Nasal Colonization by Potential Bacterial Pathogens in Healthy Kindergarten Children of Nepal: A Prevalence Study
Abstract
Introduction
Methods
Study design and site
Study population
Inclusion and exclusion criteria
- Inclusion criteria
- Primarily, children aged 2–5 years old fulfilling health-related criteria mentioned in the questionnaire and most importantly with signed consent.
- Exclusion criteria
- Participants with acute respiratory infections (presence of two or more signs or symptoms such as fever, runny nose or nasal congestion, cough, or sore throat) [20], with any sort of respiratory tract infection or nasal abscesses, with immune-compromised state, those unwilling to participate and those without consent, were excluded.
Sample collection and processing
Bacterial isolation and characterization
Antimicrobial susceptibility test
Statistical analysis
Ethical clearance
Results
Demographic study and the prevalence of the target bacterial pathogens among participants
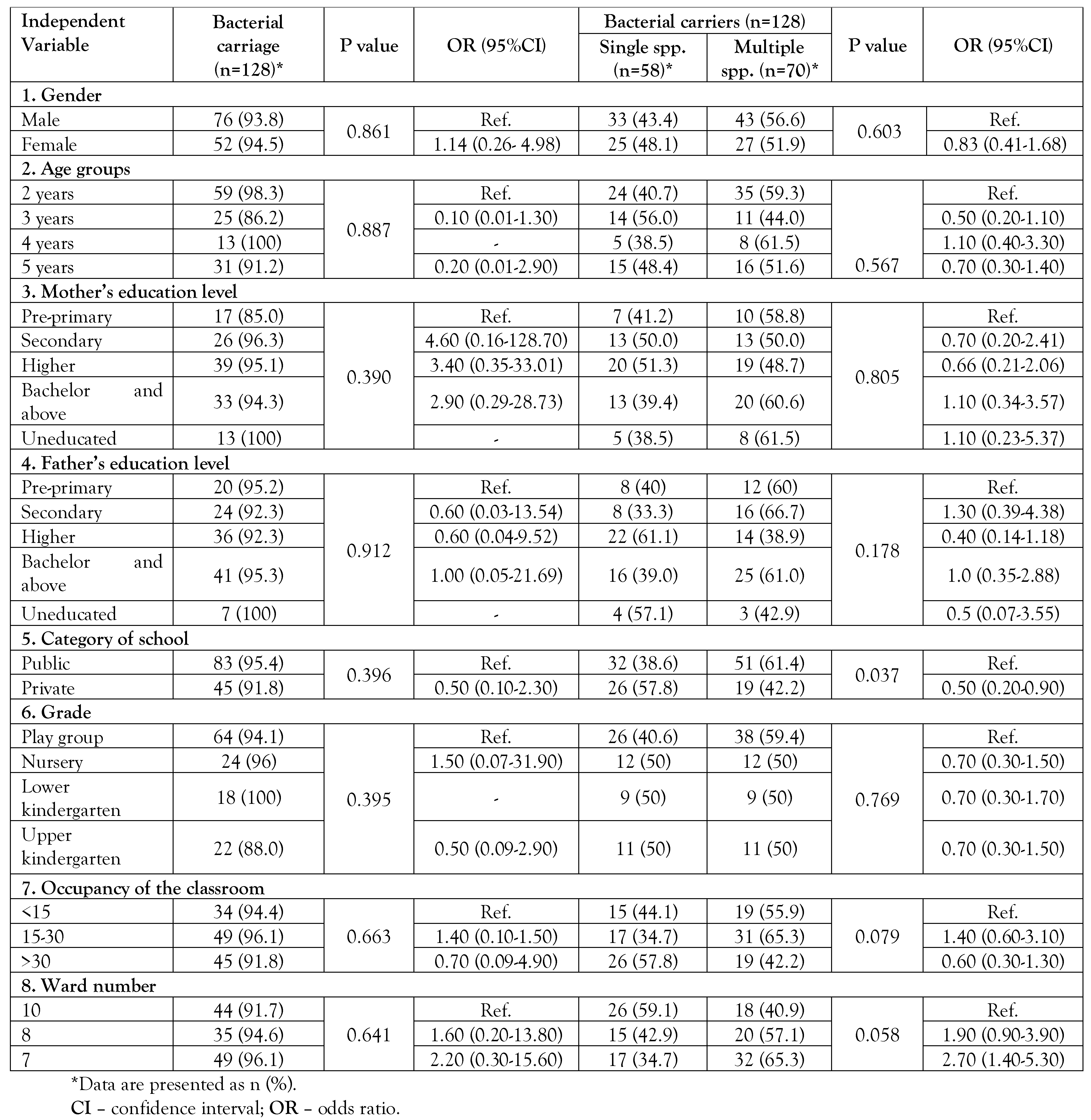
Independent variables and distribution of bacterial carriage
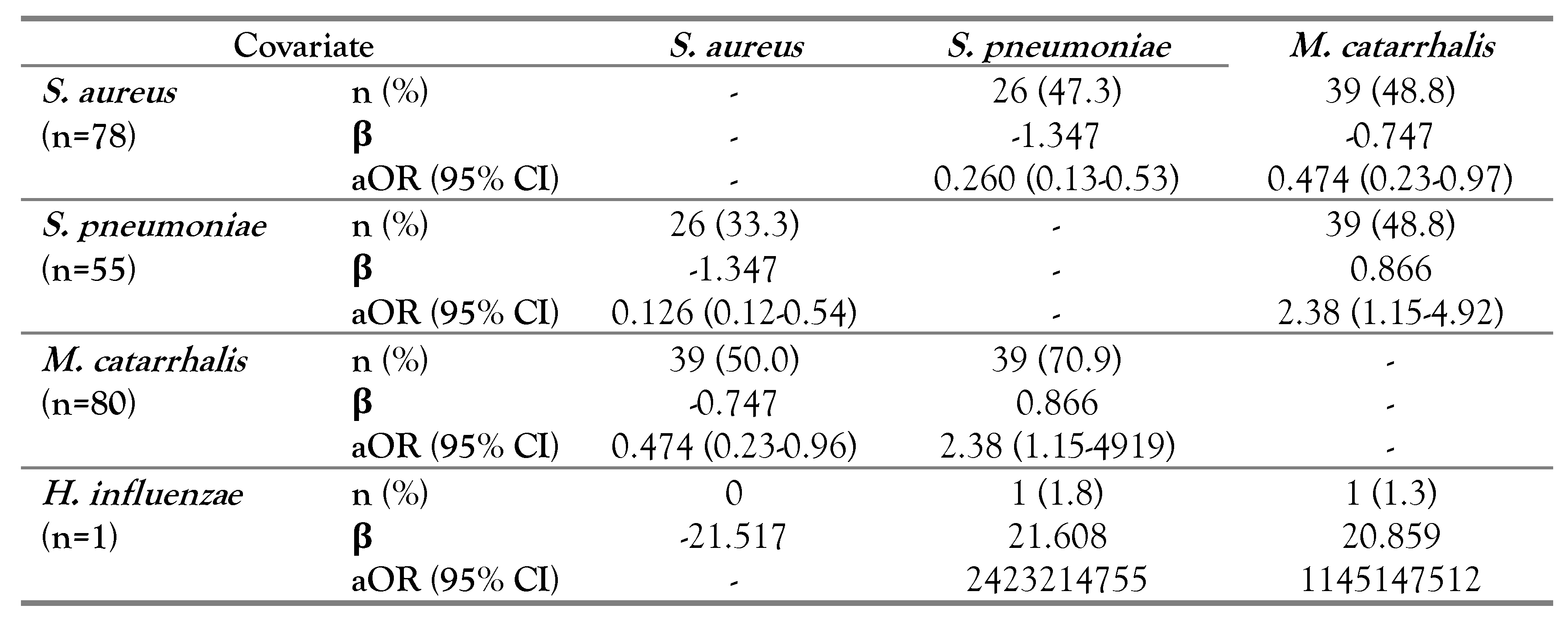
Multiple logistic regression analysis of bacterial co-existence
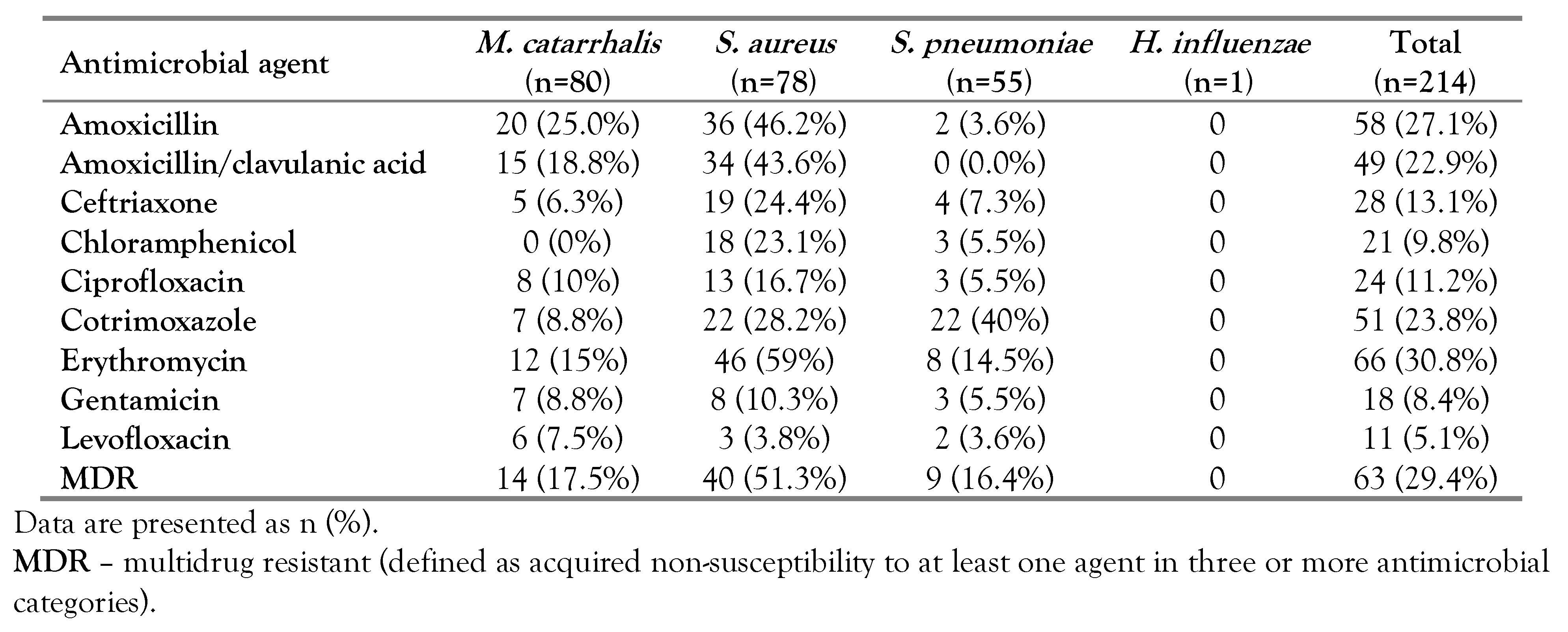
Antimicrobial pattern of target carrier bacteria
Multidrug resistance in isolates
Discussion
Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of interest
References
- Assane, D.; Makhtar, C.; Abdoulaye, D.; et al. Viral and bacterial etiologies of acute respiratory infections among children under 5 years in Senegal. Microbiol Insights 2018, 11, 1178636118758651. [Google Scholar] [CrossRef]
- Thomas, M.; Bomar, P.A. Upper respiratory tract infection. In StatPearls; StatPearls Publishing: Treasure Islan, FL, 2021. [Google Scholar]
- Dasaraju, P.V.; Liu, C. Infections of the respiratory system. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston Galveston: TX, 1996. [Google Scholar]
- Jin, X.; Ren, J.; Li, R.; et al. Global burden of upper respiratory infections in 204 countries and territories, from 1990 to 2019. EClinicalMedicine 2021, 37, 100986. [Google Scholar] [CrossRef]
- GBD 2017 Lower Respiratory Infections Collaborators. Quantifying risks and interventions that have affected the burden of lower respiratory infections among children younger than 5 years: An analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis. 2020, 20, 60–79. [Google Scholar] [CrossRef]
- Tazinya, A.A.; Halle-Ekane, G.E.; Mbuagbaw, L.T.; Abanda, M.; Atashili, J.; Obama, M.T. Risk factors for acute respiratory infections in children under five years attending the Bamenda Regional Hospital in Cameroon. BMC Pulm Med. 2018, 18, 7. [Google Scholar] [CrossRef]
- Taylor, S.; Lopez, P.; Weckx, L.; et al. Respiratory viruses and influenza-like illness: Epidemiology and outcomes in children aged 6 months to 10 years in a multi-country population sample. J Infect. 2017, 74, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.F.; Parameswaran, G.I. Moraxella catarrhalis, a human respiratory tract pathogen. Clin Infect Dis. 2009, 49, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Siegel, S.J.; Weiser, J.N. Mechanisms of bacterial colonization of the respiratory tract. Annu Rev Microbiol. 2015, 69, 425–444. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.E.; Cleary, D.W.; Clarke, S.C. Secondary bacterial infections associated with influenza pandemics. Front Microbiol. 2017, 8, 1041. [Google Scholar] [CrossRef]
- Dunne, E.M.; Murad, C.; Sudigdoadi, S.; et al. Carriage of Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and Staphylococcus aureus in Indonesian children: A cross-sectional study. PLoS ONE 2018, 14, e0195098. [Google Scholar] [CrossRef]
- Thapa, S.; Gokhale, S.; Sharma, A.L.; et al. Burden of bacterial upper respiratory tract pathogens in school children of Nepal. BMJ Open Respir Res. 2017, 4, e000203. [Google Scholar] [CrossRef]
- Wahl, B.; O’Brien, K.L.; Greenbaum, A.; et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: Global, regional, and national estimates for 2000-15. Lancet Glob Health 2018, 6, e744–e757. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Factsheet About Pneumococcal Disease. Available online: https://www.ecdc.europa.eu/en/pneumococcal-disease/facts (accessed on 22 January 2022).
- Centers for Disease Control and Prevention. 2020. Global pneumococcal disease and vaccine. 2020. Available online: https://www.cdc.gov/ncird/ (accessed on 15 June 2021).
- Pan, H.; Cui, B.; Huang, Y.; Yang, J.; Ba-Thein, W. Nasal carriage of common bacterial pathogens among healthy kindergarten children in Chaoshan region, southern China: A cross-sectional study. BMC Pediatr. 2016, 16, 161. [Google Scholar] [CrossRef]
- Government of Nepal. Ministry of Health and Population. National Immunization Programme. Available online: https://mohp.gov.np/eng/program/child-health-services/nip (accessed on 15 June 2021).
- Bhatta, D.R.; Gokhale, S.; Sharma, A.L.; et al. Carrier state of Haemophilus influenzae type b (Hib), Streptococcus pneumoniae, Streptococcus pyogenes, Neisseria meningitidis and Corynebacterium diphtheriae among school children in Pokhara, Nepal. Asian Pacific J Trop Dis. 2014, 4, 45–49. [Google Scholar] [CrossRef]
- District Coordination Committee Office Bhaktapur, Nepal. Bhaktapur at a Glance. Available online: https://dccbhaktapur.gov.np/en/brief-introduction/ (accessed on 15 June 2020).
- Centers for Disease Control and Prevention. National Center for Immunization and Respiratory Diseases (NCIRD). 2017. Available online: https://www.cdc.gov/flu/about/glossary.htm (accessed on 20 June 2021).
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. 2000. Available online: https://www.cdc.gov/nchs/data/nhanes/nhanes_01_02/specimen_collection_year_3.pdf (accessed on 20 June 2021).
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Document M100-S10; CLSI: Wayne, PA, 2018. [Google Scholar]
- Liu, L.; Xie, W.; Chunmei, J. Survey of respiratory tract microbial population in children. Chin. J Microecol. 2007, 19, 22–26. [Google Scholar]
- Naaber, P.; Tamm, E.; Pütsepp, A.; Kõljalg, S.; Maimets, M. Nasopharyngeal carriage and antibacterial susceptibility of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis in Estonian children. Clin Microbiol Infect. 2000, 6, 675–677. [Google Scholar] [CrossRef]
- Zemlicková, H.; Urbásková, P.; Adámková, V.; Motlová, J.; Lebedová, V.; Procházka, B. Characteristics of Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis and Staphylococcus aureus isolated from the nasopharynx of healthy children attending day-care centres in the Czech Republic. Epidemiol Infect. 2006, 134, 1179–1187. [Google Scholar] [CrossRef]
- Quiñones, D.; Llanes, R.; Toraño, G.; Pérez, M. Nasopharyngeal colonization by Moraxella catarrhalis and study of antimicrobial susceptibility in healthy children from Cuban day-care centers. Arch Med Res. 2005, 36, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Jourdain, S.; Smeesters, P.R.; Denis, O.; et al. Differences in nasopharyngeal bacterial carriage in preschool children from different socio-economic origins. Clin Microbiol Infect. 2011, 17, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Kovács, E.; Sahin-Tóth, J.; Tóthpál, A.; van der Linden, M.; Tirczka, T.; Dobay, O. Co-carriage of Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis among three different age categories of children in Hungary. PLoS ONE 2020, 15, e0229021. [Google Scholar] [CrossRef]
- Adegbola, R.A.; DeAntonio, R.; Hill, P.C.; et al. Carriage of Streptococcus pneumoniae and other respiratory bacterial pathogens in low and lower-middle income countries: A systematic review and meta-analysis. PLoS ONE 2014, 9, e103293. [Google Scholar] [CrossRef]
- Bae, S.; Yu, J.Y.; Lee, K.; Lee, S.; Park, B.; Kang, Y. Nasal colonization by four potential respiratory bacteria in healthy children attending kindergarten or elementary school in Seoul, Korea. J Med Microbiol. 2012, 51 Pt 5, 678–685. [Google Scholar] [CrossRef]
- Luo, X.; Liang, J.; Gao, S.; Li, Z.; Dong, Z.R. Surveys on bacteria nasopharyngeal carriage prevalence in 186 children. Chin. J Microecol. 2006, 18, 204–208. [Google Scholar]
- Littorin, N.; Rünow, E.; Ahl, J.; Resman, F.; Riesbeck, K. Decreased prevalence of Moraxella catarrhalis in addition to Streptococcus pneumoniae in children with upper respiratory tract infection after introduction of conjugated pneumococcal vaccine: A retrospective cohort study. Clin Microbiol Infect. 2021, 27, 630.e1–630.e6. [Google Scholar] [CrossRef]
- Cura Yayla, B.C.; Özsürekçi, Y.; Aykaç, K.; et al. Characteristics and management of children with COVID-19 in Turkey. Balk Med J. 2020, 37, 341–347. [Google Scholar] [CrossRef]
- Gönüllü, N.; Yıldız, S.; Aydoğan, O.; et al. Nasopharyngeal carriage of potential pathogenic bacteria in healthy children living in İstanbul. Med Bull Haseki. 2020, 58, 470–476. [Google Scholar] [CrossRef]
- Shaffer, T.L.; Balder, R.; Buskirk, S.W.; Hogan, R.J.; Lafontaine, E.R. Use of the Chinchilla model to evaluate the vaccinogenic potential of the Moraxella catarrhalis filamentous hemagglutinin-like proteins MhaB1 and MhaB2. PLoS ONE 2013, 8, e67881. [Google Scholar] [CrossRef]
- Pathak, A.; Marothi, Y.; Iyer, R.V.; et al. Nasal carriage and antimicrobial susceptibility of Staphylococcus aureus in healthy preschool children in Ujjain, India. BMC Pediatr. 2010, 10, 100. [Google Scholar] [CrossRef]
- Lamaro-Cardoso, J.; de Lencastre, H.; Kipnis, A.; et al. Molecular epidemiology and risk factors for nasal carriage of Staphylococcus aureus and methicillin-resistant S. aureus in infants attending day care centers in Brazil. J Clin Microbiol. 2009, 47, 3991–3997. [Google Scholar] [CrossRef]
- Brugger, S.D.; Frey, P.; Aebi, S.; Hinds, J.; Mühlemann, K. Multiple colonization with S. pneumoniae before and after introduction of the seven-valent conjugated pneumococcal polysaccharide vaccine. PLoS ONE 2010, 5, e11638. [Google Scholar] [CrossRef]
- Gebre, T.; Tadesse, M.; Aragaw, D.; et al. Nasopharyngeal carriage and antimicrobial susceptibility patterns of Streptococcus pneumoniae among children under five in Southwest Ethiopia. Children (Basel) 2017, 4, 27. [Google Scholar] [CrossRef]
- Luminos, M.; Dorobat, O.; Jugulete, G.; et al. Nasopharyngeal carriage of Streptococcus pneumoniae in Romanian children before the introduction of the pneumococcal conjugated vaccination into the national immunization programme: A national, multi-centre, cross-sectional observational study. Int J Infect Dis. 2014, 29, 169–173. [Google Scholar] [CrossRef]
- Arvas, A.; Çokuğraş, H.; Gür, E.; Gönüllü, N.; Taner, Z.; Tokman, H.B. Pneumococcal nasopharyngeal carriage in young healthy children after pneumococcal conjugate vaccine in Turkey. Balk Med J. 2017, 34, 362–366. [Google Scholar] [CrossRef]
- Ordeno, J.M. Anti-pneumonia vaccine PCV13 ‘more superior’ than PCV10. Available online: https://www.gmanetwork.com/news/scitech/science/725651/anti-pneumonia-vaccine-pcv13-more-superior-than-pcv10-expert/story/ (accessed on 20 June 2021).
- Shrestha, B.; Thorson, S.; Gurung, M.; et al. Impact of PCV10 on nasopharyngeal carriage of Streptococcus pneumoniae in community children of Nepal. Pneumo Nepal Proj. 2017, 7, 1000348. [Google Scholar]
- Yang, Y.; Cheng, W.; Pan, X.; et al. Prevalence of Haemophilus influenzae type b infection in Chinese children: A systematic review and meta-analysis. Lancet 2017, 390, S42. [Google Scholar] [CrossRef]
- Shrestha, S.; Stockdale, L.K.; Gautam, M.C.; et al. Impact of vaccination on Haemophilus influenzae type b carriage in healthy children less than 5 years of age in an urban population in Nepal. J Infect Dis. 2021, 224 (Suppl. 3), 267–274. [Google Scholar] [CrossRef]
- Williams, E.J.; Lewis, J.; John, T.; et al. Haemophilus influenzae type b carriage and novel bacterial population structure among children in urban Kathmandu, Nepal. J Clin Microbiol. 2011, 49, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Dunne, E.M.; Smith-Vaughan, H.C.; Robins-Browne, R.M.; Mulholland, E.K.; Satzke, C. Nasopharyngeal microbial interactions in the era of pneumococcal conjugate vaccination. Vaccine 2013, 31, 2333–2342. [Google Scholar] [CrossRef]
- Regev-Yochay, G.; Trzcin, K.; Thompson, C.M.; Malley, R.; Lipsitch, M. Interference between Streptococcus pneumoniae and Staphylococcus aureus: In vitro hydrogen peroxide-mediated killing by Streptococcus pneumoniae. J Bacteriol. 2006, 188, 4996–5001. [Google Scholar] [CrossRef]
- Devine, V.T.; Jefferies, J.M.; Clarke, S.C.; Faust, S.N. Nasopharyngeal bacterial carriage in the conjugate vaccine era with a focus on pneumococci. J Immunol Res. 2015, 2015, 394368. [Google Scholar] [CrossRef]
- Bradley, J.S.; Kauffman, R.E.; Balis, D.A.; et al. Assessment of musculoskeletal toxicity 5 years after therapy with levofloxacin. Pediatrics 2014, 134, e146–e153. [Google Scholar] [CrossRef]
- University of Minnesota. Antimicrobial resistance learning site. Antibiotics in veterinary medicine. Available online: https://amrls.umn.edu/antibiotics-veterinary-medicine (accessed on 27 January 2022).
- Onanuga, A.; Adamu, O.J.; Odetoyin, B.; Hamza, J.A. Nasal carriage of multi-drug resistant Panton Valentine leukocidin positive Staphylococcus aureus in healthy individuals of Tudun-Wada, Gombe State, Nigeria. Afr J Infect Dis. 2020, 15, 24–33. [Google Scholar] [CrossRef] [PubMed]

 |
 |
 |
 |
© GERMS 2022.
Share and Cite
Paudel, G.; Amatya, N.; Saud, B.; Wagle, S.; Shrestha, V.; Adhikari, B. Nasal Colonization by Potential Bacterial Pathogens in Healthy Kindergarten Children of Nepal: A Prevalence Study. GERMS 2022, 12, 86-98. https://doi.org/10.18683/germs.2022.1309
Paudel G, Amatya N, Saud B, Wagle S, Shrestha V, Adhikari B. Nasal Colonization by Potential Bacterial Pathogens in Healthy Kindergarten Children of Nepal: A Prevalence Study. GERMS. 2022; 12(1):86-98. https://doi.org/10.18683/germs.2022.1309
Chicago/Turabian StylePaudel, Govinda, Neetu Amatya, Bhuvan Saud, Sunita Wagle, Vikram Shrestha, and Bibhav Adhikari. 2022. "Nasal Colonization by Potential Bacterial Pathogens in Healthy Kindergarten Children of Nepal: A Prevalence Study" GERMS 12, no. 1: 86-98. https://doi.org/10.18683/germs.2022.1309
APA StylePaudel, G., Amatya, N., Saud, B., Wagle, S., Shrestha, V., & Adhikari, B. (2022). Nasal Colonization by Potential Bacterial Pathogens in Healthy Kindergarten Children of Nepal: A Prevalence Study. GERMS, 12(1), 86-98. https://doi.org/10.18683/germs.2022.1309




