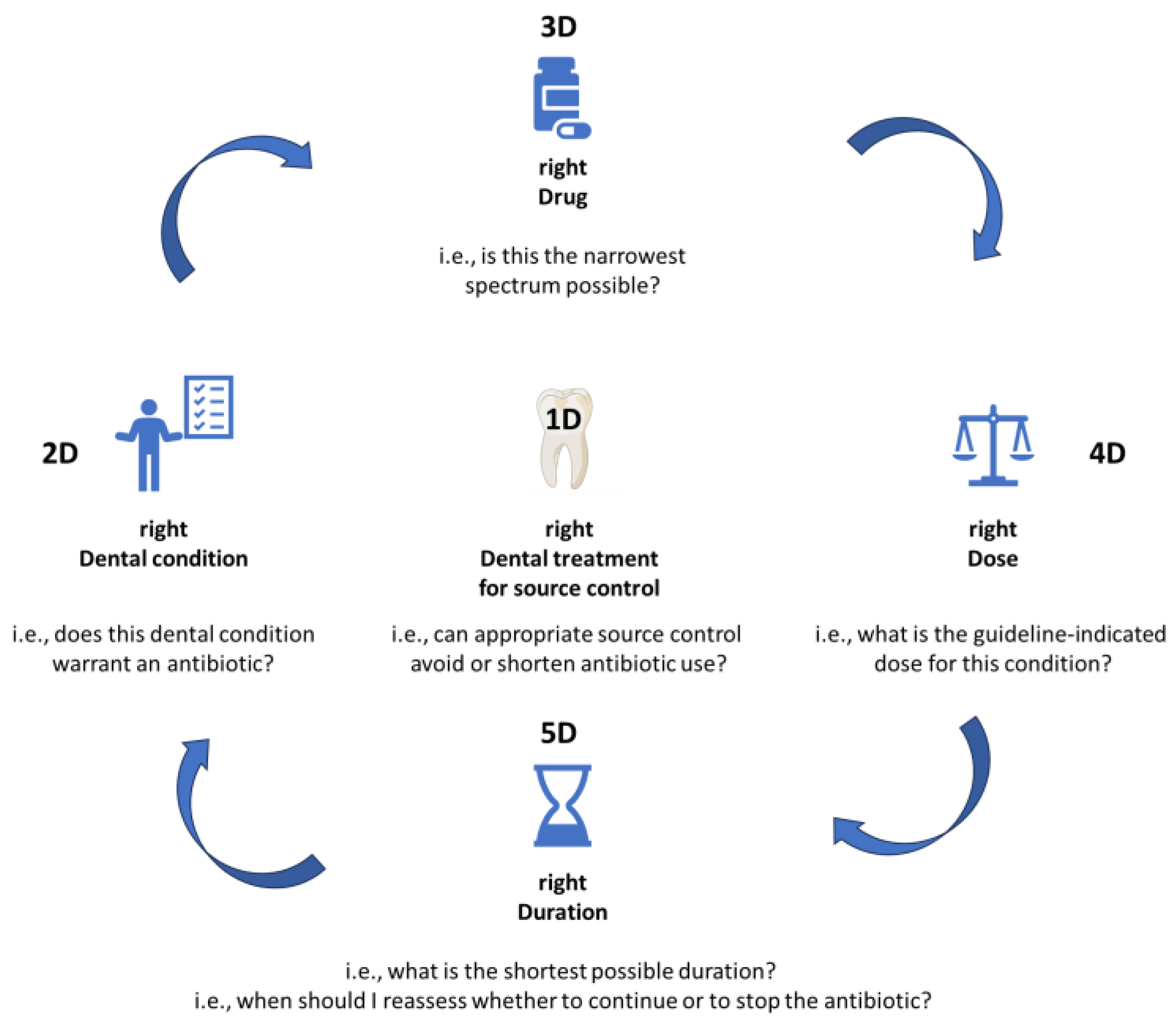
The 4Ds of optimal antimicrobial prescription have been described more than 15 years ago by Joseph et al. but are still very much true to this day. These represent: choosing the right Drug, in the right Dose, for the right Duration, with De-escalation to pathogen-directed therapy as soon as possible [8].
We hereby propose a slight adaptation for dental medicine (Figure 1), in order to fully address all steps needed to optimize antimicrobial prescription in this field of practice. The proposed adaptation includes the 5Ds described below.
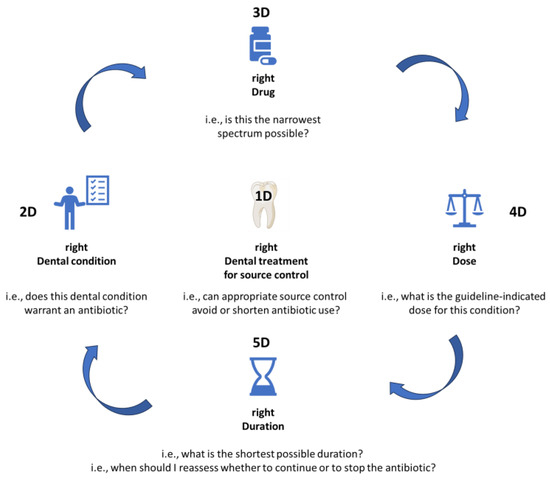
Figure 1.
The 5Ds of optimized antimicrobial prescription in dental medicine.
1D. right Dental treatment (source control)
In many instances, a correct dental treatment will allow full resolution of the dental condition, even in the absence of antibiotic treatment [9]. For many clinical conditions, antibiotics can be avoided by performing prompt source control. Even in situations where an antibiotic is warranted, its required duration of administration will be much shorter if concurrent dental treatment is also performed.
This is the first and the most essential step of the rational antibiotic prescribing practice, and antibiotics should never be prescribed without first considering what type of dental treatment is warranted, and performing appropriate source control.
2D. right Dental condition (indication)
The next step in the clinical reasoning process is establishing whether or not an indication to prescribe an antibiotic truly exists for the patient’s Dental condition. If the answer is “No”, then in most cases the process will be stopped here. If the answer is “Yes”, then the pathway will be continued to address the following Ds.
3D. right Drug (antibiotic choice)
If an antibiotic is indeed warranted, the right Drug should be chosen, by asking the question: which is the narrowest spectrum antibiotic that will offer cover for the pathogens most likely involved in this infectious process?
4D. right Dose
Depending on the site of infection, a different dose or a different frequency of administration might be warranted to ensure penetration and adequate levels in the infectious site. In particular, “adequate levels” should not be confused with “high levels”, as high antibiotic doses may be just as deleterious as suboptimal levels. Whenever possible, condition-specific guidelines should be consulted if they exist, to check the exact dose for each particular clinical indication.
4D. right Duration
Treatment duration should be established for each individual case, based on the type of infection, and the extent to which source control was obtained through dental treatment. In all cases, the shortest possible duration should be selected, as every extra dose of antibiotic leads to cumulated resistance pressure. Treatment duration should be based on guideline indications, but also on clinical response. Quite often, an antibiotic can be stopped as soon as source control has been appropriately performed, and the general trend in all fields of medicine is to gradually validate shorter treatment durations for most clinical indications, i.e., 3 days instead of 8 for pneumonia [10], or 7 days instead of 14 for Gram-negative bacteremia [11,12].
To ensure that antibiotic treatment is stopped as soon as clinically warranted, it is essential for the prescriber to reevaluate the patient every few days to identify stopping queues in timely manner.
Conclusions
Antibiotic prescription is a delicate process that requires fine tuning and clinical reasoning at all steps. In all instances, it is essential to make sure that for each prescription, the antibiotic is both needed and appropriately administered. We propose a prescription algorithm based on the adapted 5Ds to ensure optimized antimicrobial use in dentistry.
Funding
None to declare.
Conflicts of Interest
All authors – none to declare.
References
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: past, present and future. Curr Opin Microbiol 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.R.; Gewitz, M.; Lockhart, P.B.; et al. Prevention of viridans group streptococcal infective endocarditis: a scientific statement from the American Heart Association. Circulation 2021, 143, e963–e978. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; et al. 2020 ACC/AHA Guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72-227. [Google Scholar] [CrossRef]
- Nakatani, S.; Ohara, T.; Ashihara, K.; et al. JCS 2017 Guideline on prevention and treatment of infective endocarditis. Circ J 2019, 83, 1767–1809. [Google Scholar] [CrossRef] [PubMed]
- Şimşek-Yavuz, S.; Rüçhan Akar, A.; Aydoğdu, S.; et al. Diagnosis, treatment and prevention of infective endocarditis: Turkish consensus report-2019. Turk Kardiyol Dern Ars 2020, 48, 187–226. [Google Scholar] [CrossRef] [PubMed]
- Šutej, I.; Par, M.; Lepur, D.; et al. Dentists’ practice and compliance with current guidelines of infective endocarditis prophylaxis- National survey study. J Clin Exp Dent 2021, 13, e648–e652. [Google Scholar] [CrossRef] [PubMed]
- Delgado, V.; Marsan, N.; de Waha, S.; et al. 2023 ESC Guidelines for the management of endocarditis: Developed by the task force on the management of endocarditis of the European Society of Cardiology (ESC) Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS) and the European Association of Nuclear Medicine (EANM). Eur Heart J 2023, ehad193. [Google Scholar] [CrossRef]
- Joseph, J.; Rodvold, K.A. The role of carbapenems in the treatment of severe nosocomial respiratory tract infections. Expert Opin Pharmacother 2008, 9, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, P.B.; Tampi, M.P.; Abt, E.; et al. Evidence-based clinical practice guideline on antibiotic use for the urgent management of pulpal- and periapical-related dental pain and intraoral swelling: A report from the American Dental Association. J Am Dent Assoc 2019, 150, 906–921.e12. [Google Scholar] [CrossRef] [PubMed]
- Dinh, A.; Ropers, J.; Duran, C.; et al. Discontinuing β-lactam treatment after 3 days for patients with community-acquired pneumonia in non-critical care wards (PTC): a double-blind, randomised, placebo-controlled, non-inferiority trial. Lancet 2021, 397, 1195203. [Google Scholar] [CrossRef] [PubMed]
- Yahav, D.; Franceschini, E.; Koppel, F.; et al. Seven versus 14 days of antibiotic therapy for uncomplicated Gram-negative bacteremia: a noninferiority randomized controlled trial. Clin Infect Dis 2019, 69, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Turjeman, A.; von Dach, E.; Molina, J.; et al. Duration of antibiotic treatment for Gram-negative bacteremia—Systematic review and individual participant data (IPD) meta-analysis. EClinicalMedicine 2023, 55, 101750. [Google Scholar] [CrossRef] [PubMed]
© GERMS 2023.