Prevalence of Multidrug-Resistant Gram-Negative Bacteria From Blood Cultures and Rapid Detection of Beta-Lactamase-Encoding Genes by Multiplex PCR Assay
Abstract
Introduction
Methods
Isolation and identification of GNB from blood cultures
Antimicrobial susceptibility testing and the detection of ESBLs
Detection of beta-lactamases-encoding genes by multiplex PCR assay
Classification of isolates
Results
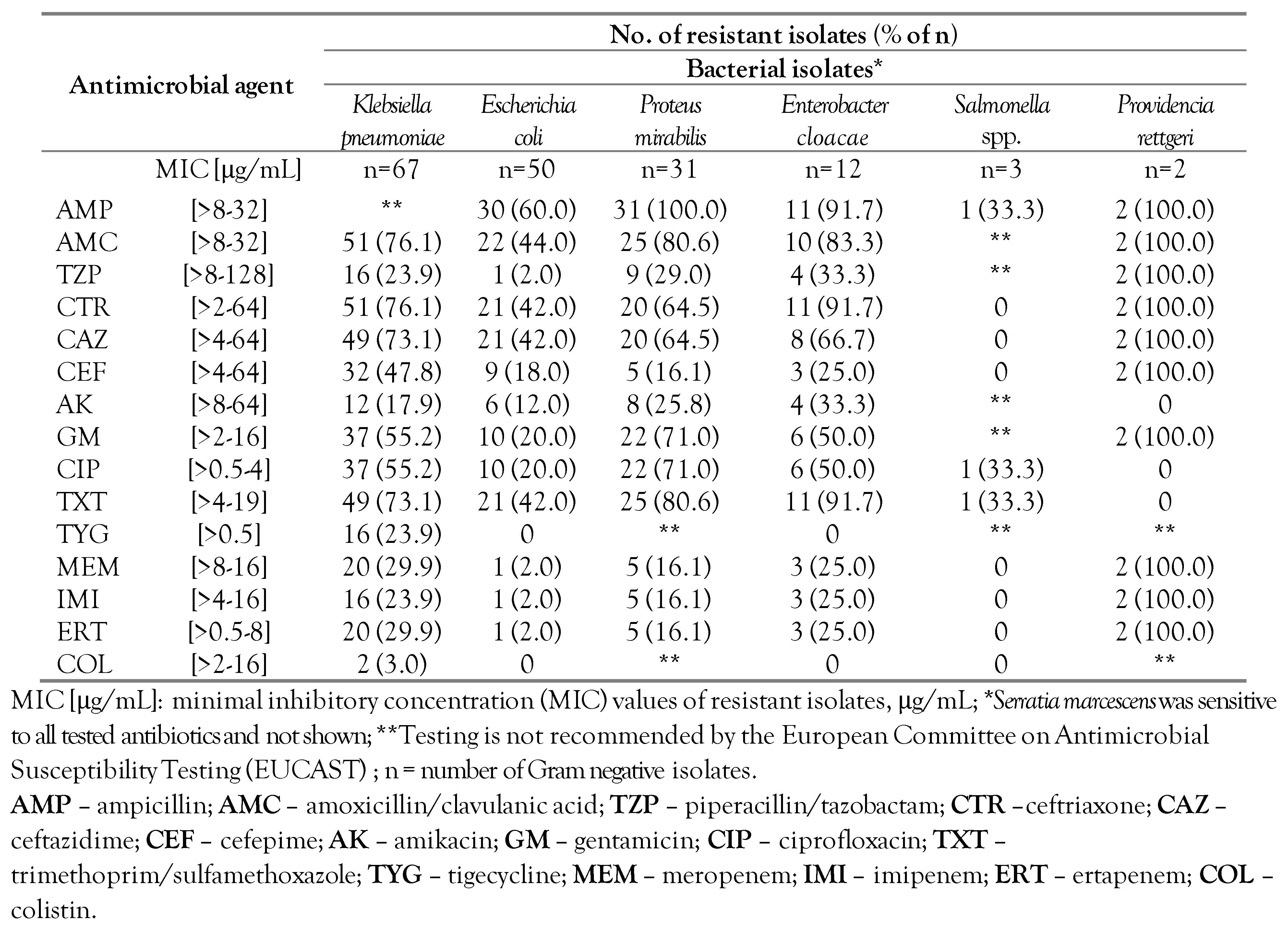
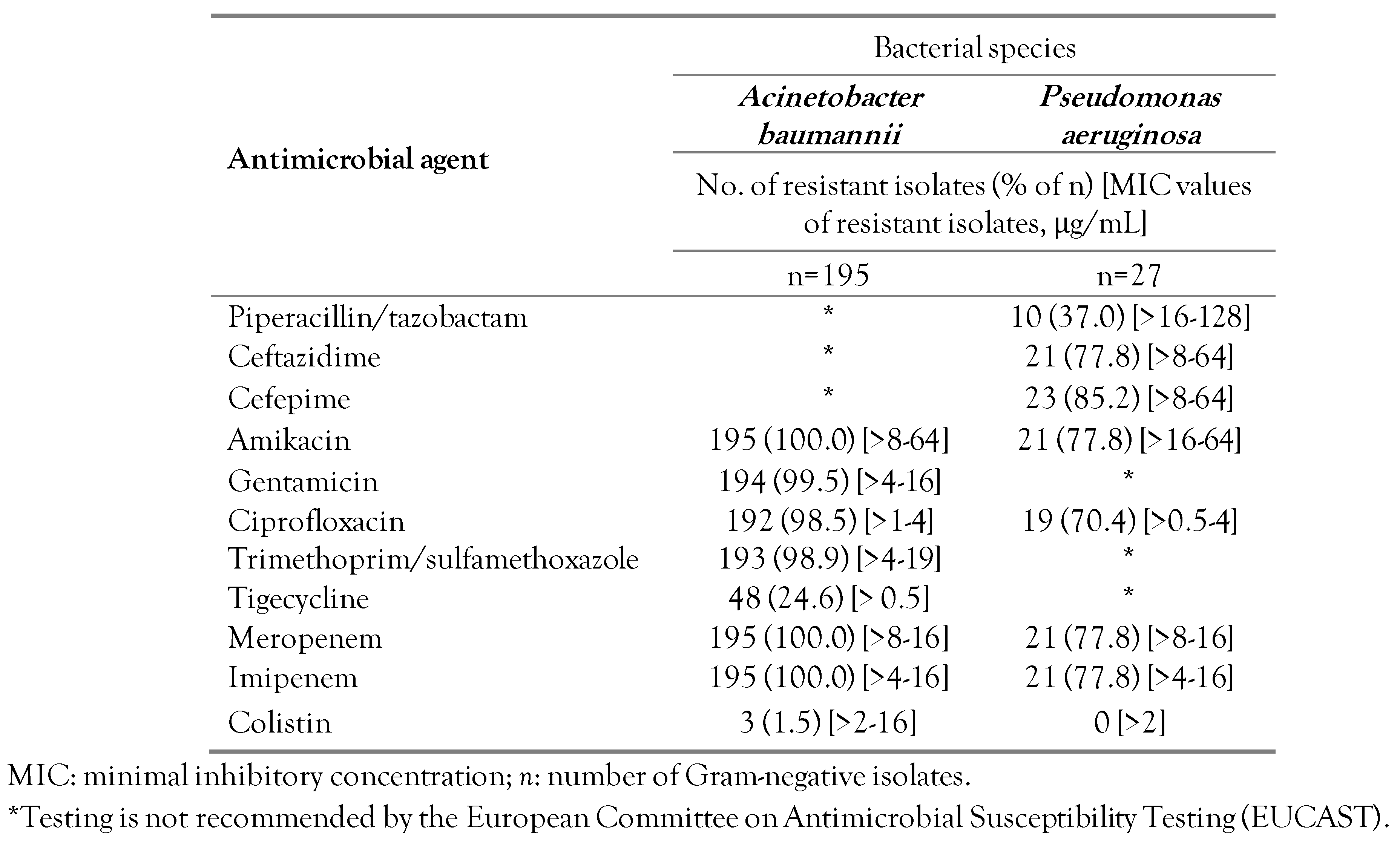
Antimicrobial resistance
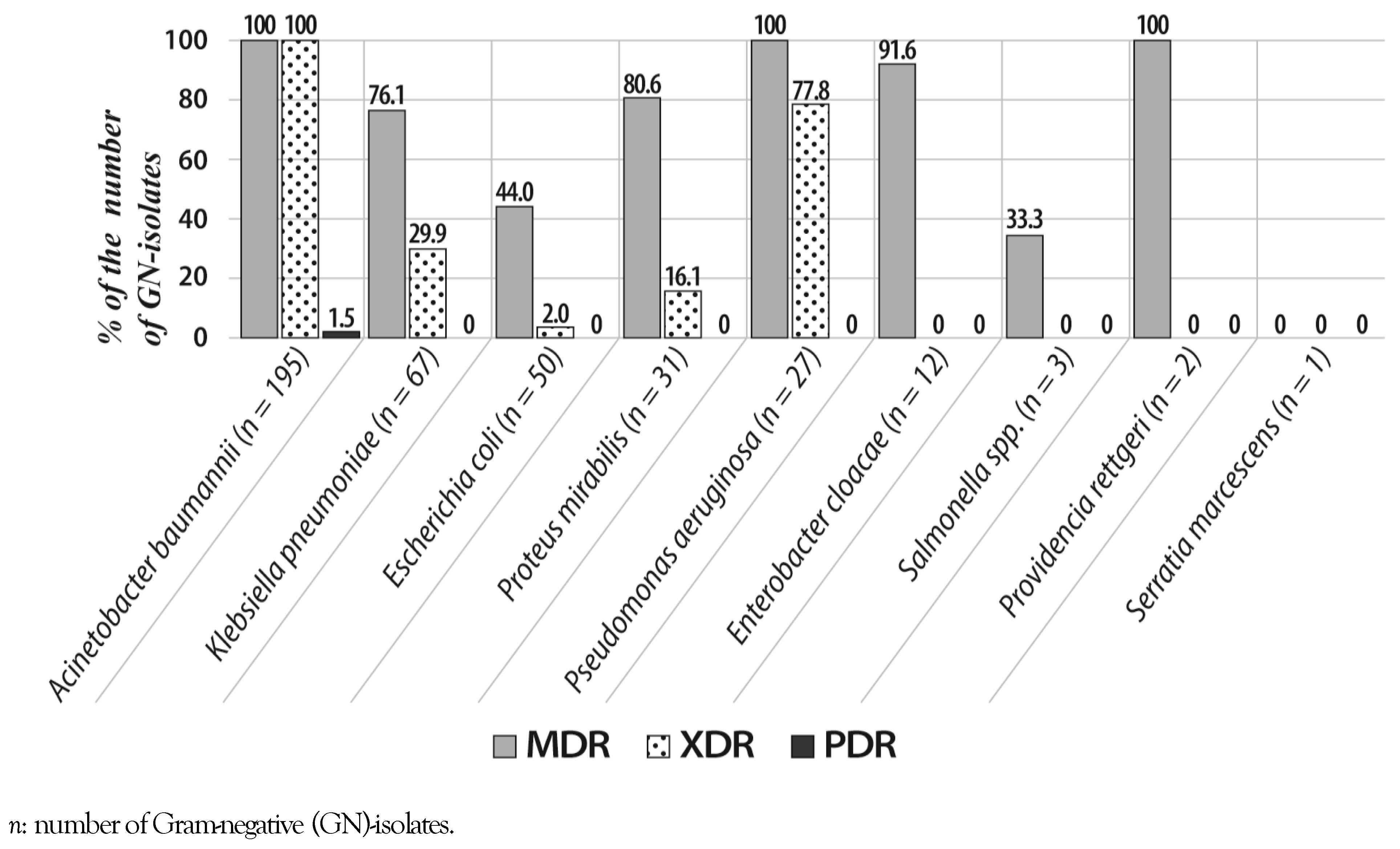
Prevalence of MDR, XDR and PDR isolates
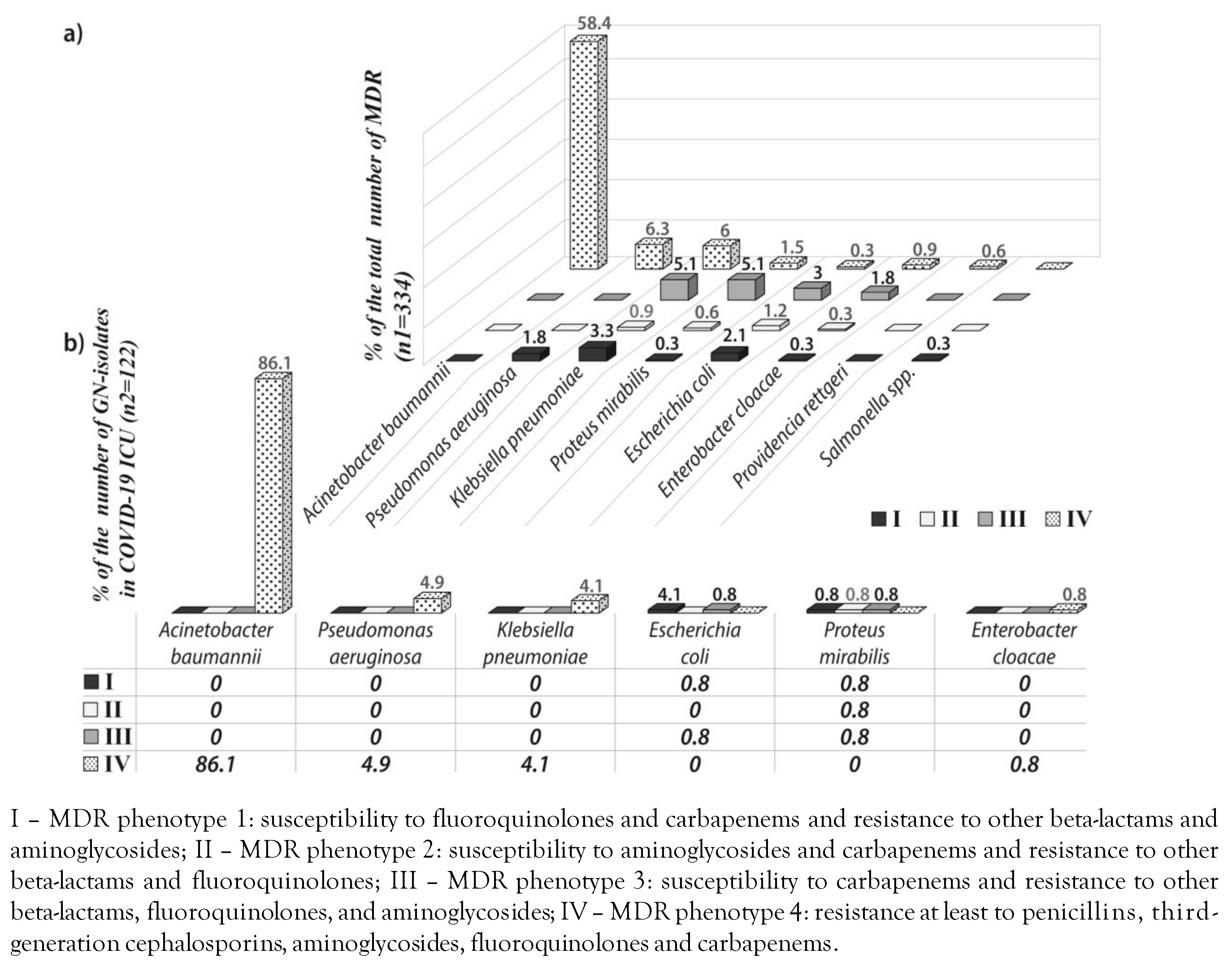
MDR phenotypes and the distribution of GNB species in various hospital wards
Evaluation of beta-lactamase-encoding genes detection and ESBLs production
Discussion
Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of interest
References
- World Health Organization. Antimicrobial resistance. World Health Organization (WHO), Geneva, 2021. Available online: https://www.who.int/health-topics/antimicrobial-resistance (accessed on 17 October 2022).
- Karvouniaris, M.; Poulakou, G.; Tsiakos, K.; et al. ICU-associated Gram-negative bloodstream infection: risk factors affecting the outcome following the emergence of colistin-resistant isolates in a regional Greek Hospital. Antibiotics (Basel). 2022, 11, 405. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Bradford, P.A. Interplay between β-lactamases and new β-lactamase inhibitors. Nat Rev Microbiol. 2019, 17, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; Sanguinetti, M.; Montuori, E.; et al. Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob Agents Chemother. 2007, 51, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Timsit, J.F.; Ruppé, E.; Barbier, F.; Tabah, A.; Bassetti, M. Bloodstream infections in critically ill patients: an expert statement. Intensive Care Med. 2020, 46, 266–284. [Google Scholar] [CrossRef] [PubMed]
- Feretzakis, G.; Loupelis, E.; Sakagianni, A.; et al. A 2-year single-centre audit on antibiotic resistance of Pseudomonas aeruginosa, Acinetobacter baumannii and Klebsiella pneumoniae strains from an intensive care unit and other wards in a general public hospital in Greece. Antibiotics (Basel). 2019, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Giannella, M.; Bussini, L.; Pascale, R.; et al. Prognostic utility of the new definition of difficult-to-treat resistance among patients with Gram-negative bloodstream infections. Open Forum Infect Dis. 2019, 6, ofz505. [Google Scholar] [CrossRef] [PubMed]
- Buetti, N.; Ruckly, S.; de Montmollin, E.; et al. COVID-19 increased the risk of ICU-acquired bloodstream infections: a case-cohort study from the multicentric OUTCOMEREA network. Intensive Care Med. 2021, 47, 180–187. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Antimicrobial Susceptibility Testing. EUCAST breakpoint tables for interpretation of MICs and zone diameters, version 10.0. The European Committee on Antimicrobial Susceptibility Testing (EUCAST), 2022. Available online: https://www.eucast.org/clinical_breakpoints/ (accessed on 17 October 2022).
- European Committee on Antimicrobial Susceptibility Testing. EUCAST reading guide for broth microdilution. The European Committee on Antimicrobial Susceptibility Testing (EUCAST), 2022. Available online: https://www.eucast.org/ast_of_bacteria/mic_determination/ (accessed on 17 October 2022).
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Plachouras, D.; Eckmanns, T.; et al. Burden of six healthcare-associated infections on European population health: estimating incidence-based disability-adjusted life years through a population prevalence-based modelling study. PLoS Med. 2016, 13, e1002150. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Central Asian and European surveillance of antimicrobial resistance network (CAESAR). Annual report 2019. World Health Organization (WHO): Geneva, 2020. Available online: https://apps.who.int/iris/handle/10665/345873 (accessed on 17 October 2022).
- European Centre for Disease Prevention and Control. Surveillance of antimicrobial resistance in Europe. Annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) 2019. European Centre for Disease Prevention and Control (ECDC): Stockholm; 2020.
- World Health Organization (WHO) Regional Office for Europe/European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2022 – 2020 data. Copenhagen: WHO Regional Office for Europe; 2022.
- Grundmann, H.; Glasner, C.; Albiger, B.; et al. European Survey of Carbapenemase-Producing Enterobacteriaceae (EuSCAPE) Working Group. Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): a prospective, multinational study. Lancet Infect Dis. 2017, 17, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Ripabelli, G.; Sammarco, M.L.; Scutellà, M.; Felice, V.; Tamburro, M. Carbapenem-resistant KPC- and TEM-producing Escherichia coli ST131 isolated from a hospitalized patient with urinary tract infection: first isolation in Molise Region, Central Italy, July 2018. Microb Drug Resist. 2020, 26, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Mairi, A.; Pantel, A.; Sotto, A.; Lavigne, J.P.; Touati, A. OXA-48-like carbapenemases producing Enterobacteriaceae in different niches. Eur J Clin Microbiol Infect Dis. 2018, 37, 587–604. [Google Scholar] [CrossRef] [PubMed]
- Nasser, M.; Palwe, S.; Bhargava, R.N.; Feuilloley, M.G.J.; Kharat, A.S. Retrospective analysis on antimicrobial resistance trends and prevalence of β-lactamases in Escherichia coli and ESKAPE pathogens isolated from Arabian patients during 2000-2020. Microorganisms. 2020, 8, 1626. [Google Scholar] [CrossRef] [PubMed]
- Girlich, D.; Bonnin, R.A.; Dortet, L.; Naas, T. Genetics of acquired antibiotic resistance genes in Proteus spp. Front Microbiol. 2020, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Recio, R.; Mancheño, M.; Viedma, E.; et al. Predictors of mortality in bloodstream infections caused by Pseudomonas aeruginosa and impact of antimicrobial resistance and bacterial virulence. Antimicrob Agents Chemother. 2020, 64, e01759-19. [Google Scholar] [CrossRef] [PubMed]
- Romanin, P.; Palermo, R.L.; Cavalini, J.F.; et al. Multidrug- and extensively drug-resistant Acinetobacter baumannii in a tertiary hospital from Brazil: the importance of carbapenemase encoding genes and epidemic clonal complexes in a 10-year study. Microb Drug Resist. 2019, 25, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Di Tella, D.; Tamburro, M.; Guerrizio, G.; et al. Molecular epidemiological insights into colistin-resistant and carbapenemases-producing clinical Klebsiella pneumoniae isolates. Infect Drug Resist. 2019, 12, 3783–3795. [Google Scholar] [CrossRef] [PubMed]
- Lukovic, B.; Gajic, I.; Dimkic, I.; et al. The first nationwide multicenter study of Acinetobacter baumannii recovered in Serbia: emergence of OXA-72, OXA-23 and NDM-1-producing isolates. Antimicrob Resist Infect Control. 2020, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, N.; Vihari, N.; Meena, D.S.; et al. Clinical profile of bloodstream infections in COVID-19 patients: a retrospective cohort study. BMC Infect Dis. 2021, 21, 933. [Google Scholar] [CrossRef] [PubMed]
 |
 |
 |
© GERMS 2022.
Share and Cite
Zornic, S.; Lukovic, B.; Petrovic, I.; Jencic, A. Prevalence of Multidrug-Resistant Gram-Negative Bacteria From Blood Cultures and Rapid Detection of Beta-Lactamase-Encoding Genes by Multiplex PCR Assay. GERMS 2022, 12, 434-443. https://doi.org/10.18683/germs.2022.1349
Zornic S, Lukovic B, Petrovic I, Jencic A. Prevalence of Multidrug-Resistant Gram-Negative Bacteria From Blood Cultures and Rapid Detection of Beta-Lactamase-Encoding Genes by Multiplex PCR Assay. GERMS. 2022; 12(4):434-443. https://doi.org/10.18683/germs.2022.1349
Chicago/Turabian StyleZornic, Sanja, Bojana Lukovic, Ivana Petrovic, and Aleksandra Jencic. 2022. "Prevalence of Multidrug-Resistant Gram-Negative Bacteria From Blood Cultures and Rapid Detection of Beta-Lactamase-Encoding Genes by Multiplex PCR Assay" GERMS 12, no. 4: 434-443. https://doi.org/10.18683/germs.2022.1349
APA StyleZornic, S., Lukovic, B., Petrovic, I., & Jencic, A. (2022). Prevalence of Multidrug-Resistant Gram-Negative Bacteria From Blood Cultures and Rapid Detection of Beta-Lactamase-Encoding Genes by Multiplex PCR Assay. GERMS, 12(4), 434-443. https://doi.org/10.18683/germs.2022.1349




