Abstract
Introduction: The study objective was to compare the prevalence of antimicrobial resistance (AMR) in clinical Escherichia coli and Pseudomonas aeruginosa isolates obtained from a secondary-care hospital prior to and during the COVID-19 pandemic in Kuwait. Methods: A retrospective descriptive study was conducted based on AMR profiles of clinical Escherichia coli and Pseudomonas aeruginosa isolates. The AMR data represented isolates from five specimen types (body fluids; blood; respiratory; wound, bone, or other tissues; and urine) of patients admitted to four wards (surgical, medical, pediatric, and maternal-postnatal). Tested isolates between January 2019 and February 2020 represented the pre-COVID-19 pandemic period in Kuwait, whereas those from February 2020 until April 2021 represented the ‘during COVID-19’ period. Results: A total of 1303 isolates (57.2% E. coli and 42.8% P. aeruginosa) were analyzed. For ceftazidime, ertapenem and meropenem, the prevalence of AMR in E. coli was significantly (p < 0.05) lower in pre-COVID-19 wards compared to that during COVID-19, whereas for other antibiotics (i.e., cefepime, gentamicin, and trimethoprim/sulfamethoxazole), the prevalence of AMR in pre-COVID-19 was significantly higher than that during COVID-19. The prevalence of AMR to gentamicin in P. aeruginosa isolates from non-COVID-19 wards (52.8%) was significantly higher (p < 0.001) than that from COVID-19 wards (35.0%) and from the pre-COVID-19 period (32.9%). The multidrug-resistance (MDR) prevalence was 37.4% for E. coli and 32.1% for P. aeruginosa isolates. The odds of MDR in E. coli isolates from the COVID-19 medical wards were significantly lower (OR = 0.27, [95%CI: 0.09–0.80], p = 0.018) compared to the pre-COVID-19 wards. The odds of MDR E. coli and P. aeruginosa isolates by COVID-19 status stratified by specimen type were not different (p > 0.05). Conclusions: No major differences in AMR in E. coli and P. aeruginosa prevalence by specimen type and wards prior to and during the COVID-19 pandemic was observed at this hospital. The high reported MDR prevalence calls for better infection control and prevention.
Introduction
Infections with multidrug-resistant (MDR) bacteria continue to be a major cause of morbidity and mortality worldwide [1,2]. The misuse and abuse of antimicrobial agents in human and veterinary medicine have contributed to the propagation, development and spread of antimicrobial resistance (AMR) genes among bacteria in hospital settings, the community, and the environment [3].
The impact of COVID-19 on AMR phenotypes and genotypes in bacteria is still not clear. There are a number of reports that have shown an increase in number of infections with AMR bacteria during the COVID-19 pandemic [4,5,6,7], whereas other reports have revealed a decrease or no significant change in AMR infections in relation to the pandemic [8,9,10]. It is possible that several factors such as changes in antibiotic use/prescription, improvement in infection control and prevention practices in the hospital and in the community, reduction in outpatient and inpatient visits during the pandemic, and variations in workload among healthcare staff might have played a role in the increase, no change, or decrease of AMR levels during the pandemic [11,12]. It is necessary to evaluate AMR trends over time in response to changes in healthcare facilities. For instance, modification to the antibiotic stewardship program, changes in hospital management or infection control programs, and emergence of disease outbreaks might affect trends in AMR commensal bacteria over the short and/or long term. Therefore, periodic longitudinal analysis of AMR data is important to evaluate changes in trend and to inform health personnel. Ultimately, the risk of AMR bacterial infection among patients can be reduced.
In Kuwait and the Arab Gulf region, there are currently no published reports on the prevalence and trends of AMR clinical isolates during the COVID-19 pandemic. The objective of this study was to determine and compare the prevalence and distribution of AMR patterns of Escherichia coli and Pseudomonas aeruginosa isolates clinical isolates prior to and during COVID-19 (2019-2021) at a secondary-care hospital in Kuwait. Understanding the changes in prevalence, trends, and distribution of clinical AMR phenotypes among bacterial isolates is important for antimicrobial stewardship programs.
Methods
Study site and data collection
A retrospective descriptive study was conducted based on antimicrobial susceptibility data for E. coli and P. aeruginosa isolates collected between January 2019 and April 2021. The data on AMR profiles of these clinical isolates were extracted from the microbiology laboratory information system (LIS) at Farwaniya Hospital. This hospital is one of the major secondary-care hospitals in Kuwait that offers healthcare services to nearly one-fourth of the country’s 4.5 million people. The hospital has 866 beds with multiple medical and surgical specialties including outpatient clinics. Available antimicrobial susceptibility profiles of E. coli and P. aeruginosa from patients admitted to different wards (i.e., surgical, medical, pediatric, and maternal-postnatal) at the hospital were included in the study. During the COVID-19 pandemic (2020-2021), some of the wards within multiple hospital locations were converted to COVID-19 wards while other wards continued to operate as non-COVID-19. The conversion from a non-COVID-19 ward to a COVID-19 ward was due to the surge in COVID-19 cases during the pandemic to accommodate inpatients. The wards that were converted to COVID-19 continued to operate that way for a minimum of six months before they were closed and re-instated as non-COVID-19 wards. There were no E. coli or P. aeruginosa isolates available in the LIS from the post-COVID-19 wards (i.e., those wards that were closed as COVID-19 and returned to operate as non-COVID-19 wards). Furthermore, there were very few isolates from the COVID-19 intensive care unit locations at this hospital during the study period; hence, we did not include them in this study.
During the study period, all consecutive non-duplicated E. coli and P. aeruginosa isolates tested for antimicrobial susceptibility were used in this study. Tested isolates between 1st January 2019 to 23rd February 2020 represented the pre-COVID-19 pandemic period in Kuwait, whereas those from 24th February 2020 until the end of the study period (15th April 2021) represented the ‘during COVID-19’ period.
Sample collection, isolation and identification of E. coli and P. aeruginosa
To differentiate healthcare associated infections (HAI) from contamination, Center for Disease Control and Prevention (CDC) protocols (for bloodstream, respiratory, urinary tract infection, surgical site infection, and other site infection) were adopted by the infection control team [13,14,15,16]. Isolates of presumed clinical significance (i.e., any patient case presenting to the treating physician whether accompanied by signs/symptoms or at an early stage of a suspected infection) and as determined by the hospital infection control program were included. One isolate per bacterial species per patient was used for the analysis. The clinical information extracted from the LIS included patient identification number, patient’s location at the time of isolation, specimen type: (a) body fluids, b) blood, c) respiratory samples including sputum, tracheal aspirate, or bronchoalveolar lavage, d) wound, bone, or other tissues (such as external tissue infections around the eye, ear, and mouth), and e) urine, and the antimicrobial resistance profile. The identity of the patients was removed prior to sharing the data with the authors. The demographic information (e.g., age, sex, nationality) and clinical diagnosis were not available. All the clinical information was confirmed by the laboratory technician and reconfirmed by the laboratory manager.
Antimicrobial susceptibility testing
Using the standard operational procedures, the isolates were identified, and antimicrobial susceptibility (AST) testing was performed by Vitek 2 instrument (bioMérieux, France). For each isolate, appropriate cards were inoculated according to the manufacturer’s instructions. The resulting AST were interpreted according to the breakpoints provided by the Clinical and Laboratory Standards Institute (CLSI) [17] using the Vitek software VTK-R01.02. The results of AST were reported as susceptible, intermediate, or resistant. The E. coli or P. aeruginosa isolates were tested against 12 antibiotics (except for those to which they were intrinsically resistant) that belong to 6 antimicrobial classes as follows: penicillins (ampicillin and piperacillin/tazobactam), cephalosporins (ceftazidime, cefotaxime, cefuroxime, and cefepime), fluoroquinolones (ciprofloxacin), carbapenems (ertapenem, meropenem), aminoglycosides (amikacin and gentamicin), and sulfonamides (trimethoprim/sulfamethoxazole). Pseudomonas aeruginosa isolates were not tested for antimicrobial susceptibility against ertapenem. Quality control testing was performed at the laboratory using ATCC quality control strains (i.e., P. aeruginosa 27853 and E. coli 25922) to check the consistency of AST. For the purpose of data analyses, intermediate antimicrobial susceptibility test results were reclassified as susceptible.
Ethical considerations
This study was approved by the Standing Committee for the Coordination of Health and Medical Research (i.e., the Research Ethics Committee) of the Ministry of Health, Kuwait City, Kuwait (approval number: 1236/2019). Since no contact with human subjects occurred and the study was based on existing data, a waiver of informed consent was granted by the Committee. All data collected were anonymized and no identifying information were recorded.
Statistical analysis
The 12 individual antimicrobial resistance outcomes (binary) as well as the multidrug resistance totals (resistance to three or more antibiotics belonging to different drug classes) for each organism were cross-tabulated by location, specimen type, and COVID-19 status (i.e., pre-COVID-19 and during COVID-19–non-COVID-19 wards or COVID-19 wards). The prevalence of individual antimicrobial resistance as well as the multidrug resistance were compared by location, specimen type, and COVID-19 status using Chi-square statistic in STATA statistical software version 15.1 (STATA corp., USA). Furthermore, the relationship between MDR isolates (binary outcome: MDR or not) and the study variables was assessed using a logistic regression model in STATA.
Results
A total of 1303 isolates (57.2% E. coli and 42.8% P. aeruginosa) were analyzed for antimicrobial susceptibility. Most of the isolates were cultured from urine specimens (40.1%), followed by wound, bone, or other tissue (32.8%), respiratory specimens (19.7%), blood (6.2%), and body fluids (1.2%).
Individual antibiotic resistance
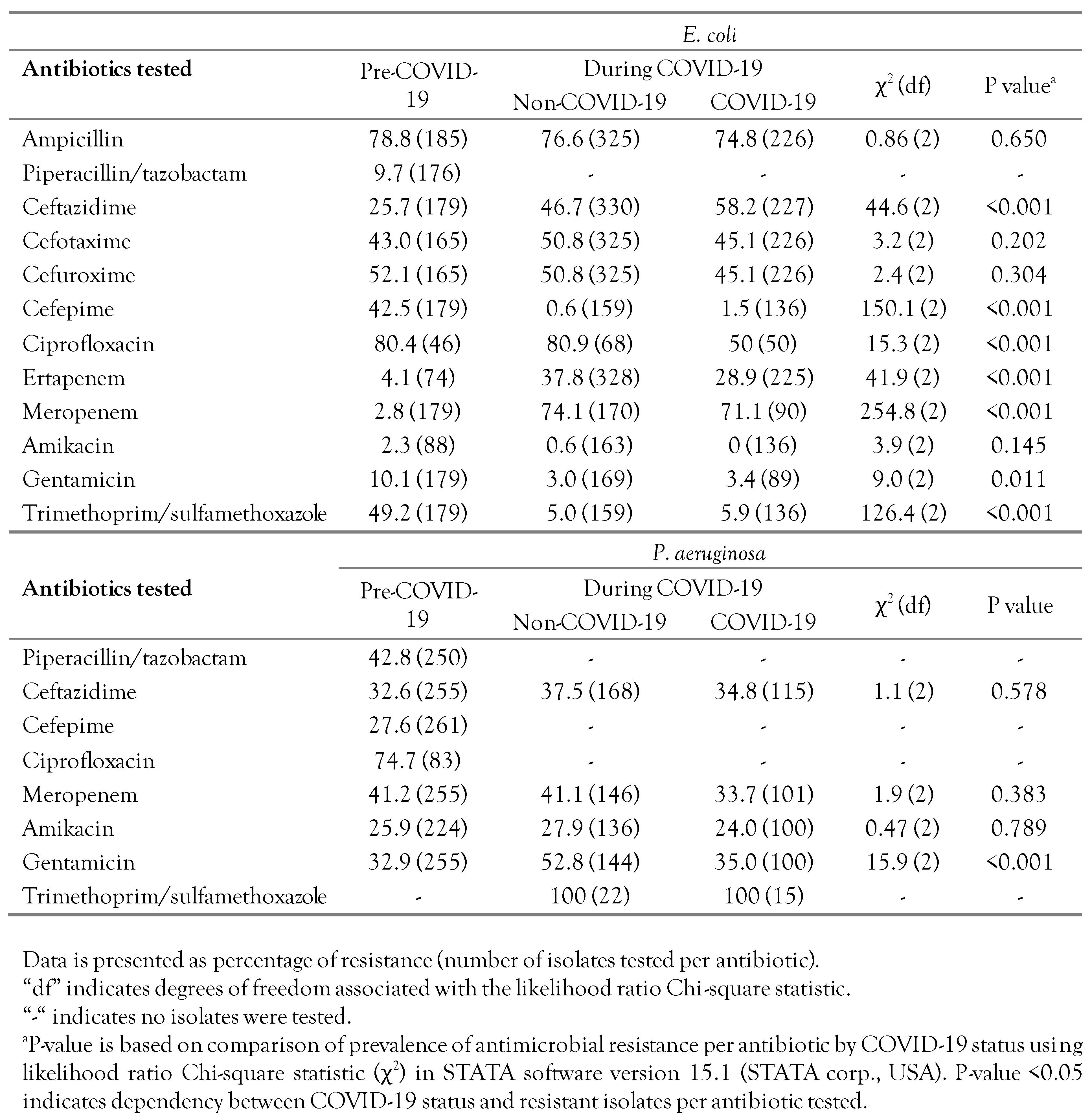
The individual AMR for E. coli and P. aeruginosa, cross-tabulated by COVID-19 status (pre-COVID-19 and during COVID-19) are shown in Table 1. In general, the prevalence of individual AMR among E. coli isolates to ampicillin, cephalosporin drugs (except for cefepime), ciprofloxacin, meropenem, and trimethoprim/sulfamethoxazole was high (>40%) throughout the study period, whereas the lowest AMR prevalence (<10%) was mostly to piperacillin/tazobactam, amikacin, and gentamicin. For ceftazidime, ertapenem and meropenem, the prevalence AMR in E. coli for isolates from pre-COVID-19 wards was significantly (p<0.001) lower compared to that during COVID-19 (for both COVID-19 and non-COVID-19 wards), whereas for other antibiotics (i.e., cefepime, gentamicin, and trimethoprim/sulfamethoxazole) the prevalence of AMR in E. coli in pre-COVID-19 wards was significantly higher than that during COVID-19 (for both COVID-19 and non-COVID-19 wards).

Table 1.
Comparison of phenotypic resistance of E. coli and P. aeruginosa sampled prior to the COVID-19 pandemic and during the pandemic at a secondary care hospital in Kuwait.
The overall prevalence among P. aeruginosa isolates of individual AMR to piperacillin/tazobactam, ciprofloxacin, meropenem, and trimethoprim/sulfamethoxazole was high (>40%), whereas AMR prevalence was still >25% for the other tested antibiotics (Table 1). The prevalence of AMR in P. aeruginosa isolates to gentamicin obtained from non-COVID-19 wards (52.8%) was significantly higher (p<0.001) than that from COVID-19 wards (35.0%) and from the pre-COVID-19 period (32.9%). There were no significant differences in AMR prevalence among P. aeruginosa by COVID-19 status for the other antibiotics (Table 1).
Multidrug resistance
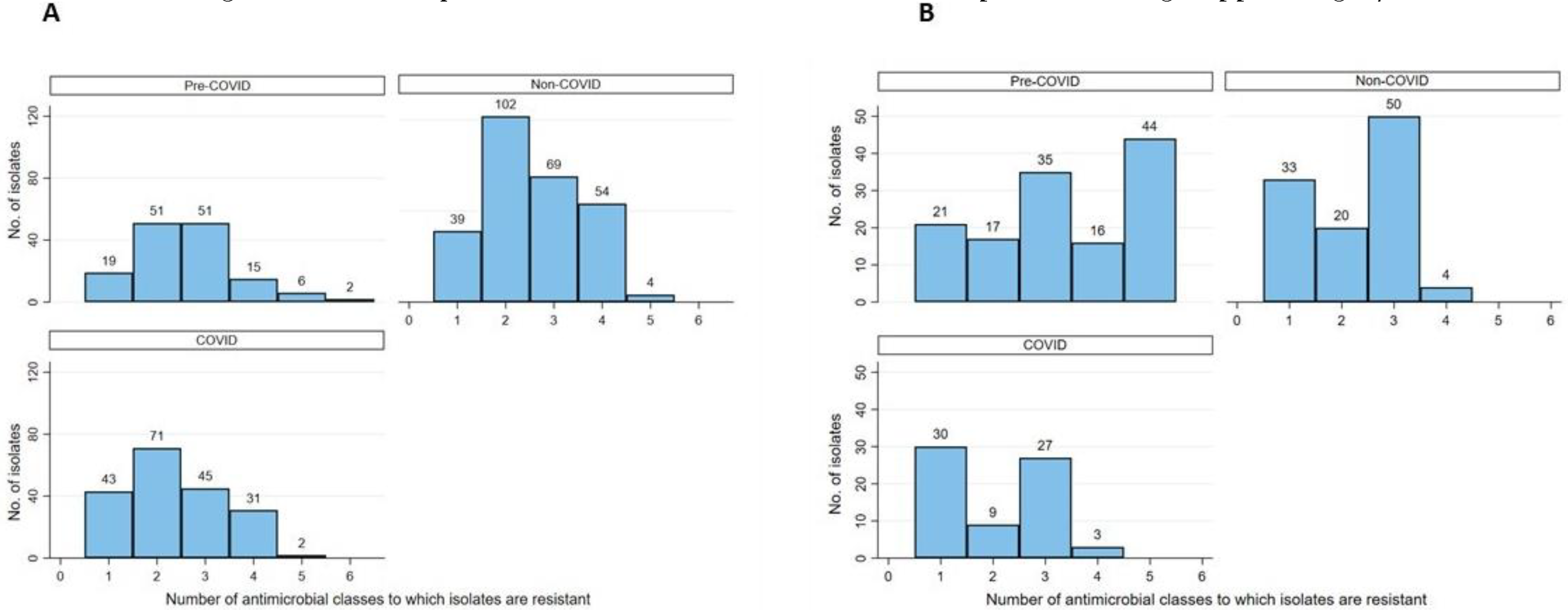
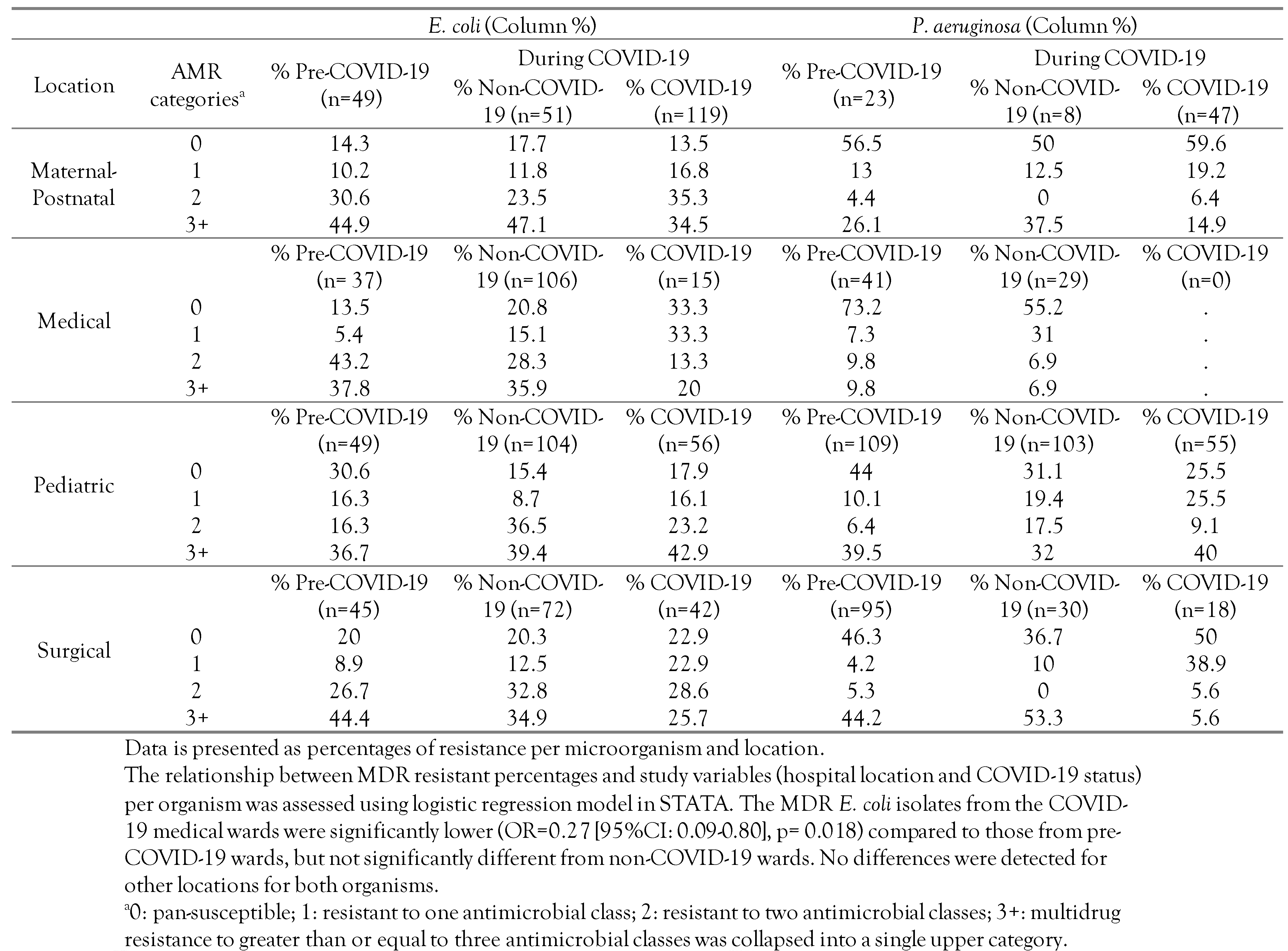
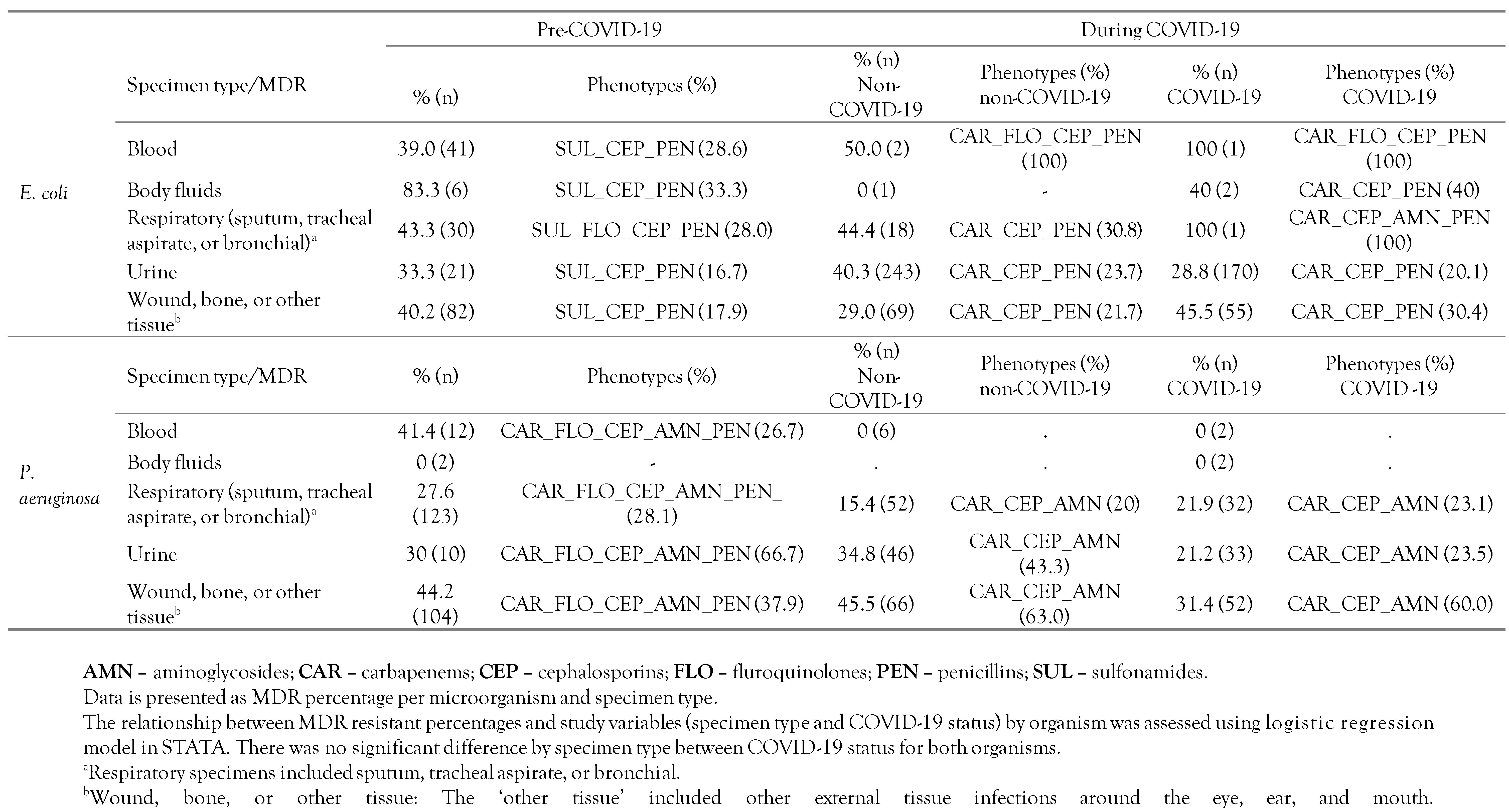
The distribution of multidrug resistance (MDR) was categorized as follows: 0: Pan-susceptible, 1: Resistant to one antimicrobial class, 2: Resistant to two antimicrobial classes, 3+: Multidrug resistance to greater than or equal three (3+) antimicrobial classes was collapsed into a single upper category. The MDR categories were stratified by hospital location and COVID-19 status as shown in Table 2. Furthermore, the distribution of resistant phenotypes including MDR totals and the frequency of MDR phenotypes, cross-tabulated by specimen type and COVID-19 status, are shown in Table 3. Moreover, the frequency distribution of AMR by antibiotic class, COVID-19 status, and organism, not collapsed into the 3+ categories, to better show the maximum MDR phenotype for each isolate, is shown in Figure 1A,B.

Table 2.
Distribution of resistant phenotypes of E. coli and P. aeruginosa isolates by number of resistant antimicrobial classes and different locations prior to the COVID-19 pandemic and during the pandemic at a secondary care hospital in Kuwait.

Table 3.
Distribution of resistant phenotypes of E. coli and P. aeruginosa isolates by number of resistant antimicrobial classes and specimen type prior to the COVID-19 pandemic and during the pandemic at a secondary care hospital in Kuwait.
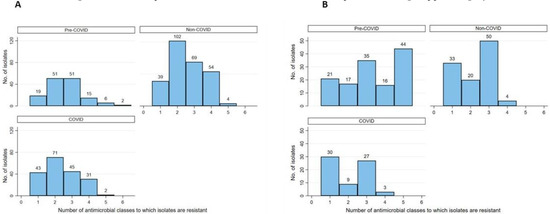
Figure 1.
Frequency bar chart illustrating the distribution of phenotypic resistance to up to 6 antimicrobial classes among of E. coli (A) and P. aeruginosa (B) bacterial isolates prior to the COVID-19 pandemic and during the pandemic at a secondary care hospital in Kuwait.
Overall, the MDR prevalence was 37.2% (95%CI: 34.7-39.8) across all the isolates with 37.4% (95%CI: 34.0-41.0) for E. coli isolates and 32.1% (95%CI: 28.3-36.1) for P. aeruginosa isolates. As shown in Table 2, the overall trend of MDR E. coli prevalence was high (mostly >30%) for each of the hospital locations with relatively lower values from COVID-19 wards compared to both non-COVID-19 wards and pre-COVID-19. Moreover, we revealed that the odds of MDR in E. coli isolates from the COVID-19 medical wards were significantly lower (OR=0.27 [95%CI: 0.09-0.80], p=0.018) compared to that from pre-COVID-19 wards, but not significantly different from the non-COVID-19 wards. The difference in the MDR prevalence in E. coli by COVID-19 status for the other hospital locations was not significant (p>0.05). For P. aeruginosa, the prevalence of MDR isolates was high for all hospital locations except for medical. The differences in MDR prevalence in P. aeruginosa by COVID-19 status and hospital locations were not significant (p>0.05).
In Table 3, we revealed that MDR prevalence among E. coli isolates from pre-COVID-19 and during COVID-19 across all specimen types was high, particularly that from respiratory specimens; wounds, bones, or other tissues; and urine. However, the odds of MDR in E. coli isolates by COVID-19 status stratified by specimen type were not significantly different (p>0.05). Interestingly, while the most frequent MDR phenotypes among E. coli isolates from pre-COVID-19 included three antimicrobial drug classes (i.e., sulfonamide, penicillins, and cephalosporins), the most frequent MDR phenotype during COVID-19 (regardless of COVID-19 status) included the same three drug classes except for having carbapenems replacing sulfonamide (Table 3). As for P. aeruginosa, the MDR prevalence among the isolates from pre-COVID-19 and during COVID-19 across all specimen types was high, particularly from specimens obtained from wounds, bones, or other tissues. However, the odds of MDR in P. aeruginosa isolates by COVID-19 status and specimen type were not significantly different (p>0.05). Interestingly, while the most frequent MDR phenotype among P. aeruginosa isolates from pre-COVID-19 included five antimicrobial drug classes (i.e., carbapenems, fluoroquinolones, cephalosporins, aminoglycosides and penicillins), the most frequent MDR phenotype during COVID-19 (regardless of COVID-19 status) included three antimicrobial drug classes (i.e., carbapenems, cephalosporins, and aminoglycosides).
Discussion
The impact of the COVID-19 pandemic on the epidemiology of AMR is still debatable as discussed by several authors [11,18,19]. It was hypothesized that because of the increased number of hospitalized COVID-19 patients during the pandemic and the consequently higher empirical use of antibiotics to treat patients, an increase in AMR bacterial prevalence in hospital settings will occur [20,21]. Moreover, it was anticipated that the COVID-19 pandemic might have had a different effect on the potential spread of community-acquired AMR bacteria to hospitals. Since hospitals were busy dealing with COVID-19 patients, non-COVID-19 patients mostly stayed home, hence, antibiotic use declined and influx of community-acquired AMR bacteria to the hospital was likely reduced. Moreover, travel restrictions and social distancing might have had a positive impact on AMR transmission within the community and consequently to the hospitals. Our study showed that when comparing the prevalence of AMR among E. coli and P. aeruginosa isolated from patients in pre-COVID-19 wards, COVID-19 wards and non-COVID-19 wards, there were generally no significant differences observed. It is worth mentioning that although we compared prevalence (positive percentages), the number of isolates tested for antimicrobial susceptibility during COVID-19 was lower than that in the pre-COVID-19 period. This might be because of the reduction in the laboratory capacity to conduct regular AST during the pandemic that was caused by shortage in staff and shortage in reagents to carry out the testing.
In this study, AMR prevalence in E. coli by COVID-19 status varied by antibiotic with some (i.e., ceftazidime, ertapenem and meropenem) having significantly lower AMR prevalence in pre-COVID-19 compared to during COVID-19, whereas for other antibiotics (i.e., cefepime, gentamicin, and trimethoprim/sulfamethoxazole), the prevalence of AMR in pre-COVID-19 was significantly higher than that during COVID-19. In a recent study from Mexico that compared changes in AMR in multiple organisms prior to the COVID-19 pandemic and during the pandemic, authors found increased percentages of E. coli isolates resistant to most antibiotics tested (i.e., ampicillin-sulbactam, ampicillin, cefoxitin, ceftazidime, cefepime, imipenem, meropenem, ciprofloxacin, levofloxacin, gentamicin, and trimethoprim-sulfamethoxazole) during the pandemic [22]. Their study was based on 35 hospital-based laboratories that attended COVID-19 patients with comparing AMR E. coli and P. aeruginosa across laboratories with one year (pre-COVID-19) and another year (during COVID-19). However, our study was based on a single center/laboratory, comparing AMR prevalence between wards (COVID-19 and non-COVID-19) over one year (pre-COVID-19) to another 1.5 year (during COVID-19). This may explain the difference in our results vs. López-Jácome et al. [22] Contrary to the study from Mexico, a study conducted in Indonesia revealed that the overall prevalence of extended-spectrum betalactamase (ESBL)-producing E. coli was reduced significantly from 50% (pre-COVID-19) to 29.9% (during COVID-19) [10] We observed a reduction in AMR E. coli percentages for some of the antibiotics when comparing pre-COVID-19 to during COVID-19 (Table 1). Interestingly, the study from Indonesia was based on a single center as well and compared two periods (pre-COVID-19 and during COVID-19). However, it was not clear from the study whether all isolates resulted from COVID-19 wards or patients. Furthermore, the number of isolates used during the two periods was low, which could impact the results reported. Moreover, the antibiotic use data in relation to AMR trends in these studies were not included, similar to our study.
We stated that prevalence of AMR P. aeruginosa isolates obtained from the non-COVID-19 wards (52.8%) was significantly higher than that from COVID-19 wards (35.0%) and from the pre-COVID-19 period (32.9%) for gentamicin only. This disagrees with López-Jácome et al. [22], a study from Mexico where authors reported a significant increase in resistant P. aeruginosa prevalence from pre-COVID-19 compared to during COVID-19 for the following antibiotics: piperacillin-tazobactam, cefepime, imipenem, meropenem, ciprofloxacin, levofloxacin, and gentamicin. The main difference between their study and ours is that the former was based on AMR data from 46 centers in Mexico, while this study was based on a single center.
Overall, the MDR prevalence was 37.4% and 32.1% for E. coli and P. aeruginosa isolates, respectively. The overall MDR prevalence for both organisms was high for most hospital locations regardless of the COVID-19 status (i.e., pre-COVID-19 or during COVID-19) in this study (Table 2). Furthermore, we reported that MDR E. coli prevalence was generally high across all specimen types particularly respiratory; urine; and wounds, bones, or other tissues (Table 3). Similarly, MDR P. aeruginosa prevalence among the isolates across all specimen types regardless of the COVID-19 status was high particularly from specimens obtained from wounds, bones, or other tissues (Table 3). However, we did not detect statistically significant differences between MDR E. coli or MDR P. aeruginosa isolates by specimen type stratified by COVID-19 status. In a study conducted at a single hospital in Italy (similar to our study setting) that compared MDR organisms before the pandemic (2017-2019) to one year during the pandemic (2020), authors showed that the prevalence of MDR bacteria was very high over the study period but with no significant difference (p>0.05) in MDR before and during the pandemic [9]. The authors explained the findings as possibly due to data obtained from a single center and/or due to the high MDR prevalence present before the pandemic at the center, which continued to be the case during the pandemic. This explanation is similar to what we observed in our study (i.e., a single center study and the prior high MDR prevalence). Furthermore, in a study conducted by Patel et al. [23] in May 2020 at University of Maryland Medical Center (USA), authors showed an increase in incidence of MDR E. coli and P. aeruginosa based on a retrospective analysis of a hospital wide data. While their findings are different from what we observed, the study by Patel et al. was limited by the number of isolates tested and the short study period. In another study that compared MDR isolates of Gram-negative and Gram-positive organisms pre-COVID-19 (2017-2018) and during the COVID-19 pandemic (2019-2020) at a hospital in Rome (Italy), authors reported a significantly higher incidence of MDR bacterial infections in COVID-19 wards as compared to other medical wards [24], which disagrees with our findings. Moreover, in another study conducted at two geriatric wards in Italy [24], authors reported very high prevalence (nearly 50% of the samples) of MDR bacteria from the pre- and during COVID-19 periods. Specifically, they revealed that E. coli was the primary MDR organism (38.6% of the 83 samples) during the study period, with higher prevalence in the pre-COVID-19 period than during COVID-19. While their study population is different than ours, we observed a similar trend in our MDR isolates. The presence of MDR organisms in a hospital poses a substantial transmission risk to susceptible patients, and treatment failure.
We revealed that the most frequent MDR phenotype among E. coli isolates from pre-COVID-19 included sulfonamide, penicillins, and cephalosporins compared to that during COVID-19, which included carbapenems, penicillins, and cephalosporins (Table 3). Moreover, for P. aeruginosa, the frequent MDR phenotypes from pre-COVID-19 included carbapenems, fluoroquinolones, cephalosporins, aminoglycosides and penicillins compared to those during COVID-19 (carbapenems, cephalosporins, and aminoglycosides)—Table 3. While there is limited literature that compared phenotypes in relation to the COVID-19 pandemic, this change in MDR phenotypes in E. coli isolates might be related to increase in carbapenem use (personal communication with infection control team). However, for P. aeruginosa, the reduction in MDR phenotype during COVID-19 might be linked to a decrease in hospital-acquired P. aeruginosa infections. This observation might to due to the polices implemented during the COVID-19 pandemic, which might have affected the number of patients attending hospitals (outpatients and inpatients). The reduction in the number of patients attending hospitals was mostly due to stay-at-home mandates and prioritizing COVID-19 patients over non-emergency patients.
This study has several limitations. First, the study was based on retrospective AMR data from one hospital in Kuwait, which might not represent other public hospitals in the country. However, this participating hospital is a major health institution that serves about a quarter of Kuwait’s population. Second, data on sociodemographic and historical antibiotic use data were not available. Nonetheless, we believe the study findings reveal, for the first time, the magnitude of AMR prior to and during the COVID-19 pandemic. Furthermore, the findings are important for decision makers to improve AMR control and prevention as well as to establish a surveillance program to monitor trends. Third, the available data represent only a 1.5-year period during the pandemic. It might take longer to establish trends of AMR during and post-pandemic times. Furthermore, changes in the number of non-COVID-19 patient visits might have impacted the number and heterogeneity of AMR isolates in 2020 and 2021 compared to 2019.
Conclusions
In this study, we reported the prevalence and distribution of AMR in E. coli and P. aeruginosa isolated from clinical samples of patients admitted at different hospital locations and from various specimen types prior to the COVID-19 pandemic (2019) and during the pandemic (2020 -2021). Overall, we found that most individual-level AMR and MDR for the two organisms were not significantly different before the pandemic compared to during the pandemic across hospital locations and specimen types. Nonetheless, the general trend from our data indicates that AMR prevalence (particularly MDR) continues to be high (overall 37.2%) in 2019 through 2021. This agrees with our previous AMR prevalence study in 2018 in the same hospital where the overall MDR prevalence was 38.7% [25].
Overall, we did not observe major differences in AMR prevalence in E. coli and P. aeruginosa isolates prior to the COVID-19 pandemic vs. COVID-19 and non-COVID-19 wards during the pandemic. This may indicate the lack of effect of changes associated with the pandemic on AMR prevalence at this hospital. Nonetheless, the observed minor differences that we report were limited to specific antibiotics or antimicrobial classes, which might be associated with specific antibiotic use and/or changes in infection control programs during the pandemic. Importantly, the establishment of an integrated AMR surveillance program at the hospital to monitor AMR prevalence and changes in trends is needed to assess the long-term impact of the COVID-19 pandemic on the burden of AMR bacterial infections and consequently public health.
Author Contributions
All authors (WQA, NMA, WA, and RD) were involved in the scholarly creativity and design of the study. WA, NMA, and RD were involved in data extraction and data quality check. WQA conducted statistical analysis and write up. All authors read and approved the final version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author, upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant—Associated orthopedic infections. J Orthop Res. 2018, 36, 22–32. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Evolving Threat of Antimicrobial Resistance: Options for Action; World Health Organization: Geneva, Switzerland, 2012. Available online: http://apps.who.int/iris/bitstream/10665/44812/1/9789241503181eng.pdf (accessed on 8 August 2019).
- Woolhouse, M.; Ward, M.; van Bunnik, B.; Farrar, J. Antimicrobial resistance in humans, livestock and the wider environment. Philos Trans R Soc Lond B Biol Sci. 2015, 370, 20140083. [Google Scholar] [CrossRef] [PubMed]
- Rawson, T.M.; Ming, D.; Ahmad, R.; Moore, L.S.P.; Holmes, A.H. Antimicrobial use, drug-resistant infections and COVID-19. Nat Rev Microbiol. 2020, 18, 409–410. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Record number of countries contribute data revealing disturbing rates of antimicrobial resistance. 2020. Available online: https://www.who.int/news-room/detail/01-06-2020-record-number-of-countries-contribute-data-revealing-disturbing-rates-of-antimicrobial-resistance (accessed on 13 February 2022).
- He, Y.; Li, W.; Wang, Z.; Chen, H.; Tian, L.; Liu, D. Nosocomial infection among patients with COVID-19: A retrospective data analysis of 918 cases from a single center in Wuhan, China. Infect Control Hosp Epidemiol. 2020, 41, 982–983. [Google Scholar] [CrossRef] [PubMed]
- Polly, M.; de Almeida, B.L.; Lennon, R.P.; Cortês, M.F.; Costa, S.F.; Guimarães, T. Impact of the COVID-19 pandemic on the incidence of multidrug-resistant bacterial infections in an acute care hospital in Brazil. Am J Infect Control. 2022, 50, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Bentivegna, E.; Luciani, M.; Arcari, L.; Santino, I.; Simmaco, M.; Martelletti, P. Reduction of multidrug-resistant (mdr) bacterial infections during the COVID-19 pandemic: A retrospective study. Int J Environ Res Public Health. 2021, 18, 1003. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, M.; Barilaro, G.; Zanini, U.; Giuliani, G. Impact of the COVID-19 pandemic on multidrug-resistant hospital-acquired bacterial infections. J Hosp Infect. 2022, 123, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Wardoyo, E.H.; Suardana, I.W.; Yasa, I.W.P.S.; Sukrama, I.D.M. Antibiotics susceptibility of Escherichia coli isolates from clinical specimens before and during COVID-19 pandemic. Iran J Microbiol. 2021, 13, 156–160. [Google Scholar] [PubMed]
- Collignon, P.; Beggs, J.J. CON: COVID-19 will not result in increased antimicrobial resistance prevalence. JAC Antimicrob Resist. 2020, 2, dlaa051. [Google Scholar] [CrossRef] [PubMed]
- Donà, D.; Di Chiara, C.; Sharland, M. Multi-drug-resistant infections in the COVID-19 era: A framework for considering the potential impact. J Hosp Infect. 2020, 106, 198–199. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Bloodstream infection event (central line-associated bloodstream infection and non-central line associated bloodstream infection). 2020. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf (accessed on 28 November 2020).
- Centers for Disease Control and Prevention. Pneumonia (ventilator-associated [VAP] and nonventilator-associated pneumonia [PNEU]) event. 2020. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/6pscvapcurrent.pdf (accessed on 28 November 2020).
- Centers for Disease Control and Prevention. Urinary tract infection (catheter-associated urinary tract infection [CAUTI] and non-catheter-associated urinary tract infection [UTI]) events. 2021. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/7psccauticurrent.pdf (accessed on 28 November 2020).
- Centers for Disease Control and Prevention. CDC/NHSN surveillance definitions for specific types of infections. 2022. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf (accessed on 28 May 2022).
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; CLSI: Wayne, PA, USA, 2017; Volume M100, pp. 21–244. [Google Scholar]
- van Duin, D.; Barlow, G.; Nathwani, D. The impact of the COVID-19 pandemic on antimicrobial resistance: A debate. JAC Antimicrob Resist. 2020, 2, dlaa053. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.J.; Buehrle, D.J.; Nguyen, M.H. PRO: The COVID-19 pandemic will result in increased antimicrobial resistance rates. JAC Antimicrob Resist. 2020, 2, dlaa049. [Google Scholar] [CrossRef] [PubMed]
- Nori, P.; Cowman, K.; Chen, V.; et al. Bacterial and fungal coinfections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect Control Hosp Epidemiol. 2021, 42, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; et al. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin Microbiol Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef] [PubMed]
- López-Jácome, L.E.; Fernández-Rodríguez, D.; Franco-Cendejas, R.; et al. Increment antimicrobial resistance during the COVID-19 pandemic: Results from the Invifar Network. Microb Drug Resist. 2022, 28, 338–345. [Google Scholar] [PubMed]
- Patel, A.; Emerick, M.; Cabunoc, M.K.; et al. Rapid spread and control of multidrug-resistant Gram-negative bacteria in COVID-19 patient care units. Emerg Infect Dis. 2021, 27, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Gasperini, B.; Cherubini, A.; Lucarelli, M.; Espinosa, E.; Prospero, E. Multidrug-resistant bacterial infections in geriatric hospitalized patients before and after the COVID-19 outbreak: Results from a retrospective observational study in two geriatric wards. Antibiotics 2021, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Alali, W.Q.; AlFouzan, W.; Dhar, R. Prevalence of antimicrobial resistance in Gram-negative clinical isolates from a major secondary hospital in Kuwait: A retrospective descriptive study. Germs 2021, 11, 498–511. [Google Scholar] [CrossRef] [PubMed]
© GERMS 2022.