Introduction
Nowadays, viral hepatitis represents one of the most common pathological entities: over 21 million cases of acute hepatitis (A and E) and 400 million cases of chronic hepatitis B (CHB) and chronic hepatitis C (CHC) (about 240 million persons with hepatitis B virus (HBV) infections have been communicated and, respectively, about 130–150 million persons with hepatitis C virus (HCV) infection), with an annual death-rate around 1 million [
1].
Abdominal ultrasonographies are largely accessible with small costs and, therefore, are frequently performed in the diagnosis process of all abdominal pathologies. For this reason, it is very useful to identify elements which could allow the early diagnosis and treatment of CH, as early detection can reduce considerably the costs and duration of treatment and the occurrence of severe complications. In advanced forms of CH, when parenchymal or vascular decompensation occurs, ultrasound changes are easily identified and, thus, a positive diagnosis can be rapidly met. The main problem is represented by an asymptomatic CH, when most frequently, the liver has a normal aspect on ultrasonography and stiffness test, the patient being unaware of the disease, showing no suggestive symptoms. Considering these aspects, our research aims to establish the value of ultrasonography in early diagnosis of asymptomatic CH.
Methods
We conducted a prospective study in the National Institute for Infectious Diseases “Prof. Dr. Matei Balș”, Bucharest, Romania on a group of 111 patients diagnosed with CH, from January 2016 to December 2019.
We considered the asymptomatic chronic hepatitis an asymptomatic early form of chronic hepatitis with normal liver structure on ultrasonography and normal stiffness test.
Inclusion criteria were: age over 18 years, asymptomatic patients with CHB or CHC, with normal liver structure on ultrasonography and normal stiffness test, who agreed to sign an informed consent.
Exclusion criteria were: abdominal inflammatory pathologies (within 6 months before enrollment), tumoral abdominal pathologies, abdominal surgery (within 6 months before enrollment), inadequate ultrasound window, interferon therapy (within 6 months before enrollment), cirrhosis, viral coinfections (B+C, B+D, B+C+D, Epstein Barr virus infection, cytomegalovirus infection, HIV infection), fatty liver, autoimmune or cholestatic liver diseases and food or fluids ingestion (within 4 h before examination).
The study was approved by the institution’s Ethics Committee and the informed consent was obtained from all enrolled subjects, according to the World Medical Association Declaration of Helsinki, revised in 2000, Edinburg. All patients were invited to participate in the trial and have signed an informed consent.
Patients were divided into two groups according to the etiology of CH: group A (53 patients with CHB) and group B (58 patients with CHC). The recorded data of CH patients were compared with those obtained from the control group evaluation of 50 healthy subjects.
Ultrasonographies were performed by the same doctor, on an Aloka ProSound α7 device (HITACHI-ALOKA, Japan), using a multi-frequency convex probe. We used B-mode exam for parenchymal structures, color Doppler and pulsed Doppler to assess portal vein. Patients were evaluated in fasting, positioned in both supine and left lateral decubitus. All measurements were taken three times, the average value being noted. The doctor who performed the ultrasonographies was blinded regarding the inclusion of the subjects in the study or control group, in order to increase the accuracy of the recorded data.
We determined the liver sizes, as follows: longitudinal diameter of the right hepatic lobe (RHL) measured in the axis of the right hepatic vein, longitudinal diameter of the left hepatic lobe (LHL) and also, longitudinal diameter of the caudate segment (CS); the hepatic assessments were performed during full inspiratory apnea. We considered, in our study, increased liver size, dimensions larger than those found in the control group (151 mm for RHL, 118 mm for LHL, respectively 46 mm for CS). The portal vein was measured in subcostal approach, in the hepatic hilum during full inspiratory apnea. The perihepatic lymphadenopathies measured in our study included: the hepatic hilum lymphadenopathies found anterior to the portal vein and between the portal vein and the inferior vena cava, and also, the gastrohepatic ligament lymphadenopathies. We considered, in our study, as lymphadenopathies, all the lymphadenopathies larger than those found in the control group (at least 8 mm in long axis and 3 mm in short axis).
Statistical analyses
We used a dedicated software (S-Plus 8.0, TIBCO Software Inc., USA) for statistical data processing, nonparametric statistical Kruskal-Wallis test (to evaluate the distribution of values across categories), ANOVA test (used in the regression models), ROC curves (receiver operating characteristic curve—nonparametric estimation of two distribution functions), positive predictive value (PPV) and Youden index (YI). YI represents the optimal threshold above which the patient has a type of pathology and under which the patient has another pathology.
Results
Studied patients had a median age of 43.7 years with IQR of 22 (31.5; 53.5) years in group A, respectively, 44.48 years with IQR of 21.5 (34; 54.25) years in group B, with a sex ratio males:women of 25:28 in group A, and respectively, 24:34 in group B, showing a higher proportion of women in the group of patients with hepatitis C. In the control group patients had a median age of 33 years with IQR of 15.5 (26.5; 42) years with a sex ratio males:women of 23:27.
We found mild elevations for ALT for 56.6% patients in group A with median value of 62 UI/L with IQR of 50 (39.5; 89.5) UI/L and for 63.79% patients in group B with median value of 64 UI/L with IQR of 38.75 (48; 86.75) UI/L. Mild elevations for AST were observed for 54.71% patients in group A with median value of 47 UI/L with IQR of 38.5 (29; 67.5) UI/L and for 60.34% patients in group B with median value of 50.5 UI/L with IQR of 34.5 (37.75; 72.25) UI/L. In the control group we found mild elevations of AST in 2 cases (4% patients) of 56 UI/L respectively 68 UI/L while ALT levels were in normal range for all patients.
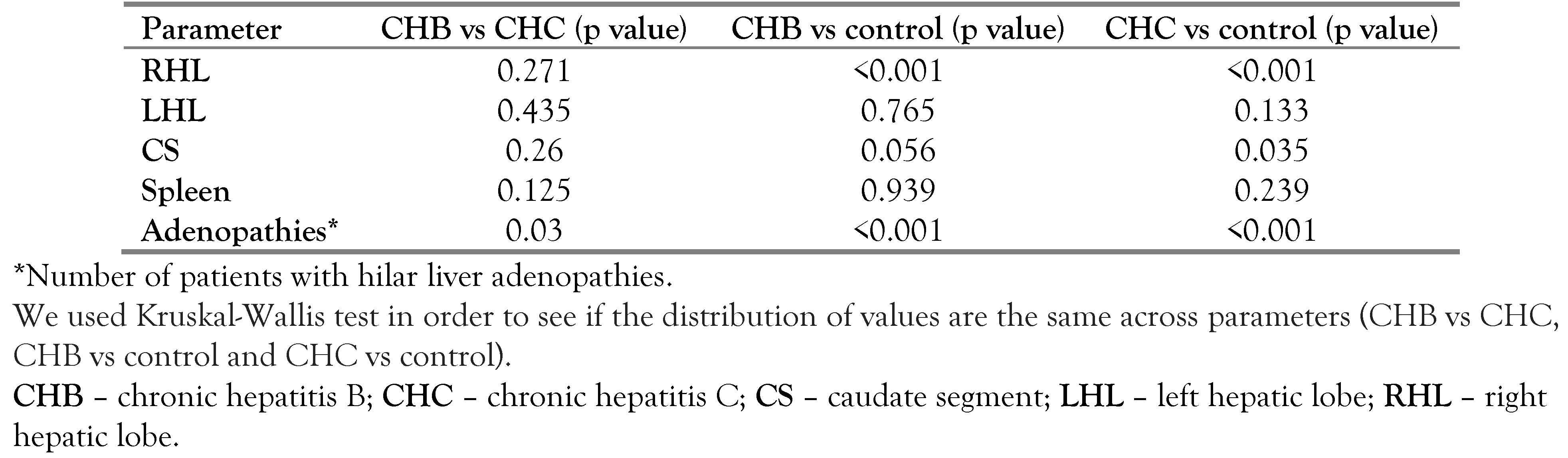
We registered an enlarged portal vein in 3.77% of group A patients and in 3.45% of group B patients (
Table 1), with no significant difference from the control group.
Increased liver sizes were found as follows: for RHL–15.09% of group A patients and in 20.69% of group B patients; for LHL–in 16.98% of group A patients and in 17.24% of group B patients; and for CS—in 16.98% of group A patients and 22.41% of group B patients (
Table 2).
The large number of patients with liver hilar adenopathies in both study groups compared to control presented statistical significance (
Table 3), leading to a more detailed evaluation of the specific findings.
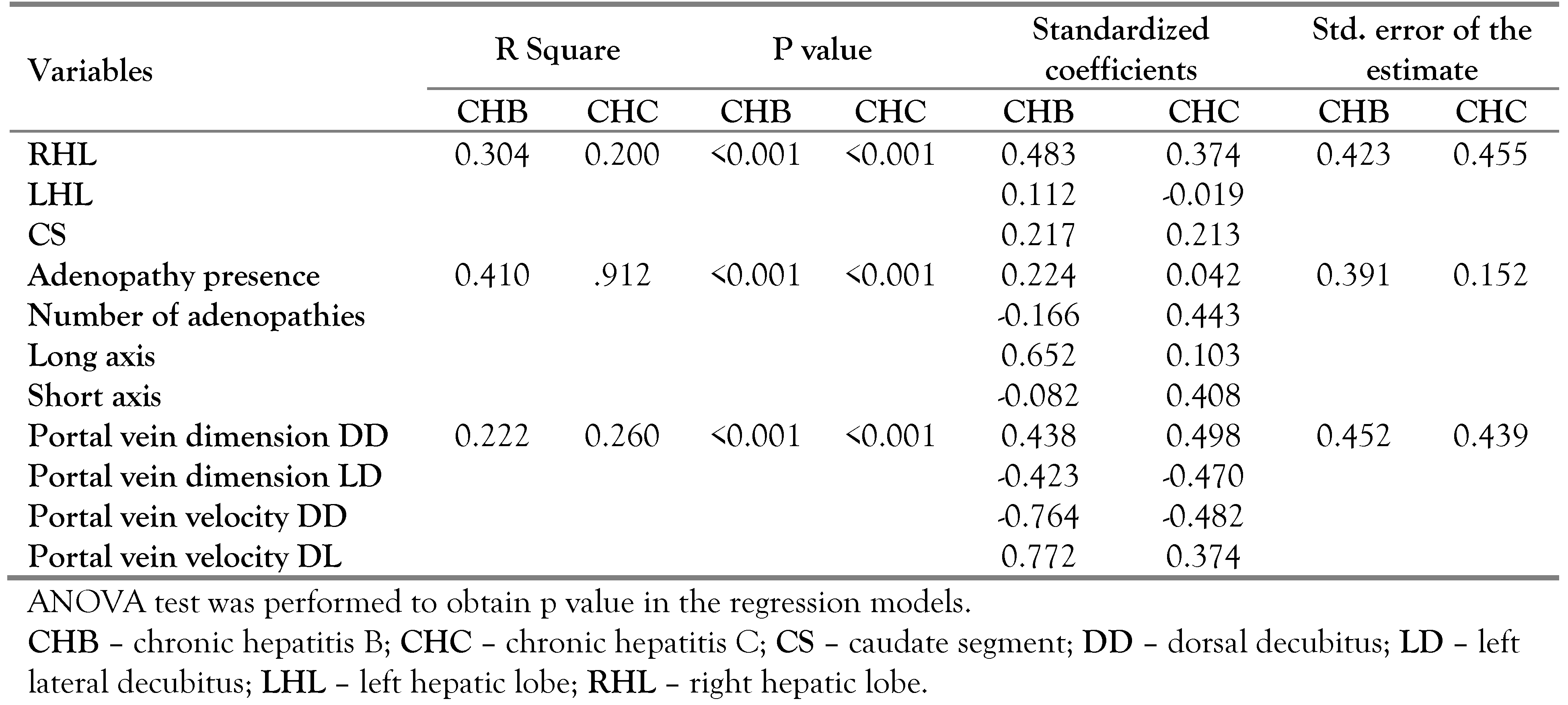
In order to estimate how much the evaluated parameters explain the presence of asymptomatic chronic hepatitis we performed linear regressions for different parameter categories (liver size, portal parameters and liver hilar adenopathies), registering the best regression model when we used hilar adenopathies (
Table 4).
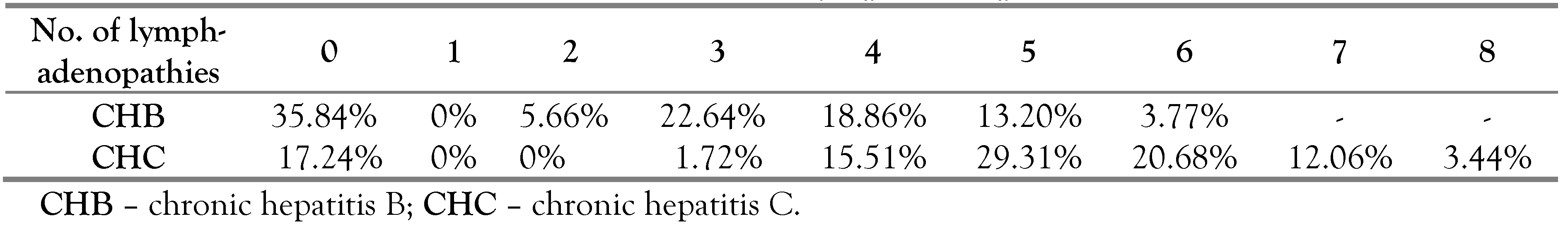
We found perihepatic lymphadenopathies in 64.16% cases in group A and 82.76% in group B (
Table 5). Considering these results we further evaluated the parameter „lymphadenopathies” in two directions: to establish if the lymphadenopathies have a diagnostic or prognostic value for CH and at the same time to establish if we can differentiate the two types of CH (B and C) based on the characteristics of these lymphadenopathies.
Regarding the number of enlarged lymph nodes, we detected that in CHC the number of lymphadenopathies was slightly higher (4–7) compared to the number of lymphadenopathies found in patients with CHB (3–5).
We investigated further if an association between the number lymphadenopathies and their sizes (long axis, short axis) can be found for the patients in both study groups.
Comparing the lymphadenopathies axes in the two study groups, significant differences were obtained for the long axis (p<0.001), while for the short axis the measurements were similar (p value=0.243).
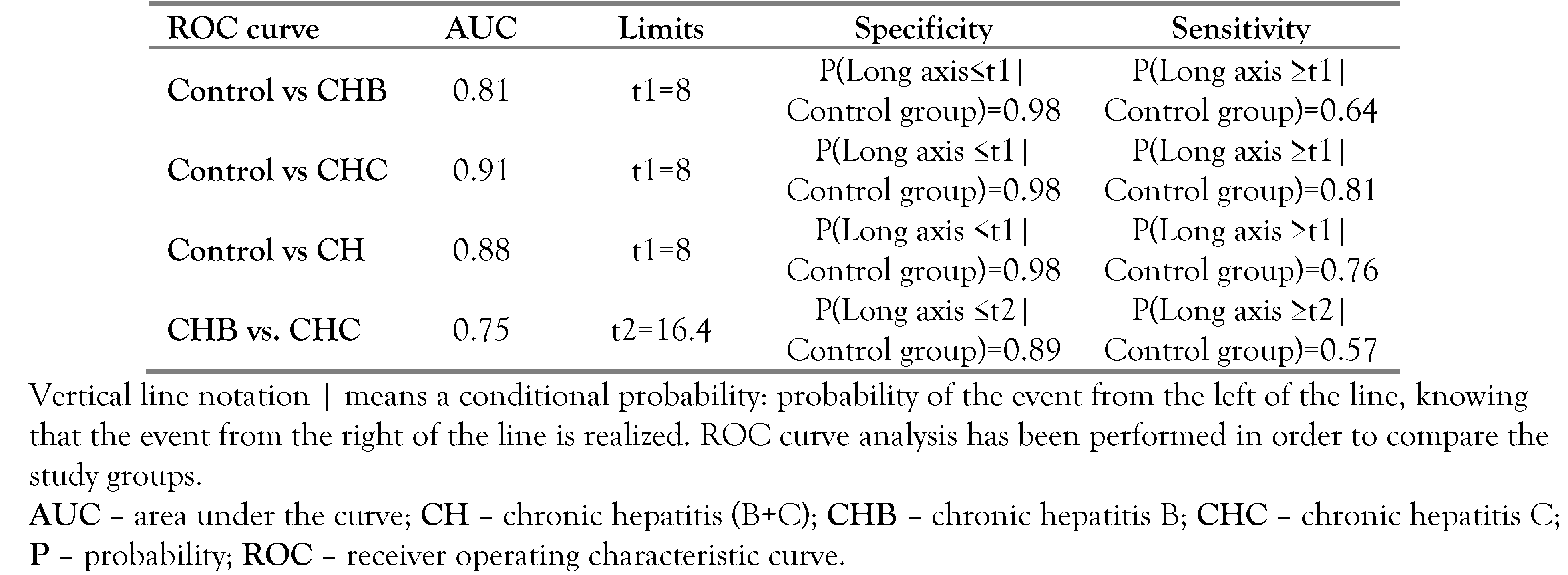
We evaluated the accuracy of lymphadenopathy long axis in differentiating the two types of CH, by constructing a ROC curve. AUC (area under curve) was estimated equal with 0.747 with a confidence interval of 95% equal with [0.69; 0.80]. For this curve we calculated the Youden index (YI). In our study, the estimated threshold is 16.4; meaning that a long axis over 16.4 mm indicates CHC, and respectively, a long axis less than 16.4 mm indicates CHB. The confidence interval calculated for 95% is [15; 18], with sensitivity and specificity of 0.572, and respectively, of 0.89.
In order to analyze the diagnostic probability of the lymphadenopathies for CHB, respectively CHC, we used ROC curves, obtaining the results listed in
Table 6.
Using the control group vs. CHB ROC curve, it results that a patient has CHB if the long axis is over 8 mm; and if the long axis is shorter than 8 mm, the patient is healthy. Similarly, for control group vs. CHC ROC curve we obtained the same threshold, with a higher sensitivity than for CHB. Furthermore, the third ROC curve (CHB vs. CHC ROC curve) demonstrates that a higher value of the long axis increases the chance of a patient to have CHC, instead of CHB. The results of the three ROC curves indicate the following rule: if the long axis is t2, the patient has CHC (where t1=8 mm and t2=16.4 mm).
Further, we conducted a similar analysis for the short axis of perihepatic lymphadenopathies (obtained thresholds are t1=3 mm and t2=5 mm) and for the number of adenopathies (obtained thresholds are t1=1 and t2=4).
For CHC, the long axis is the marker which provides the higher PPV. As for CHB, the long axis is the marker to use below a certain prevalence (0.18), while over this specified prevalence the number of lymphadenopathies should be chosen as marker.
If we use as marker the lymphadenopathies’ long axis, the PPV value is over 80% for CHC; in the case of CHB, the PPV value is over 60. This means that if the marker (long axis) is found between t1 and t2, there are over 60% chances for the patient to have CHB, and if the marker is higher than t2, there are over 80% chances for the patient to have CHC.
Discussion
The dimensions for both left and right hepatic lobes were slightly increased, but we found only the small differences compared to the control group (2 mm–5 mm), and therefore we don’t recommend using liver dimension as positive diagnostic marker in early chronic hepatitis. Our results were concordant with other published data—Afzal S et al. evaluated chronic liver disease and found normal liver sizes in 84% of the patients, while 9% had enlarged liver sizes and 7% had reduced liver sizes [
2].
We registered changes for the portal parameters (dimension and flow velocity) only in a small percentage of the patients, with no statistical relevance in the positive diagnosis of asymptomatic CH. However, ultrasound evaluation can be a useful screening tool in this early stage of hepatitis, that helps exclude other liver pathological entities (tumoral, inflammatory or posttraumatic).
Abbas et al. [
3] concluded that approximately 80–90% of patients with acute hepatitis C virus developed a persistent infection and only about 50% had elevated transaminase indicating ongoing liver inflammation; in our study we detected perihepatic lymphadenopathies in 64.16% of the patients with asymptomatic CHB, and respectively, in 82.76% of asymptomatic patients with CHC, thus lymphadenopathies can represent a useful diagnostic tool in asymptomatic early CH, a viable alternative to the transaminases levels which might often be normal. Evaluating the lymphadenopathies, Kuo HT et al. [
4] also found a significantly increased incidence in both CHB and CHC patients (56.9% and 69.4%, respectively; both p<0.001), compared with the non-viral group. This rate was independent of the transaminase levels and the width of lymphadenopathies was the only significant parameter when viral and non-viral groups were compared (p<0.05). A positive predictive rate of 88% and a specificity of 89% were found when a width of more than 5 mm was used to predict CHB or the CHC.
Choi MS et al. [
5] detected perihepatic lymphadenopathies in 48 of 50 patients with CH, which presented significant correlation with serum level of aspartate-transaminase (r=0.66), alanine-transaminase (r=0.63), gamma-glutamyl-transpeptidase (r=0.53), histological activity index (r=0.59) and necroinflammatory score (r=0.59), with statistical significance (p<0.05) for all parameters; but no correlation was found with fibrosis score or viral load. They concluded that enlarged perihepatic lymphadenopathies in CH can represent a useful indicator for both histological and biochemical inflammatory activity of the liver, but not for serological parameter (as viral load).
Perihepatic lymphadenopathies should be carefully evaluated because this may be the only change recorded in patients with CH. It is possible to continue the ultrasound investigation with other imaging modalities like computerized tomography (CT) or magnetic resonance imaging (MRI) [
6], but these are expensive techniques and often require contrast administration. Even if these exams are performed, lymphadenopathies adjacent to the portal vein and/or in retroperitoneal space may be the only indication of hepatic disease in 35% cases [
7].
The presence and detection of perihepatic lymphadenopathies can contribute to a better assessment of the disease. Pal Set al. [
8] confirmed the presence of virus C infection in 17 of 20 (85%) lymphadenopathies assessed by hybridization in situ; and also, virus C replication in 50% of cases by detection of replicative intermediate virus C ribonucleic acid (RNA), demonstrating the lymph tropism of this virus, and the implication of lymphadenopathies in the extrahepatic virus C replication. These results represent a supplementary argument for a more severe involvement of the lymphatic system in CHC and for a higher prevalence of lymphadenopathies in this infection. At the same time the existence of a chronic inflammatory process, continuous or intermittent, induces release of cytokines with persistent stimulation of lymphocyte production in local lymph nodes. Both mechanisms (viral replication in local lymph nodes and persistent inflammatory stimulation of local lymph nodes) can explain the occurrence of lymphadenopathies in patients with asymptomatic chronic hepatitis. The data obtained in our research allow us to affirm that if the patient has an increased number of perihepatic lymphadenopathies (over than 4), increased in size (at least one lymphadenopathy with long axis over 16.4 mm and short axis over 5 mm), the probability of the patient to have CHC exceeds 90%. In case of fewer or smaller lymphadenopathies, a differentiation between CHB and CHC cannot be made accurately, using ultrasound as a diagnostic tool.
In the advanced stages of chronic hepatitis, lymphadenopathies can be caused also by altered lymphatic drainage: altered hepatic cytoarchitecture causes a decrease in the intensity of lymphatic drainage through the liver, which can lead to the accumulation of additional lymph in the adjacent lymphatic circulation and lymph nodes enlargement. At the same time, if portal hypertension is present, it can accentuate the process of intestinal bacterial translocation, increasing the amount of toxins, antigens and pathogens that reach both the portal circulation and the lymphatic circulation, intensifying the immune stimulation of local lymphatic stations. Both mechanisms can accentuate the lymphadenopathies present in the early phases of chronic hepatitis.
The importance of evaluating the presence, number and sizes of perihepatic lymphadenopathies resides first of all in the exclusion of a possible CH. Thus, an asymptomatic patient without lymphadenopathies has a probability of about 97% to be healthy; if lymphadenopathies are present and their long axis is under 8 mm and the short axis is under 3 mm, the patient has a probability between 90% and 97.7% to be healthy.
Data from the medical literature regarding the association of lymphadenopathies with CHC varies, most authors reporting a strong correlation between CH and perihepatic lymphadenopathies. Forsberg L et al. detected lymphadenopathies in all 114 studied patients (100%) with CHC [
9], Muller P et al. detected lymphadenopathies in 90 from 104 patients (86.53%) with CHC [
10], while Grier S et al. found that only 30–40% of the patients with CH had detectable lymphadenopathies [
11]. Casani F et al. described also lymphadenopathies only in 19% (42 of 227) of patients with chronic liver disease and no evidence of tumor, upper gastrointestinal carcinoma or lymphoproliferative disorders [
12]. Sorenzi M et al. showed a prevalence of 22% of abdominal lymphadenopathies in subjects with hepatitis virus C infection with normal alanine-aminotransferase values, suggesting that the evidence of abdominal lymphadenopathies in these patients could be an indication to perform liver biopsy [
13]. Neri S et al. communicated also a strong association between perihepatic lymphadenopathies and hepatitis virus C infection, the biochemical tests revealing the positivity of HCV antibodies in 528 from 684 subjects with lymphadenopathies and in 8 of 7290 subjects without lymphadenopathies, showing a significant difference (p<0.001) between the two groups [
14].
In our study, we found perihepatic lymphadenopathies in 82.76% of the patients with CHC (group B). Evaluating the relevance of perihepatic lymphadenopathies, Braden B et al. showed that in most of the patients with acute viral hepatitis (94%) perihepatic lymphadenopathies were found, while in chronic liver disease, perihepatic lymphadenopathies were present in 86% of CH and only in very small percentages in other chronic hepatic pathologies (6% of hemochromatosis, 1% of fatty liver disease, and in 4% of cholecystolithiasis), concluding that perihepatic lymphadenopathies are found mostly in infectious liver diseases, but not in metabolic or toxic liver pathologies [
15].
In larger perihepatic lymphadenopathies encountered in CHB patients with acute flare (positive HBe antigen), the magnitude of lymphadenopathies’ width change may predict HBe antigen seroconversion with recovery [
16], while in patients with HCV genotype 1 and very high HCV RNA, perihepatic lymphadenopathies are a negative predictor for sustained virological response independent of other known predictors [
17]. Longo S et al. communicated a significantly lymphadenopathy volume for perihepatic lymphadenopathies in non-responder patients with CHC, compared to the responders group [
18].
Conclusions
In conclusion, ultrasonography represents a useful screening tool for asymptomatic CH. Perihepatic lymphadenopathies have a superior diagnostic value in asymptomatic CH patients, than portal vein diameter, portal flow velocity or liver sizes. If perihepatic lymph nodes cannot be detected or if they are smaller than 8 mm in long axis and the short axis is under 3 mm, the patient has a probability between 90% and 97.7% to be healthy. The presence of perihepatic lymphadenopathies with a short axis over 5 mm and long axis over 16.4 mm is highly suggestive of CHC.