Abstract
Introduction: Mortality due to carbapenem-resistant Pseudomonas aeruginosa (CRPA) infection has increased worldwide in recent years. The risk factors associated with hospital settings in Iran and the role of strain resistance mechanisms in many studies are unclear. Methods: A retrospective study was conducted on consecutive non-repetitive patients with CRPA infections isolated from seven major hospitals from northwest of Iran. We evaluated different risk factors and characteristics of bacteria for the death or survival of patients. Results: In this study, 116 CRPA isolates were obtained from patients admitted to seven hospitals. Forty-one (35.3%) patients were enrolled in the study of mortality risk factors. Significant risk factors associated with mortality included the site of infection, hospitalization in different wards, the use of invasive devices, and the type of carbapenem resistance mechanisms. Conclusions: ICU admission, the use of mechanical ventilation and chest tube and infection with pandrug-resistant strains were the most important factors in increasing mortality due to CRPA infection. These results suggested that the clinicians should emphasize the proper use of antibiotic and invasive procedures.
Introduction
Pseudomonas aeruginosa, one of the most common bacteria in nosocomial infections, is linked to greater rates of mortality and morbidity [1]. Because of their resistance to a wide range of antimicrobials, P. aeruginosa infections have always been a challenge for healthcare providers.
Carbapenems are a class of highly efficient antibiotics that are routinely used to treat severe bacterial infections. However, infections caused by carbapenem–resistant isolates are on the rise [2]. Multiple carbapenem resistance mechanisms have been identified in P. aeruginosa. These strains are a major problem all over the world and challenging to treat. Carbapenem–resistant P. aeruginosa (CRPA) results in a longer-hospital stay, greater morbidity and mortality, and therefore higher cost than infections caused by susceptible bacteria [3]. Among 20 antimicrobial-resistant bacterial species, CRPA is the second most critical-priority pathogen [4]. Today, the mortality risk factors and other aspects of CRPA infection in patients in Iran are unknown.
Therefore, we assessed the risk factors such as different mechanisms of carbapenem resistance, hospitalization days, antibiotic therapy and other clinical impacts on survivor and non-survivor hospital patients infected with CRPA strains.
Methods
Study design
This retrospective study was conducted on consecutive non-repetitive patients with CRPA infections in the two-year period from July 2013 to December 2015 in seven major hospitals from northwest of Iran (Figure 1). These hospitals have multiple intensive care units, two referrals burn wards each city and different general wards. Most patients were hospitalized due to burns, internal problems, trauma, surgeries, and kidney disease as the primary disease. According to the objectives of the study, the therapeutic dose and duration of antibiotic exposure were chosen by physicians using treatment protocols for various infections caused by P. aeruginosa.
Figure 1.
Cross-sectional study design and study flow chart. CRPA—carbapenem-resistant P. aeruginosa.
Data collection
The medical documents of CRPA infected patients were reviewed in this study. Demographic data collected included underlying diseases (trauma, burned diabetes mellitus, ischemia, infections, chronic lung disease, myocardial infract, cancer, kidney stone, surgery, chronic renal failure, transplantation, hematological disease and cerebrovascular accident), the infection site (such as blood, lung, urinary tract, soft tissue), antibiotic therapy, hospitalization days, ICU admission and mortality.
Microbiologic analysis
This study was performed on clinical information of patients extracted from medical records such as the type of infection, patients receiving antibiotics, etc. In addition, the isolates were stored in 15% glycerol and stored at −70 °C for further examinations. All P. aeruginosa isolates were identified with standard microbiological tests. The antibiotic susceptibility profile and MIC of bacteria were determined according to CLSI guidelines [5]. Multiple carbapenem resistance mechanisms (such as carbapenemases, AmpC overproduction, overexpression of efflux pump, and OprD gene down-regulation) were detected by phenotypic and genotypic methods that we described formerly [6]. The Random Amplified Polymorphic DNA (RAPD)-PCR was performed to determine the epidemiological relationships between CRPA strains [7].
Statistical analysis
Data were analyzed with the Statistical Package for Social Sciences software for Windows version 26 (IBM Corp, USA). Student’s t-test was used to analyze the numerical data and parametric continuous variables. Categorical variables were also compared using a Chi-square test. For non-parametric continuous variables, the odds ratios of all variables were analyzed by logistic regression and a p value less than 0.05 was considered to be significant. Mean ± standard deviation was used only for parametric variables.
Results
Study population
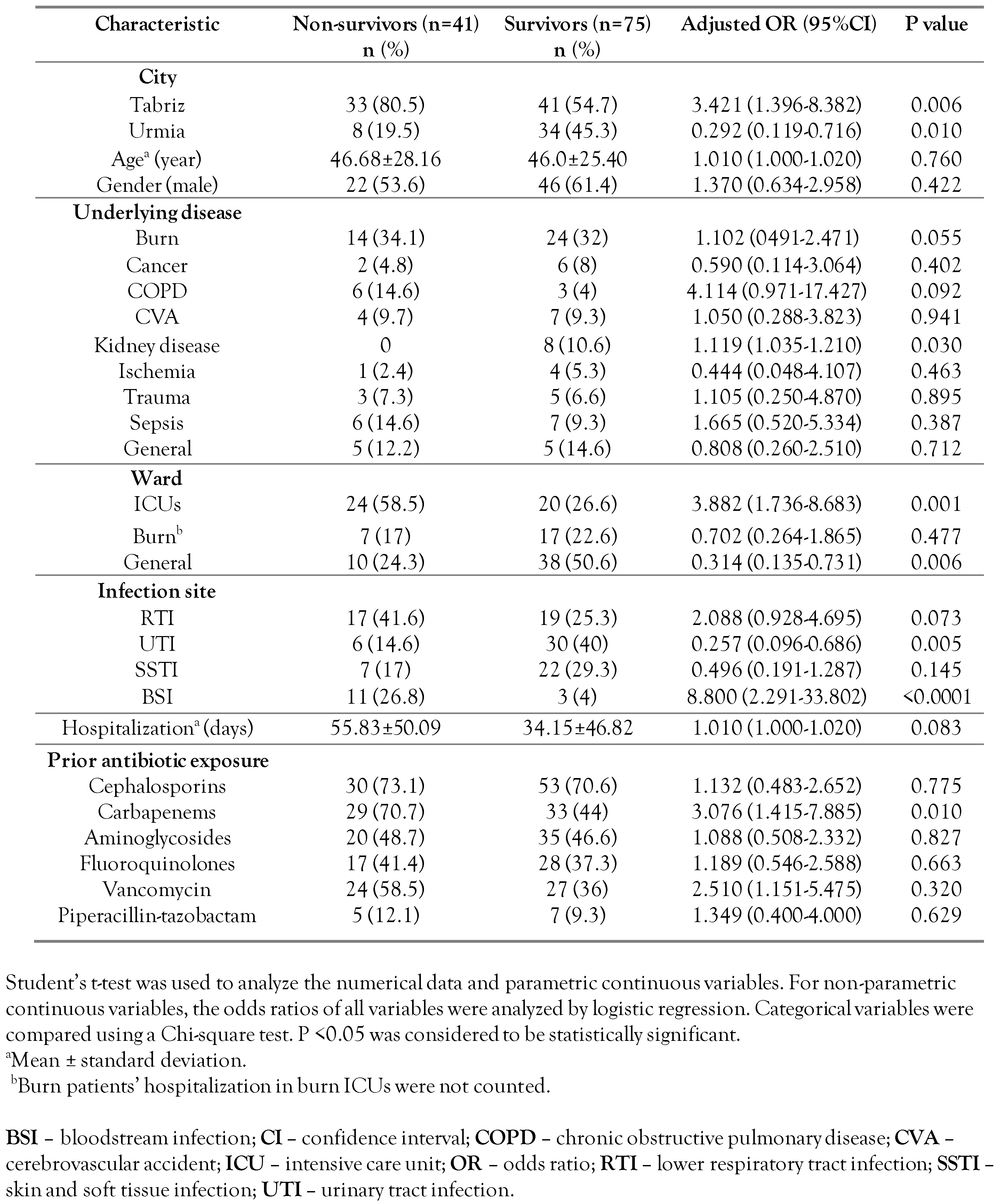
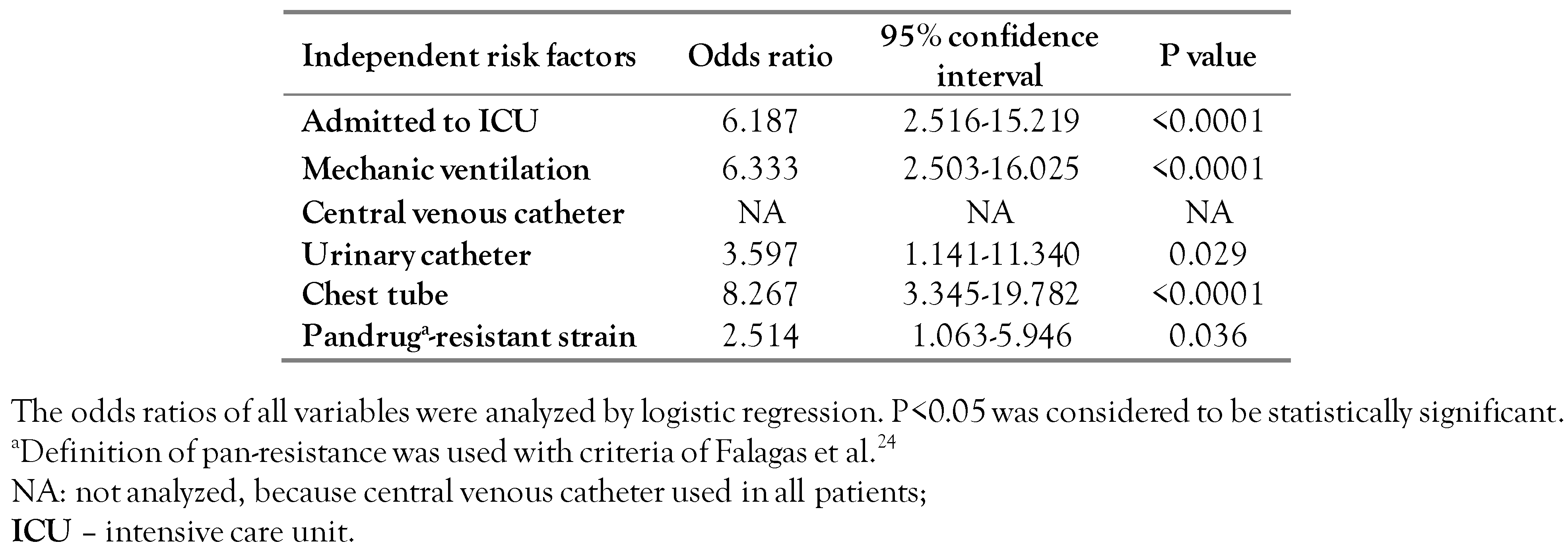
During the study periods, we collected 243 consecutive non-repetitive P. aeruginosa isolates from inpatients who became infected 48 hours after admission. A total of 116 isolates (74 isolates from Tabriz and 42 isolates from Urmia) were collected as a CRPA isolates (resistant to imipenem and/or meropenem). Of 116 infected patients, 41 died with a mortality rate of 35.34%. The mean age was 46.6 years old and 53.7% of them were male. The most common underlying disease was represented by burns (34.1%). The most frequent site of infection was the lower respiratory tract (39%) and the average hospitalization duration was 55.8 days. Further, the 41 deceased patients (case group) and 75 patients discharged alive (control group) were analyzed (Table 1). The length of hospital stay in the case and control groups were not statistically different (p=0.083). Although a similar proportion of deceased and patients discharged alive required ICU admission, deceased patients remained significantly longer in the ICU than surviving patients (p=0.001, Table 1). In the mortality risk analysis in patients with various underlying diseases, only kidney disease was statistically significant (p=0.030), because all of them survived with CRPA infection. The site of infection in most survivors was a urinary tract infection (UTI) (p=0.005) and most non-survivors had a bloodstream infection (p<0.0001, Table 1). Multivariate analysis confirmed that risk factors such as ICU admission, mechanic ventilation, urinary catheter, and infection with pandrug-resistant strain significantly increased patient mortality (Table 2).

Table 1.
Univariate comparison of the demographic and clinical characteristics of 41 non-survivor patients and 75 survivor patients with carbapenem-resistant Pseudomonas aeruginosa infection in this study.

Table 2.
Multivariate analysis (logistic regression) of risk factors for mortality with carbapenem-resistant Pseudomonas aeruginosa infection in Iran.
Microbiologic outcome
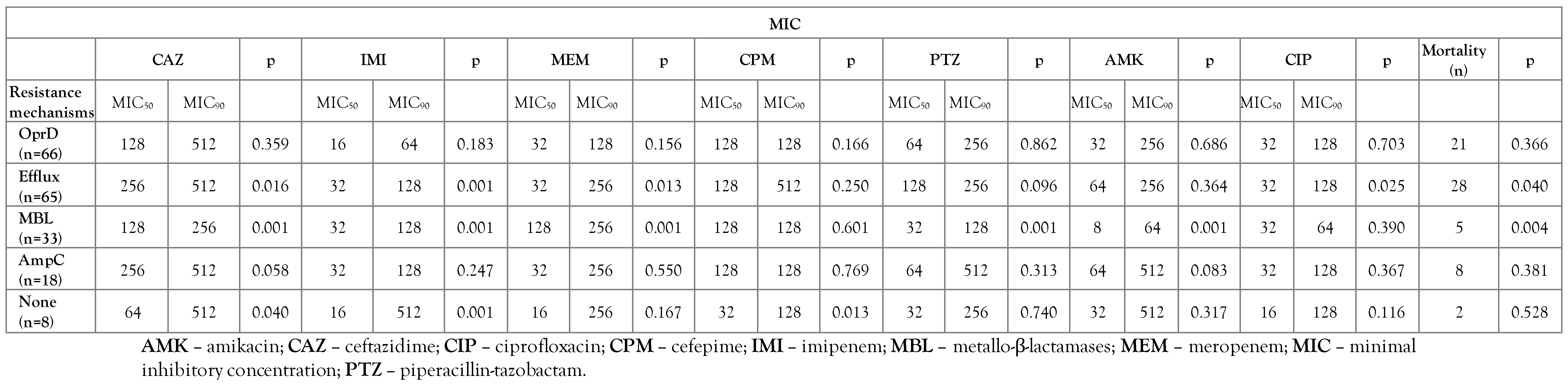
Decreased expression of the OprD gene and overexpression of efflux pumps were indicative of high-rate resistance mechanisms (56.9%, 56%), respectively. Analysis of 11 carbapenemase resistance gene showed that 33 (28.5%) isolates were positive for metallo-β-lactamases (MBL) genes including blaIMP-1 and blaVIM-2. In 18 (15.5%) isolates, overproduction of AmpC was detected. Most isolates had two or three different carbapenem resistance mechanisms. No carbapenem resistance mechanism was detected in the eight CRPA isolates (Table 3).

Table 3.
Evaluation of the carbapenem resistance mechanism with antibiotic susceptibility and mortality in collected carbapenem-resistant Pseudomonas aeruginosa isolates from Iran.
The CRPA isolates (Dice coefficient ≥80%) were distributed among 34 different RAPD-types in Tabriz and 24 RAPD-types in Urmia. A major clone of CRPA from Tabriz has 11 strains that circulate in Sina and Emam Raza hospitals. The death of 54 patients was due to strains of four main clones in Tabriz. In Urmia hospitals, the main clone had 11 carbapenemase-producing strains in the internal medicine and urology wards. All deaths of patients with CRPA strains in Urmia were related to individual clones.
Discussion
The increasing prevalence of CRPA strains is a great concern due to widespread resistance to various antibiotics [8]. Evaluation of risk factors of infection with CRPA can improve management of patients and decrease the mortality rate [9]. Our study was a retrospective study that examined various factors in death or survival due to CRPA infection. The study results may decline confounding factors from the same hospitals and help to control and manage nosocomial infections.
To our knowledge, this is the first study to address the risk factors for CRPA infection in Iran. We observed the different site of infection, hospitalization in various wards and use of invasive devices for patients, which were associated with a significantly increased mortality rate.
Several studies have described that length of hospitalization associated increased mortality with CRPA [3,9,10,11,12] We did not find any significant difference (p=0.083) in mortality rate with hospitalization days. These results were confirmed in different research [13,14]. Most of the patients with mild burns in our study needed long-term hospitalization, and some patients needed to be admitted to the ICU for various reasons, which may be due to CRPA infections. Most of the patients who died from burns were admitted to the burn intensive care unit. The highest mortality rate occurred in the intensive care unit (p=0.001) and the lowest rate of morality was in the general ward (p=0.006), which is expected because most of the critically ill patients were hospitalized in this ward. Most of our results are similar to other studies [3,9,11,15,16].
Previous use of antimicrobial agents has been associated with increased drug resistance in CRPA infection [17]. In most of the patients we studied, a combination of two to three broad-spectrum antibiotics such as fluoroquinolones, aminoglycosides, third or fourth generation cephalosporins and carbapenems was used before or after the infection. There was no statistically significant relationship between antibiotic use and mortality in our study. This result was similar to the results of Zhang et al. [18] and contrary to the results of Tsao et al. [3] Examination of patients’ records showed that carbapenem use in patients who died had a significant (p=0.016) increase in our study. Colistin, a drug used to treat CRPA isolates [8], was used in only three patients, of whom two died and one survived.
There was a significant association between the site of infection and its mortality rate in CRPA infection. Statistically, the mortality rate was lower in patients with UTIs (p=0.005), but higher in those with bloodstream infection (p<0.0001). Aminoglycoside antibiotics such as amikacin are more effective for patients with UTI and monotherapy can be used in these patients [19]. In our study of 36 patients with UTI, 27 patients were infected with amikacin-sensitive isolates, as most of these patients were treated well with the amikacin as treatment protocols. The results of our study were unlike two previous studies [3,16]. High rate of mortality in our study was from bloodstream infection that were same as in other studies [9,12,16,18].
There was no significant relationship between the assessment of the type of underlying disease and the mortality rate in our study. In this study, CRPA infection caused mortality only in patients with kidney disease (p=0.031). Also, based on laboratory data, most of these strains were sensitive to amikacin and were used in the treatment protocol. In some previous studies [3,16], some underlying diseases such as cancer or COPD caused high mortality rate. In some other studies, like our data, there was no significant relationship between mortality and the type of underlying disease [12,20,21].
Our results show that patients infected with strains that had multiple resistance mechanisms had higher mortality rates than those strains that had only one resistance mechanism. In this study, out of 15 patients infected with strains that have the OprD down-regulation mechanism, only 5 patients died, while 13 of the 23 patients infected by strains with OprD down-regulation and overexpression of efflux pumps strains died.
We confirmed that OprD down-regulation and efflux pumps hyperexpression were the main carbapenem resistance mechanisms in strains collected from patient samples. Against this study, the main mechanism of carbapenem resistance among P. aeruginosa isolates taken from many countries is carbapenemase production. Contrary to the results of other studies [22,23], the existence of carbapenemase production mechanism in our isolates was inversely related to mortality (p=0.004). In this study, we identified eight isolates that were resistant to carbapenem but had none of the studied mechanisms (efflux, OprD, carbapenemases and AmpC) of action in them (Table 3).
The presence of an efflux pump mechanism caused a significant increase in mortality in our study patients (p=0.049). In fact, the isolates that had efflux resistance mechanism were resistant to most antibiotics, while the carbapenemase-producing isolates were easily treated with aminoglycosides.
The main limitations of our study are: (I) We conducted a retrospective observational study, so there were several variables that could not be controlled at the beginning of the study design. (II) We had no control over patients’ prior exposure to antibiotics as well as the adherence and agreement to such treatments. (III) Another limitation is that the study did not consider whether the same antibiotic treatment was given to all patients. (IV) Some patients were discharged with infection and instructed to take antibiotics at home, but we have no information about the treatment outcomes of these patients.
Conclusions
We found that admission to the ICU and the use of mechanical ventilation and chest tube and infection with pandrug-resistant strains were the most important factors for increased mortality due to P. aeruginosa infection (Table 2). These outcomes suggested that the clinicians should emphasize the proper use of antibiotic and invasive procedures.
Author Contributions
YK contributed to conceptualization, design of the study, and writing–original draft preparation. PO, HRG contributed to data curation, investigation, software, validation. SZ, FB contributed to visualization, methodology, project administration. AZB contributed to supervision, methodology, reviewing and editing. All authors read and approved the final version of the manuscript.
Funding
This project was financially supported by the Immunology Research Center, Tabriz University of Medical Sciences (TBZMED) to provide all materials and supplies needed to implement this project. TBZMED was involved in the study design, collection, management, analysis, and interpretation of data.
Institutional Review Board Statement
This study was approved by the research ethics committee (TAZMED.REC.2015.161) in Tabriz University of Medical Sciences, Tabriz, Iran. In our study, we did not interfere in the treatment process of patients, we only used the information contained in the medical records of patients who were infected with CRPA.
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its tables and figure).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khalili, Y.; Omidnia, P.; Goli, H.R.; et al. Molecular characterization of carbapenem-resistant Pseudomonas aeruginosa isolated from four medical centres in Iran. Mol Biol Rep. 2022. [CrossRef] [PubMed]
- Zahedi bialvaei, A.; Rahbar, M.; Hamidi-Farahani, R.; et al. Expression of RND efflux pumps mediated antibiotic resistance in Pseudomonas aeruginosa clinical strains. Microb Pathog. 2021, 153, 104789. [Google Scholar] [CrossRef] [PubMed]
- Tsao, L.H.; Hsin, C.Y.; Liu, H.Y.; Chuang, H.C.; Chen, L.Y.; Lee, Y.J. Risk factors for healthcare-associated infection caused by carbapenem-resistant Pseudomonas aeruginosa. J Microbiol Immunol Infect. 2018, 51, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing; Twenty-fifth informational supplement M100-S25; CLSI: Wayne, PA, USA, 2015.
- Akhi, M.T.; Khalili, Y.; Ghotaslou, R.; et al. Evaluation of carbapenem resistance mechanisms and its association with Pseudomonas aeruginosa infections in the Northwest of Iran. Microb Drug Resist. 2018, 24, 126–135. [Google Scholar] [CrossRef]
- Mahenthiralingam, E.; Campbell, M.E.; Foster, J.; Lam, J.S.; Speert, D.P. Random amplified polymorphic DNA typing of Pseudomonas aeruginosa isolates recovered from patients with cystic fibrosis. J Clin Microbiol. 1996, 34, 1129–1135. [Google Scholar] [CrossRef]
- Doi, Y. Treatment options for carbapenem-resistant gram-negative bacterial infections. Clin Infect Dis. 2019, 69, S565–S75. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Cao, J.M.; Yang, Q.; et al. Risk factors for carbapenem-resistant Pseudomonas aeruginosa, Zhejiang Province, China. Emerg Infect Dis. 2019, 25, 1861–1867. [Google Scholar] [CrossRef]
- Rao, Y.B.; Ren, Z.X.; Zhong, J.J.; et al. Risk factors for imipenem-resistant Pseudomonas aeruginosa in neonatal intensive care units in south China. J Hosp Infect. 2018, 98, 305–308. [Google Scholar] [CrossRef]
- Buehrle, D.J.; Shields, R.K.; Clarke, L.G.; Potoski, B.A.; Clancy, C.J.; Nguyen, M.H. Carbapenem-resistant Pseudomonas aeruginosa bacteremia: Risk factors for mortality and microbiologic treatment failure. Antimicrob Agents Chemother. 2016, 61, e01243–16. [Google Scholar] [CrossRef]
- Kishimoto, K.; Kasai, M.; Kawamura, N.; Otake, S.; Hasegawa, D.; Kosaka, Y. Clinical characteristics and risk factors for mortality in children with Pseudomonas aeruginosa bacteraemia: A retrospective review at a paediatric tertiary centre. J Paediatr Child Health. 2021, 57, 1976–1980. [Google Scholar] [CrossRef] [PubMed]
- Ozkurt, Z.; Ertek, M.; Erol, S.; Altoparlak, U.; Akcay, M.N. The risk factors for acquisition of imipenem-resistant Pseudomonas aeruginosa in the burn unit. Burns 2005, 31, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Onguru, P.; Erbay, A.; Bodur, H.; et al. Imipenem-resistant Pseudomonas aeruginosa: Risk factors for nosocomial infections. J Korean Med Sci. 2008, 23, 982–987. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Strassle, P.D.; Williams, F.N.; Weber, D.J.; et al. Risk factors for healthcare-associated infections in adult burn patients. Infect Control Hosp Epidemiol. 2017, 38, 1441–1448. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Zeng, J.; et al. Risk factors for mortality of inpatients with Pseudomonas aeruginosa bacteremia in China: Impact of resistance profile in the mortality. Infect Drug Resist. 2020, 13, 4115–4123. [Google Scholar] [CrossRef]
- Pakyz, A.L.; Oinonen, M.; Polk, R.E. Relationship of carbapenem restriction in 22 university teaching hospitals to carbapenem use and carbapenem-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2009, 53, 1983–1986. [Google Scholar] [CrossRef]
- Zhang, D.; Cui, K.; Wang, T.; et al. Risk factors for carbapenem-resistant Pseudomonas aeruginosa infection or colonization in a Chinese teaching hospital. J Infect Dev Ctries. 2018, 12, 642–648. [Google Scholar] [CrossRef]
- Vidal, L.; Gafter-Gvili, A.; Borok, S.; Fraser, A.; Leibovici, L.; Paul, M. Efficacy and safety of aminoglycoside monotherapy: Systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2007, 60, 247–257. [Google Scholar] [CrossRef]
- Corcione, S.; Pensa, A.; Castiglione, A.; et al. Epidemiology, prevalence and risk factors for infections in burn patients: Results from a regional burn centre’s analysis. J Chemother. 2021, 33, 62–66. [Google Scholar] [CrossRef]
- Morata, L.; Cobos-Trigueros, N.; Martínez, J.A.; et al. Influence of multidrug resistance and appropriate empirical therapy on the 30-day mortality rate of Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother. 2012, 56, 4833–4837. [Google Scholar] [CrossRef]
- Ghasemian, A.; Salimian Rizi, K.; Rajabi Vardanjani, H.; Nojoomi, F. Prevalence of clinically isolated metallo-beta-lactamase-producing Pseudomonas aeruginosa, coding genes, and possible risk factors in Iran. Iran J Pathol. 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Zhang, J.F.; Chen, B.L.; Xin, X.Y.; et al. Carbapenem resistance mechanism and risk factors of Pseudomonas aeruginosa clinical isolates from a University Hospital in Xi’an, China. Microb Drug Resist. 2009, 15, 41–45. [Google Scholar] [CrossRef]
- Falagas, M.E.; Koletsi, P.K.; Bliziotis, I.A. The diversity of definitions of multidrug-resistant (MDR) and pandrug-resistant (PDR) Acinetobacter baumannii and Pseudomonas aeruginosa. J Med Microbiol. 2006, 55, 1619–1629. [Google Scholar] [CrossRef]
© GERMS 2022.