Abstract
Introduction: Worldwide, Clostridioides difficile infection is becoming one of the most common healthcare-associated infections. Management and control of this infection in healthcare facilities are associated with screening for environmental and instrumental C. difficile contamination. This systematic review and meta-analysis aimed to assess the overall prevalence of C. difficile in hospital settings, medical devices, and instruments. Methods: Four main databases, PubMed, Web of Science, Google Scholar, and Scopus, were searched using the keywords Clostridioides difficile, Clostridium difficile, C. difficile, clostridia, Clostridium spp., hospital environments, antibiotic associate colitis, intensive care unit, and ward in combination as a search strategy. The PRISMA checklist was used for selecting eligible studies. Results: A total of 11 eligible articles published between 2012 and 2021 were included. The overall pooled prevalence of C. difficile in hospital environments was 14.9%. The highest and lowest prevalence were reported for India (51.1%) and the USA (1.6%), respectively. The highest prevalence was reported for beds (46.3%). A significant heterogeneity was seen between C. difficile prevalence in hospital environments in different samples. The highest and lowest prevalence was reported for floor corners (63.2%) and privacy curtains (1.4%), respectively. Conclusions: In conclusion, hospitals’ medical devices and environmental surfaces are considered a crucial source of Clostridioides difficile infection. In this regard, we strongly recommend revising and improving the cleaning and disinfection methods in hospitals and quality control of cleaning adequacy.
Introduction
Clostridioides difficile (CD) is a toxin-producing, Gram-positive, spore-forming, anaerobic pathogen responsible for a complex clinical presentation and serious complications such as pseudomembranous colitis, acute toxic colitis with dilatation of the colon and, potentially, death [1]. The prevalence of Clostridioides difficile infection (CDI) is steadily increasing worldwide, and is becoming the most common healthcare-associated infection (HAI) [2].
CDI is characterized primarily as a HAI with diffuse diarrhea in high-risk hospitalized elderly patients taking antibiotics. Still, its epidemiology extends to all age groups, low-risk individuals without recent use of antibiotics, and community-acquired infection (CAI) [3]. More than 70% of CDI cases are related to healthcare settings, but the incidence of CAI is growing, reaching nearly a third of all CDI cases [4]. Healthcare-associated CDI provides a heavy burden on patients and the healthcare system, by increasing hospital stay length, admission rate, costs of hospitalization, risk of spreading and transmission of CD to other patients, and having significant morbidity and mortality [5,6].
Some bacterial species are epidemiologically related to antibiotic-associated diarrhea (AAD), and CD is the most prevalent bacterial etiology of AAD, accounting for more than 20% of all hospitalized cases [6]. For these reasons, the Centers for Disease Control and Prevention (CDC) classified CD as an urgent public health threat requiring immediate surveillance and outbreak control measures in 2013 [7].
Asymptomatic carriers, infected patients, contaminated surfaces, contaminated medical devices, animal products and foods are potential reservoirs of CD [8,9]. In the last decade, contamination of hospital surfaces has played an important role in the transmission of numerous healthcare-related pathogens, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus spp. (VRE), CD, and Acinetobacter spp [9].
Hospitalization is a significant risk factor for the development of CDI. It has been proven that contaminated hospital surfaces and devices play an essential role in the spread and transmission of CDI. It has also been demonstrated that hand hygiene, efficient cleaning and disinfection using sporicidal agents could reduce levels of HAI [10,11].
CDI monitoring and environmental hygiene screening in hospitals are essential strategies for reducing the incidence of healthcare-associated CDI and improving infection control.
This study aimed to assess the overall prevalence of CD in hospital settings, medical devices, instruments, and to better understand the worldwide epidemiology of CD in healthcare facilities and re-emphasize effective management of disinfection procedures using sporicidal agents.
Methods
Search strategy
Using four main databases: PubMed, Web of Science, Google Scholar, and Scopus, studies were retrieved from 01-Jan-2011 until 29-Dec-2021. The following keywords were applied: “Clostridium difficile”, “C. difficile”, “clostridia”, “Clostridium spp.”, “Clostridioides difficile”, “hospital environments”, “antibiotic associated colitis”, “intensive care unit”, and “ward”, in combination with boolean operators (‘‘AND’’ and/or ‘‘OR’’). The study was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guideline [12].
Inclusion/exclusion criteria and studies selection
The studies related to the incidence/prevalence of C. difficile in hospital wards and environments were included. The exclusion criteria were conferences, cohort studies, narrative or systematic reviews without accurate data, letters to editors, notes, books, guidelines, and non-English articles.
Two independent researchers read the full text of included studies accurately and any discrepancy was resolved by discussing with the third independent researcher. The characteristics obtained from each study were as follow: authors, publishing date, sampling year, the place of study, study size number, sample type, detection method (phenotypic and molecular methods), the total number of detected C. difficile, the place of sampling, the studied wards, and the number of detected C. difficile in each sample type.
Analysis of data
The collected data were analyzed by version 2.2.064 of Comprehensive Meta-Analysis software. The total prevalence of C. difficile and its prevalence in different sample types were demonstrated with an event rate and a 95% confidence interval (CI). The fixed or random-effects model was selected based on the heterogeneity of meta-analyses. Subgroup analyses were done to assess the source of heterogeneity in C. difficile prevalence reports based on the study place (country), and the sample type using two heterogeneity statistics of Q test and I2. The publication bias was measured by performing the Egger test. The p value <0.05 was considered as the significant threshold.
Results
Search results
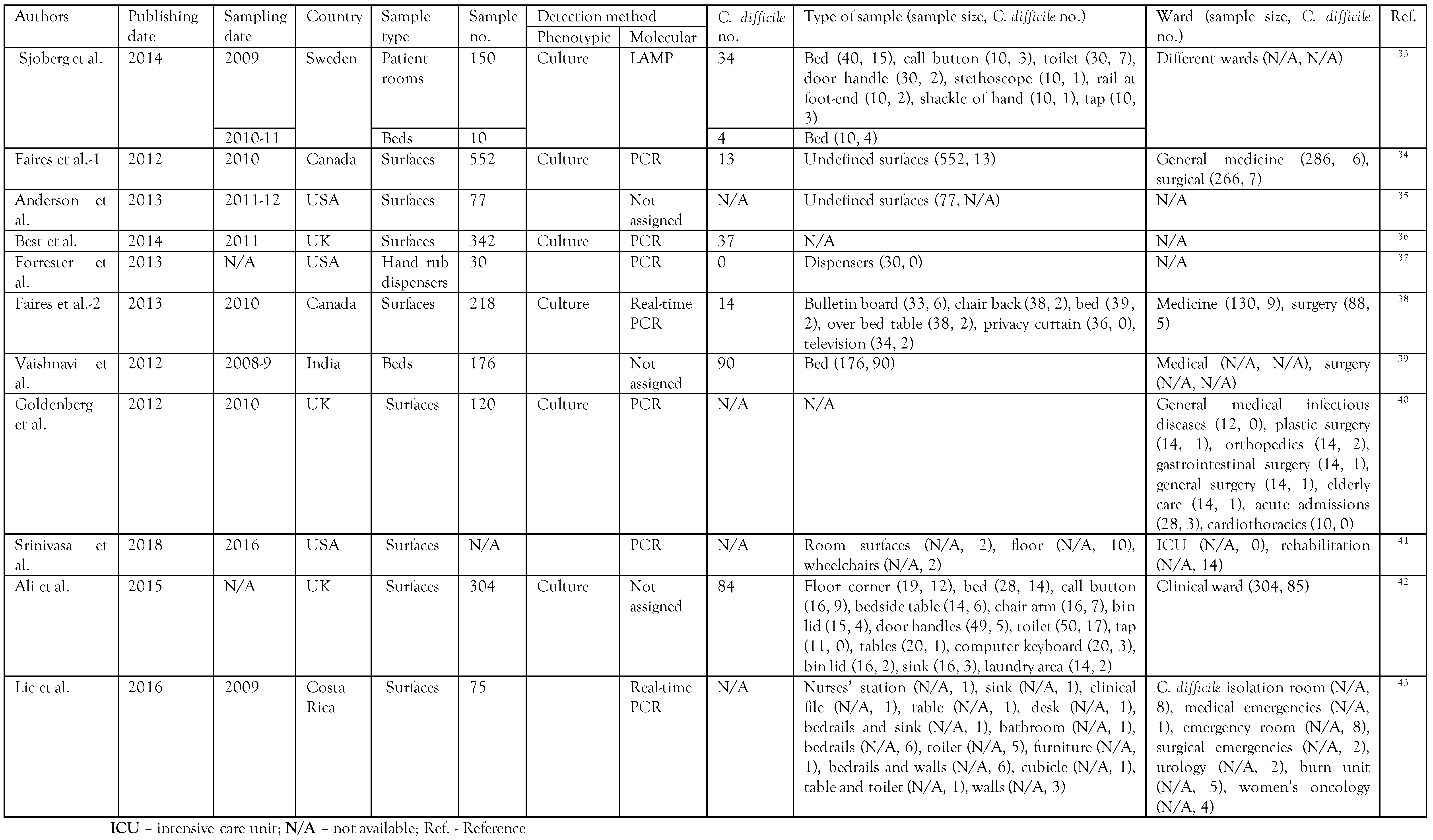
Totally, 5092 studies were retrieved from the databases. By omitting duplicates, 3338 studies remained. After excluding all articles unrelated to the prevalence of C. difficile in the hospital or healthcare setting according to the title of the retrieved article based on our inclusion/exclusion criteria, 203 studies remained. By title/abstract accurate reading, 187 studies were excluded, and 16 remained for eligibility and full-text reading. Finally, 11 studies remained for qualitative analysis and meta-analysis. The search strategy diagram is presented in Figure 1. The characteristics of the final included studies are given in Table 1. The included studies were published mainly from 2009 until 2011 (9 cases). In all studies, the culture was used as a phenotypic method for C. difficile detection, and PCR-based methods were the most frequently-used molecular detection method (Table 1).
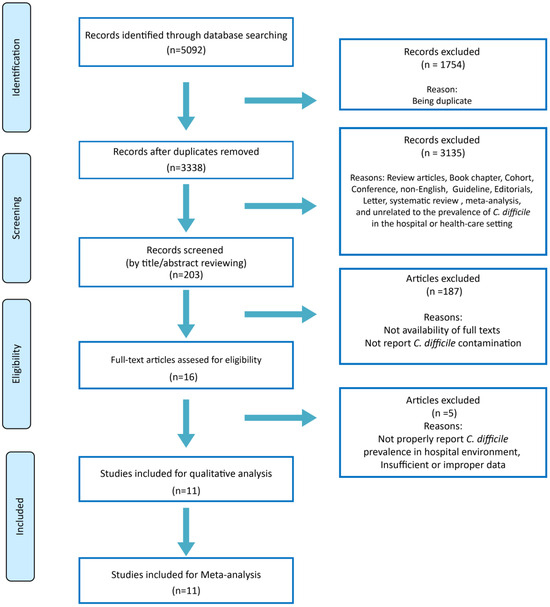
Figure 1.
PRISMA flow diagram of study selection.

Table 1.
Characteristics of the included studies.
Pooled prevalence of C. difficile in hospital environments
Using a random-effects model meta-analysis, the pooled prevalence of C. difficile in hospital environments reported in the final 11 studies was assessed. The number of C. difficile positive cases over the number of samples (called event rates) was used as the effect size index. In the text or tables to give percentages, the event rates were multiplied by 100. The overall prevalence of C. difficile in hospital environments was 14.9% (95%CI: 6.9-29.3)—Figure 2. The lowest prevalence was reported by Forrester et al. (1.6%), while the highest prevalence was seen in the study by Vashnavi et al. (51.1%)—Figure 2). A significant heterogeneity was observed between studies, where the Q-value was 213.39 and the I2 96.72, which showed that 96.72% of the variances reflect true variances between studies.
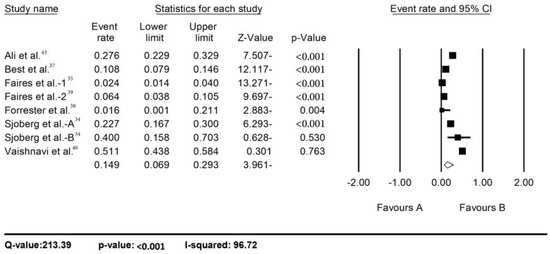
Figure 2.
Prevalence of C. difficile in hospital environment.
Subgroup analysis of C. difficile prevalence in hospital environments based on the study country
Based on the study place (country), the studies were divided into Canada (two studies), India (one study), Sweden (two studies), the UK (two studies) and the USA (one study). A significant heterogeneity was observed between C. difficile prevalence in hospital environments in different countries (Q-value=16.29, p=0.003) (Supplementary Table S1). The highest and lowest prevalence was reported for India (51.1%) and the USA (1.6%), respectively. However, it is noteworthy that the number of studies in each country is deficient, which may undermine the results of this subgroup analysis.
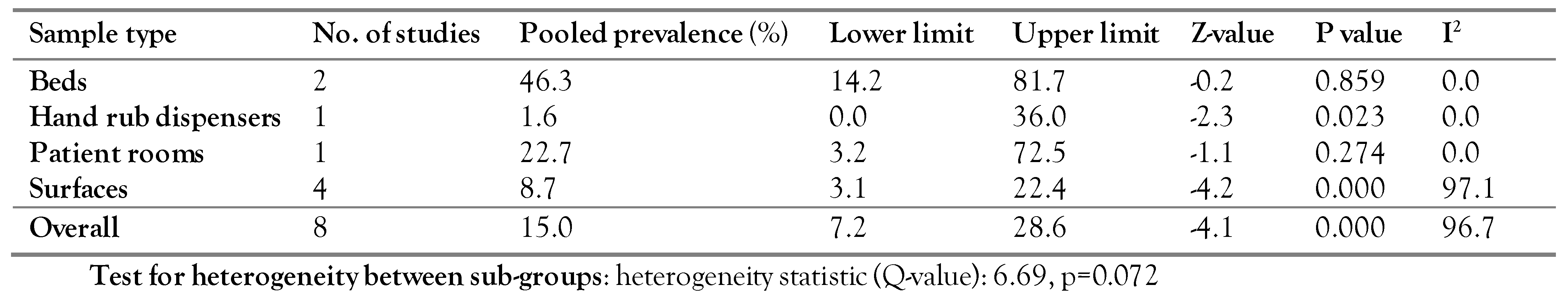
Subgroup analysis of C. difficile prevalence in hospital environments based on the study sample types
Based on the sample types, the studies were divided into Beds (two studies), Hand Rub Dispensers (one study), Patient rooms (one study), and Surfaces (four studies). Although the highest prevalence was reported for beds (46.3%), no statistically significant difference was observed between the subgroups (Q-value=6.69, p=0.072)—Table 2.

Table 2.
Subgroup analysis of C. difficile prevalence based on sample type.
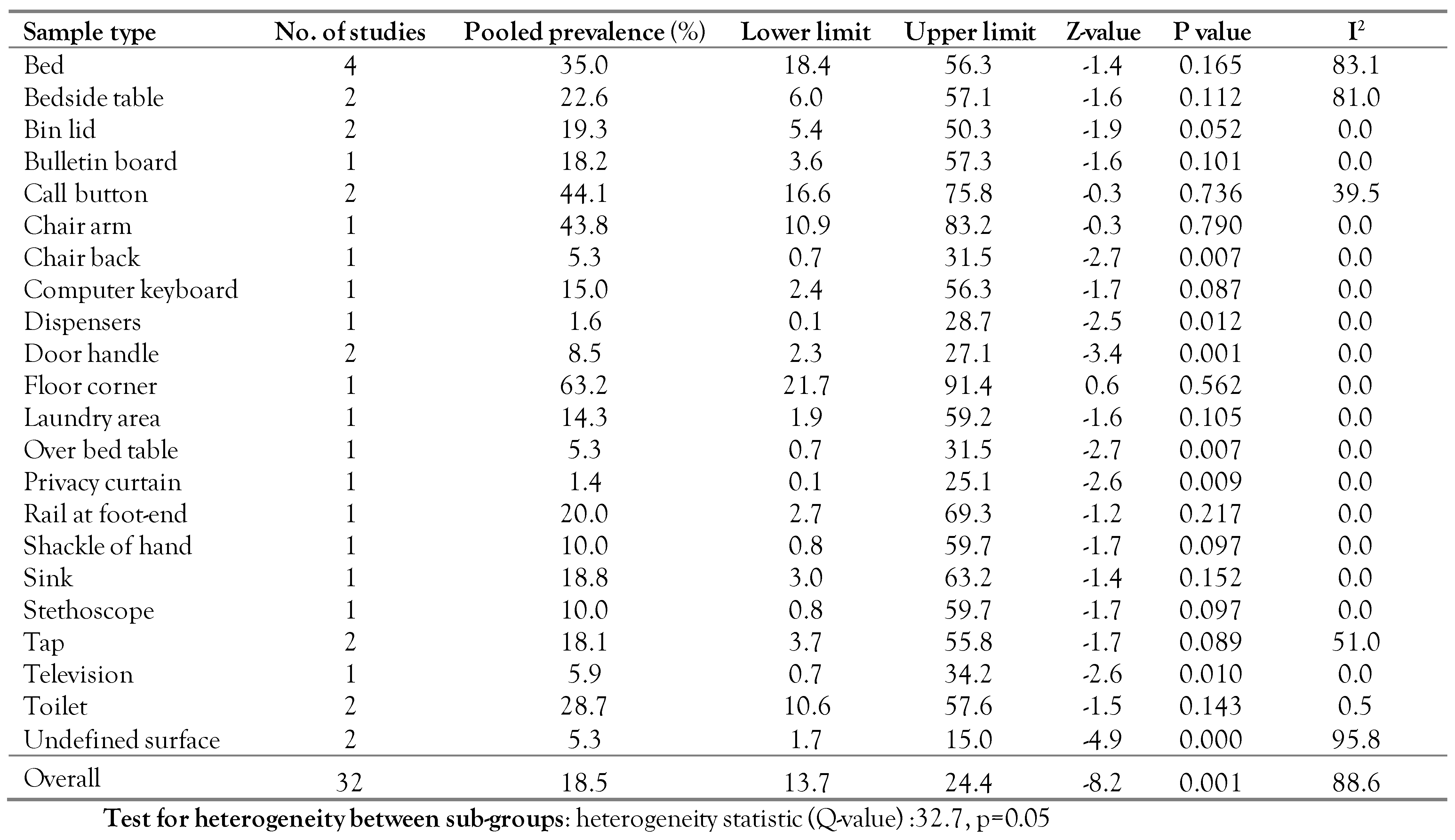
The C. difficile prevalence in each sample
To analyze C. difficile prevalence in each sample, a random-effects model meta-analysis was conducted in which the number of samples in each sample was used as sample size. In this analysis, 21 sample types were used as follows: bed, bedside table, bin lid, bulletin board, call button, chair arm, chair back, computer keyboard, dispensers, door handle, floor corner, laundry area, over bed table, privacy curtain, rail at foot-end, shackle of hand, sink, stethoscope, tap, television, toilet, and undefined surface. A significant heterogeneity was seen between C. difficile prevalence in hospital environments in different samples (Q-value=32.7, p=0.05)—Table 3. Chair back, door handle, over bed table, privacy curtain, undefined surface had p≤0.05 and are therefore significant. This indicates that the prevalence of CD is more frequent in these cases.

Table 3.
C. difficile prevalence in each sample.
The highest and lowest prevalence were reported for floor corners (63.2%) and privacy curtains (1.4%), respectively. It should be noted that the number of studies in each sample is very low, which may undermine the obtained results.
Publication bias
The prevalence of C. difficile in hospital environments was applied to check the publication bias. Egger’s test showed no significant publication bias in the studies (p=0.296).
Discussion
Clostridioides (formerly Clostridium) difficile infection (CDI) has emerged as one of the challenges in healthcare-related infectious diseases which cause high costs, and high rates of morbidity and mortality. Despite technological improvements in cleaning and disinfection, the survival of pathogens in healthcare environments increases the risk of infectious diseases and the transmission of multidrug resistant organisms to hospitalized patients. Spores, on the other hand, are forms that enter the environment by colonized bacteria or infected patients and are resistant to disinfection and persist in the environment for up to five months [13].
More than 70% of all CDI cases globally are hospital-associated [14,15]. Many studies have reported that environmental contamination by CD could increase the CDI risk in hospitalized patients receiving antibiotics, and proximity to high-touch contaminated surfaces and devices is positively correlated with CDI [16,17,18]. Therefore, the implementation of precise surveillance and screening of high-risk and high-touch surfaces in hospital wards could be helpful in the control of HAI, especially CDI.
This systematic review and meta-analysis of published studies reported the prevalence of CD in different parts of the hospital wards, rooms, devices, and instruments for the first time. Such research is pivotal for clarifying the crucial role that the environment performs in transmitting pathogens in a hospital setting.
In our extensive systematic review, of the 5,092 screened articles published over ten years between 2012 and 2021, a total of 11 eligible articles were included. The pooled overall CD prevalence was 15.5% based on the country of study. The pooled prevalence in developing countries such as India (51.1%) was higher than in developed countries such as Canada (3.9%), the UK (17.8%), and the USA (1.6%).
Epidemiological studies show that HAI prevalence in developing countries is also higher than in developed countries [18].
Interestingly, there is a direct relationship between the prevalence of environmental contamination of CD in healthcare settings and HA CDI in each country and continent [19]. The crucial reason for these differences is the lack of proper surveillance and weak infection control programs in developing countries [20].
There was a direct relationship between environmental contamination and the overall prevalence of HAIs in low-to-middle-income countries [21]. In this regard, environmental contamination is associated with the increased transmission risk of CD in hospital settings [22,23]. Furthermore, transmission of CD through environmental contamination in the healthcare setting is more important than person to person spread [13].
In other words, interestingly, any country with a high prevalence of CD isolated from hospital environments and devices, has a higher prevalence of hospital-associated CDI [13]. Of course, it must be noted that other risk factors such as antibiotic usage are also involved; therefore, colonization and the presence of a risk factor can also lead to CDI [24].
The presented study evaluated CD prevalence in the healthcare environments, and 18.5% of environmental samples were contaminated. The highest pooled prevalence belonged to the floor corner (63.2%), call button (44.1%), and the chair arm (43.8%). The last two areas are high hand-touch sites. Contamination spreading and transmission could occur through healthcare staff, patients and other caregivers. It was reported that 31% and 10% of the surfaces accessible by the physician and nurses were contaminated with CD [25]. Therefore, hand hygiene and disinfection of devices could decrease the risk of healthcare-associated infection [25], but CD spores are resistant.
Furthermore, the patient bed (35%) increases the risk of contamination due to the difficulty of cleaning. Our report was similar to a previously published study by Yui MSc et al., which reported 38% contamination of patient beds [11]. Hospital mattresses and sheets are the highest touch points when a patient is in the hospital room. Therefore, they need to be correctly decontaminated among patients. Different disinfectants such as sodium hypochlorite, hydrogen peroxide, quaternary ammonia compounds, and phenolics are used to clean and disinfect the mattress. Still, their performance in killing bacteria in the soft mattress is limited [26]. Industrial washes are inadequate to remove spores from linen sheets [27]. Unfortunately, some studies have shown that the current cleaning procedure after each patient does not adequately protect future patients and previously infected patients increase the risk of pathogen transmission. The use of a bed cover reduced the rate of HA CDIs by about 50% [28].
Certain devices also are at increased odds of contamination due to rates of stethoscope hygiene in the clinical setting being dangerously low. This may be an underappreciated vehicle for disease transmission. A study reported that stethoscope diaphragms and tubing could transmit pathogens [29]. Another study reported that the average number of colonies was 33 CFU in 8 barrier-free medical devices [30]. The present study reported a stethoscope contamination rate of 10%. The Center for Disease Control and Prevention guidelines recommend to clean for at least 1 min each time using alcohol or bleach based disinfectant, but stethoscope hygiene is poor, and spores are resistant to alcohol-based disinfectants [30]. Therefore, stethoscope hygiene must be improved to prevent transmission to patients.
Detection rates on surfaces and equipment strongly suggest the transmission of infections in the analyzed hospitals. The finding of this study emphasizes the transmission pattern of CD to be recovered from patients’ rooms and contaminated sites, which suggests the contamination is concentrated in these rooms. This raises serious concerns about hygiene measures. Preventing CDI in the hospital helps to decrease morbidity and mortality in patients. According to a recent study, CDI has a mortality rate of 9.3%. Hospitals need to find ways to reduce CDI disease.
In conclusion, hospitals’ medical devices and environmental surfaces are considered the most crucial source of CDI. In this regard, we strongly recommend revising and improving the cleaning and disinfection methods in hospitals and control of cleaning adequacy.
Conclusions
Based on the results of the present study, the importance of decontamination of floor corners call button, chair arm, bed, toilet, and bedside tables, which are known as the places with the highest level of contamination, play an essential role in preventing and reducing infections related to healthcare, especially C. difficile. Furthermore, in addition to training and emphasizing empowerment of staff, it is recommended that programs be performed to disinfect high-touch surfaces [31]. Chemical disinfectants, wipes, heat, and ultraviolet disinfection systems have potential antimicrobial activity. However, if the surfaces of patients’ rooms before and after admission are not adequately cleaned and disinfected, the reduction of microbial contamination of the surface does not occur and can contribute to the spread of healthcare CDI [32].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/doi/s1.
Author Contributions
AA designed study, SK and SB conducted data and wrote the manuscript, MR performed the statistical analysis, RA edited the article, SD, SKooti and SH searched for articles. All authors reviewed the manuscript. All authors read and approved the final version of the manuscript.
Funding
This research was financially supported by Vice-Chancellor for Research and Technology of Kermanshah University of Medical Sciences. The funding source was supported in study design.
Data Availability Statement
Data of the present study are available within the article and its supplementary materials.
Acknowledgments
The authors of this article express their gratitude and appreciation to the Vice-Chancellor for Research and Technology of the Kermanshah University of Medical Science for accepting the costs of implementing this project. The present article has been registered with grant number: 980017 and ethical code: IR.KUMS.REC.1398.017.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yakob, L.; Riley, T.V.; Paterson, D.L.; Clements, A.C. Clostridium difficile exposure as an insidious source of infection in healthcare settings: An epidemiological model. BMC Infect Dis. 2013, 13, 376. [Google Scholar] [CrossRef]
- Doll, M.; Marra, A.R.; Apisarnthanarak, A.; Al-Maani, A.S.; Abbas, S.; Rosenthal, V.D. Prevention of Clostridioides difficile in hospitals: A position paper of the International Society for Infectious Diseases. Int J Infect Dis. 2021, 102, 188–195. [Google Scholar] [CrossRef]
- Czepiel, J.; Dróżdż, M.; Pituch, H.; et al. Clostridium difficile infection: Review. Eur J Clin Microbiol Infect Dis. 2019, 38, 1211–1221. [Google Scholar] [CrossRef]
- Turner, N.A.; Anderson, D.J. Hospital infection control: Clostridioides difficile. Clin Colon Rectal Surg. 2020, 33, 98–108. [Google Scholar] [CrossRef]
- Chau, J.P.C.; Liu, X.; Lo, S.H.S.; Chien, W.T.; Wan, X. Effects of environmental cleaning bundles on reducing healthcare-associated Clostridioides difficile infection: A systematic review and meta-analysis. J Hosp Infect. 2020, 106, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, H.; Fathollahi, M.; Abiri, R.; Kadivarian, S.; Rostamian, M.; Alvandi, A. A worldwide systematic review and meta-analysis of bacteria related to antibiotic-associated diarrhea in hospitalized patients. PLoS ONE 2021, 16, e0260667. [Google Scholar] [CrossRef]
- Kociolek, L.K.; Crews, J.D.; Schwenk, H.T. Recent advances in Clostridioides difficile infection epidemiology, diagnosis and treatment in children. Curr Opin Infect Dis. 2021, 34, 527–532. [Google Scholar] [CrossRef] [PubMed]
- De Roo, A.C.; Regenbogen, S.E. Clostridium difficile infection: An epidemiology update. Clin Colon Rectal Surg. 2020, 33, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Weber, D.J. Are room decontamination units needed to prevent transmission of environmental pathogens? Infect Control Hosp Epidemiol. 2011, 32, 743–747. [Google Scholar] [CrossRef]
- Anderson, D.J.; Chen, L.F.; Weber, D.J.; et al. Enhanced terminal room disinfection and acquisition and infection caused by multidrug-resistant organisms and Clostridium difficile (the Benefits of Enhanced Terminal Room Disinfection study): A cluster-randomised, multicentre, crossover study. Lancet 2017, 389, 805–814. [Google Scholar] [CrossRef]
- Yui, S.; Ali, S.; Muzslay, M.; Jeanes, A.; Wilson, A.P.R. Identification of Clostridium difficile reservoirs in the patient environment and efficacy of aerial hydrogen peroxide decontamination. Infect Control Hosp Epidemiol. 2017, 38, 1487–1492. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Reprint-preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Phys Ther. 2009, 89, 873–880. [Google Scholar] [CrossRef]
- Balsells, E.; Shi, T.; Leese, C.; et al. Global burden of Clostridium difficile infections: A systematic review and meta-analysis. J Glob Health. 2019, 9, 010407. [Google Scholar] [CrossRef]
- Guerrero, D.M.; Nerandzic, M.M.; Jury, L.A.; Jinno, S.; Chang, S.; Donskey, C.J. Acquisition of spores on gloved hands after contact with the skin of patients with Clostridium difficile infection and with environmental surfaces in their rooms. Am J Infect Control. 2012, 40, 556–558. [Google Scholar] [CrossRef]
- Janezic, S.; Potocnik, M.; Zidaric, V.; Rupnik, M. Highly divergent Clostridium difficile strains isolated from the environment. PLoS ONE 2016, 11, e0167101. [Google Scholar] [CrossRef] [PubMed]
- Spigaglia, P. Recent advances in the understanding of antibiotic resistance in Clostridium difficile infection. Ther Adv Infect Dis. 2016, 3, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Collins, D.; Riley, T. Environmental sources of Clostridioides (Clostridium) difficile in the hospital. Int J Infect Dis. 2020, 101, 274–275. [Google Scholar] [CrossRef]
- Bauer, M.P.; Kuijper, E.J. Potential sources of Clostridium difficile in human infection. Infect Dis Clin North Am. 2015, 29, 29–35. [Google Scholar] [CrossRef]
- Guggenheim, M.; Zbinden, R.; Handschin, A.E.; Gohritz, A.; Altintas, M.A.; Giovanoli, P. Changes in bacterial isolates from burn wounds and their antibiograms: A 20-year study (1986–2005). Burns. 2009, 35, 553–560. [Google Scholar] [CrossRef]
- Vilar-Compte, D.; Camacho-Ortiz, A.; Ponce-de-León, S. Infection control in limited resources countries: Challenges and priorities. Curr Infect Dis Rep. 2017, 19, 20. [Google Scholar] [CrossRef]
- Allegranzi, B.; Pittet, D. Healthcare-associated infection in developing countries: Simple solutions to meet complex challenges. Infect Control Hosp Epidemiol. 2007, 28, 1323–1327. [Google Scholar] [CrossRef]
- Weber, D.J.; Rutala, W.A. The role of the environment in transmission of Clostridium difficile infection in healthcare facilities. Infect Control Hosp Epidemiol. 2011, 32, 207–209. [Google Scholar] [CrossRef]
- Samore, M.H.; Venkataraman, L.; DeGirolami, P.C.; Arbeit, R.D.; Karchmer, A.W. Clinical and molecular epidemiology of sporadic and clustered cases of nosocomial Clostridium difficile diarrhea. Am J Med. 1996, 100, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.; Lawrence, J.; Berry, C.; et al. Risk Factors for primary Clostridium difficile infection; results from the observational study of risk factors for Clostridium difficile infection in hospitalized patients with infective diarrhea (ORCHID). Front Public Health. 2020, 8, 293. [Google Scholar] [CrossRef] [PubMed]
- Dumford, D.M., III; Nerandzic, M.M.; Eckstein, B.C.; Donskey, C.J. What is on that keyboard? Detecting hidden environmental reservoirs of Clostridium difficile during an outbreak associated with North American pulsed-field gel electrophoresis type 1 strains. Am J Infect Control. 2009, 37, 15–19. [Google Scholar] [CrossRef]
- Hooker, E.A.; Ulrich, D.; Brooks, D. Successful removal of Clostridioides difficile spores and pathogenic bacteria from a launderable barrier using a commercial laundry process. Infect Dis. 2020, 13, 1178633720923657. [Google Scholar] [CrossRef]
- Tarrant, J.; Jenkins, R.O.; Laird, K.T. From ward to washer: The survival of Clostridium difficile spores on hospital bed sheets through a commercial UK NHS healthcare laundry process. Infect Control Hosp Epidemiol. 2018, 39, 1406–1411. [Google Scholar] [CrossRef]
- Hooker, E.A.; Bochan, M.; Reiff, T.T.; Blackwell, C.; Webb, K.W.; Hart, K.W. Decreasing Clostridium difficile health care-associated infections through use of a launderable mattress cover. Am J Infect Control. 2015, 43, 1326–1330. [Google Scholar] [CrossRef]
- Vajravelu, R.K.; Guerrero, D.M.; Jury, L.A.; Donskey, C.J. Evaluation of stethoscopes as vectors of Clostridium difficile and methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol. 2012, 33, 96–98. [Google Scholar] [CrossRef]
- Peacock, W.F.; Kalra, S.; Vasudevan, R.S.; Torriani, F. Aseptic stethoscope barriers prevent C difficile transmission in vitro. Mayo Clin Proc Innov Qual Outcomes. 2021, 5, 103–108. [Google Scholar] [CrossRef]
- Daniels, T.; Earlywine, M.; Breeding, V. Environmental services impact on healthcare-associated Clostridium difficile reduction. Am J Infect Control. 2019, 47, 400–405.e1. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Vossebein, L.; Zille, A. Efficacy of disinfectant-impregnated wipes used for surface disinfection in hospitals: A review. Antimicrob Resist Infect Control. 2019, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Sjöberg, M.; Eriksson, M.; Andersson, J.; Norén, T. Transmission of Clostridium difficile spores in isolation room environments and through hospital beds. APMIS 2014, 122, 800–803. [Google Scholar] [CrossRef] [PubMed]
- Faires, M.C.; Pearl, D.L.; Ciccotelli, W.A.; et al. A prospective study to examine the epidemiology of methicillin-resistant Staphylococcus aureus and Clostridium difficile contamination in the general environment of three community hospitals in southern Ontario, Canada. BMC Infect Dis. 2012, 12, 290. [Google Scholar] [CrossRef]
- Anderson, D.J.; Gergen, M.F.; Smathers, E.; et al. Decontamination of targeted pathogens from patient rooms using an automated ultraviolet-C-emitting device. Infect Control Hosp Epidemiol. 2013, 34, 466–471. [Google Scholar] [CrossRef]
- Best, E.L.; Parnell, P.; Thirkell, G.; et al. Effectiveness of deep cleaning followed by hydrogen peroxide decontamination during high Clostridium difficile infection incidence. J Hosp Infect. 2014, 87, 25–33. [Google Scholar] [CrossRef]
- Forrester, J.D.; Banaei, N.; Buchner, P.; Spain, D.A.; Staudenmayer, K.L. Environmental sampling for Clostridium difficile on alcohol-based hand rub dispensers in an academic medical center. Surg Infect 2014, 15, 581–584. [Google Scholar] [CrossRef]
- Faires, M.C.; Pearl, D.L.; Berke, O.; Reid-Smith, R.J.; Weese, J.S. The identification and epidemiology of meticillin-resistant Staphylococcus aureus and Clostridium difficile in patient rooms and the ward environment. BMC Infect Dis. 2013, 13, 342. [Google Scholar] [CrossRef]
- Vaishnavi, C.; Singh, M. Preliminary investigation of environmental prevalence of Clostridium difficile affecting inpatients in a north Indian hospital. Indian J Med Microbiol. 2012, 30, 89–92. [Google Scholar] [CrossRef]
- Goldenberg, S.D.; Patel, A.; Tucker, D.; French, G.L. Lack of enhanced effect of a chlorine dioxide-based cleaning regimen on environmental contamination with Clostridium difficile spores. J Hosp Infect. 2012, 82, 64–67. [Google Scholar] [CrossRef]
- Srinivasa, V.R.; Hariri, R.; Frank, L.R.; et al. Hospital-associated Clostridium difficile infection and reservoirs within the hospital environment. Am J Infect Control. 2019, 47, 780–785. [Google Scholar] [CrossRef]
- Ali, S.; Muzslay, M.; Wilson, P. A novel quantitative sampling technique for detection and monitoring of Clostridium difficile contamination in the clinical environment. J Clin Microbiol. 2015, 53, 2570–2574. [Google Scholar] [CrossRef]
- Morales, L.; Rodríguez, C.; Gamboa-Coronado, M.D.M. Molecular detection of Clostridium difficile on inert surfaces from a Costa Rican hospital during and after an outbreak. Am J Infect Control. 2016, 44, 1517–1519. [Google Scholar] [CrossRef]
© GERMS 2022.