Urinary Tract Infections in Children: Clinical and Antimicrobial Resistance Data from Bucharest Area, Romania
Abstract
Introduction
Methods
Results
Patient characteristics
Clinical and laboratory characteristics
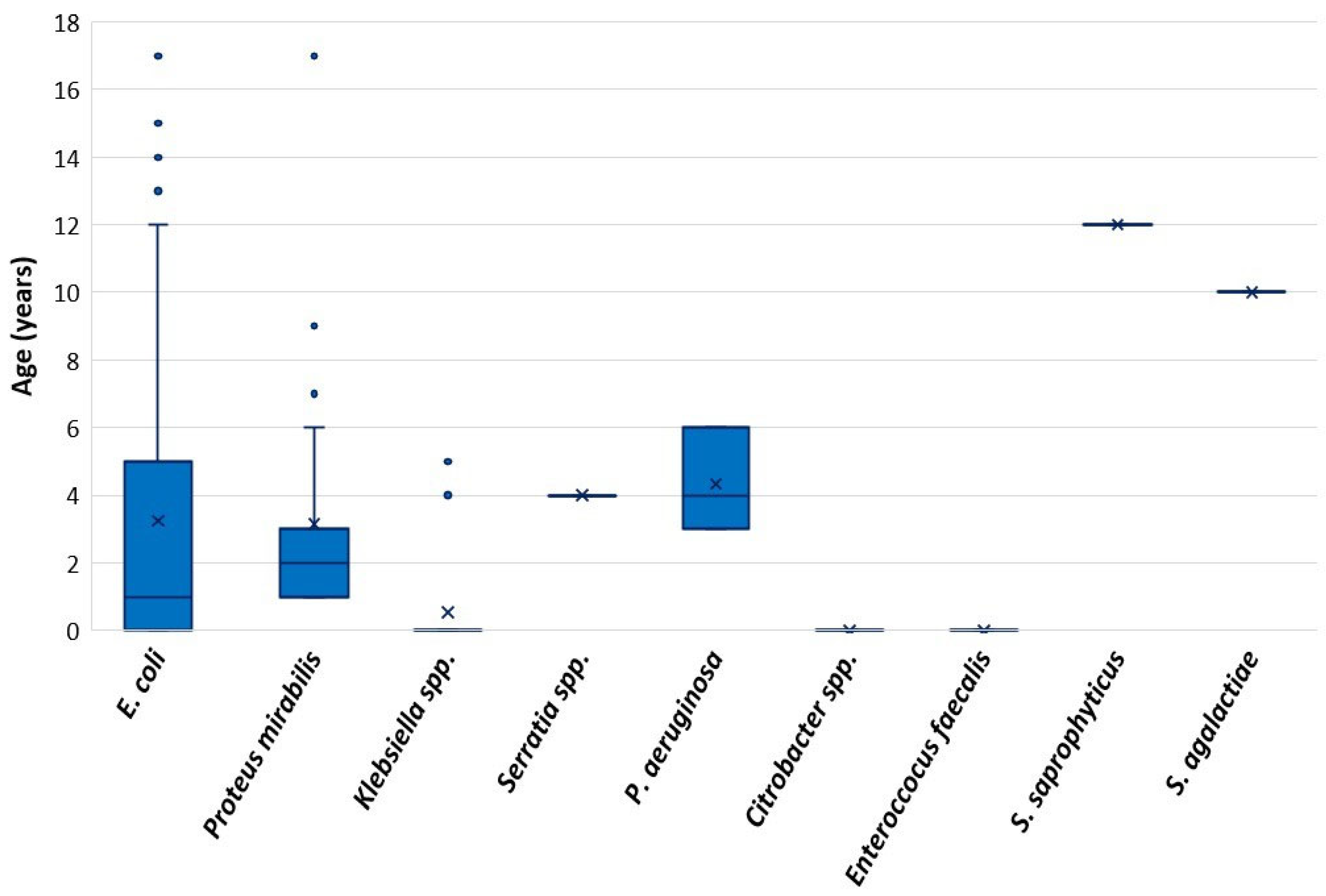
Uropathogens and antimicrobial resistance profiles
Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leung, A.K.C.; Wong, A.H.C.; Leung, A.A.M.; Hon, K.L. Urinary tract infection in children. Recent Pat Inflamm Allergy Drug Discov. 2019, 13, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Larcombe, J. Urinary tract infection in children: Recurrent infections. BMJ Clin Evid. 2015, 2015, 0306. [Google Scholar] [PubMed]
- Greenhow, T.L.; Hung, Y.Y.; Herz, A.M.; Losada, E.; Pantell, R.H. The changing epidemiology of serious bacterial infections in young infants. Pediatr Infect Dis J. 2014, 33, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.; Temple-Smith, M.; Sanci, L. Urinary tract infections in children: An overview of diagnosis and management. BMJ Paediatr Open. 2019, 3, e000487. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 27th ed; CLSI supplement M100; CLSI: Wayne, PA, USA, 2017. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed; CLSI supplement M100; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 29th ed; CLSI supplement M100; CLSI: Wayne, PA, USA, 2019. [Google Scholar]
- Hernández-Bou, S.; Trenchs, V.; Soler-Garcia, A.; Caballero, M.; Ciutad, M.; Luaces, C. Outpatient and oral management is suitable for infants 60-90 days old with urinary tract infections at low risk of bacteremia. Eur J Pediatr. 2021. [CrossRef] [PubMed]
- Duicu, C.; Cozea, I.; Delean, D.; Aldea, A.A.; Aldea, C. Antibiotic resistance patterns of urinary tract pathogens in children from Central Romania. Exp Ther Med. 2021, 22, 748. [Google Scholar] [CrossRef] [PubMed]
- Petca, R.C.; Negoiță, S.; Mareș, C.; Petca, A.; Popescu, R.I.; Chibelean, C.B. Heterogeneity of antibiotics multidrug-resistance profile of uropathogens in Romanian population. Antibiotics 2021, 10, 523. [Google Scholar] [CrossRef] [PubMed]
- Farkas, A.; Tarco, E.; Butiuc-Keul, A. Antibiotic resistance profiling of pathogenic Enterobacteriaceae from Cluj-Napoca, Romania. Germs 2019, 9, 17–27. [Google Scholar] [CrossRef] [PubMed]
- van Driel, A.A.; Notermans, D.W.; Meima, A.; et al. Antibiotic resistance of Escherichia coli isolated from uncomplicated UTI in general practice patients over a 10-year period. Eur J Clin Microbiol Infect Dis. 2019, 38, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Tot, T.; Kibel, S.; Sardelić, S.; et al. Polyclonal spread of colistin resistant Klebsiella pneumoniae in Croatian hospitals and outpatient setting. Germs 2021, 11, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Matovina, M.; Abram, M.; Repac-Antić, D.; Knežević, S.; Bubonja-Šonje, M. An outbreak of ertapenem-resistant, carbapenemase-negative and porin-deficient ESBL-producing Klebsiella pneumoniae complex. Germs 2021, 11, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Isac, R.; Basaca, D.G.; Olariu, I.C.; et al. Antibiotic resistance patterns of uropathogens causing urinary tract infections in children with congenital anomalies of kidney and urinary tract. Children 2021, 8, 585. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total Patients, n = 264 | Infants, n = 139 | Children Under 1-Year, n = 125 | Statistical Analysis |
|---|---|---|---|---|
| Clinical Characteristics | ||||
| Fever, n (%) | 211 (79.9) | 133 (95.7) | 78 (62.4) | p < 0.001, χ2 = 45.4, OR = 13.4, 95%CI: 5.5–32.7 |
| Temperature, median (IQR) °C | 39.4 (38.9, 39.8) | 39.4 (38.9, 39.8) | 39.4 (38.8, 39.9) | p = 0.695 |
| Malaise, n (%) | 185 (70.1%) | 117 (84.2%) | 68 (54.4%) | p < 0.001, χ2 = 27.8, OR = 4.5, 95%CI: 2.5–7.9 |
| Nausea, n (%) | 52 (19.7) | 30 (21.6) | 18 (17.6) | p = 0.334 |
| Diarrhea, n (%) | 64 (24.0) | 43 (30.9) | 21 (16.8) | p = 0.002, χ2 = 10.1, OR = 4.6,95%CI: 1.4–14 |
| Abdominal pain, n (%) | 86 (32.6) | NA | 86 (68.8) | NA |
| Lower back pain, n (%) | 5 (1.9) | NA | 5 (4.0) | NA |
| Dysuria, n (%) | 63 (23.9) | NA | 63 (50.4) | NA |
| Frequent urination, n (%) | 36 (13.6) | NA | 36 (28.8) | NA |
| Macroscopically cloudy urine, n (%) | 57 (21.6) | 18 (13.0) | 39 (31.2) | p < 0.001, χ2 = 11.9, OR = 3.0,95%CI: 1.6–5.7 |
| Macroscopic hematuria, n (%) | 15 (5.7) | 4 (2.9) | 11 (8.8) | p = 0.060 |
| Laboratory characteristics | ||||
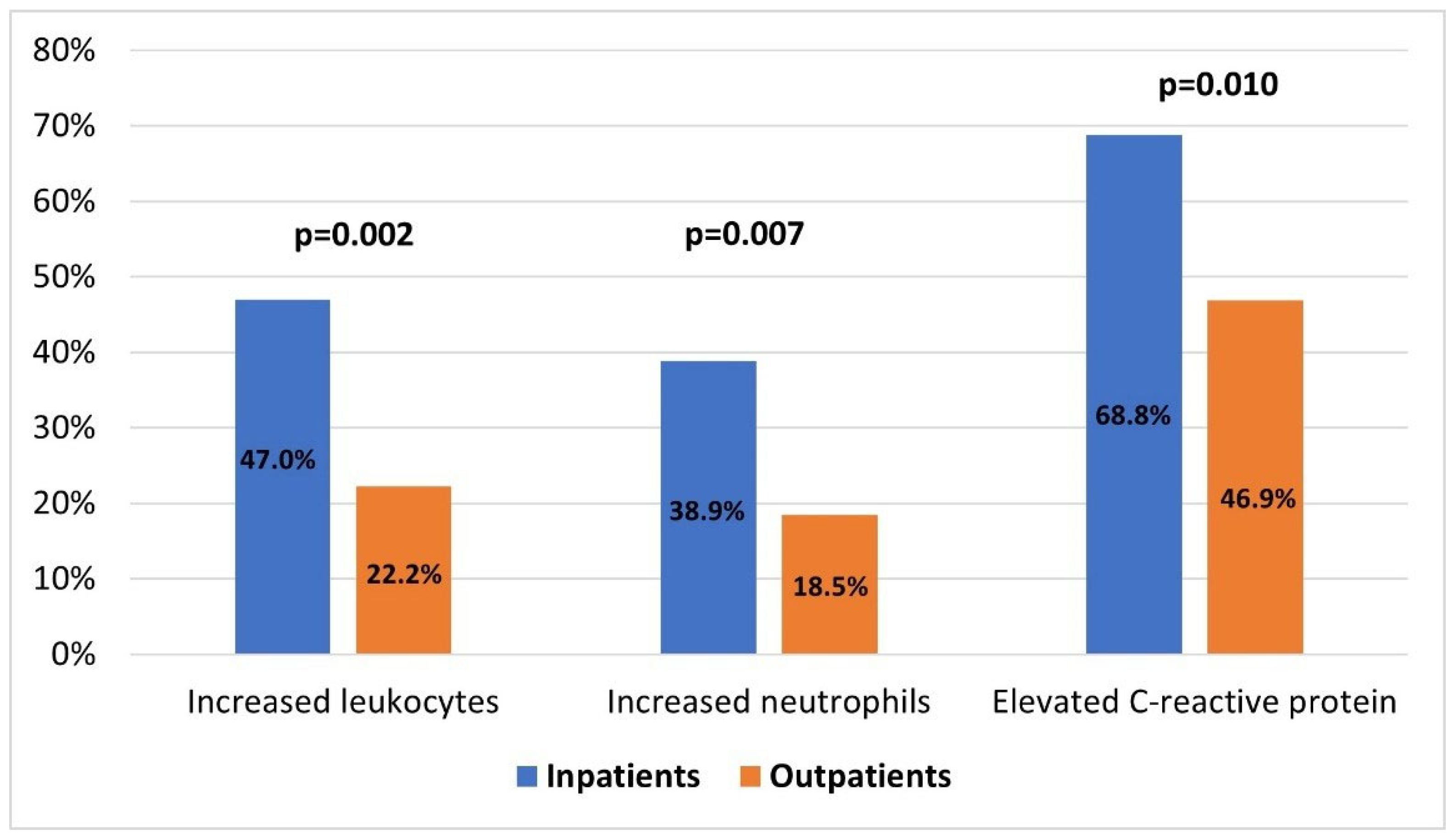
| Increased leukocytes [N = 203], n (%) | 82 (40.4) | 61/120 (50.8) | 21/83 (25.3) | p < 0.001, χ2 = 12.2, OR = 3.1, 95%CI: 1.7–5.6 |
| Increased neutrophils [N = 203], n (%) | 68 (33.5) | 46/120 (38.3) | 22/83 (26.5) | p = 0.096 |
| Anemia [N = 203], n (%) | 63 (31.0) | 46/120 (38.3) | 17/83 (20.5) | p = 0.008, χ2 = 6.5, OR = 2.4, 95%CI: 1.3–4.6 |
| Increased thrombocytes [N = 203], n (%) | 55 (27.2) | 46/120 (38.3) | 9/83 (10.8) | p < 0.001, χ2 = 19.3, OR = 5.8, 95%CI: 2.6–13.1 |
| Inflammatory syndrome [N = 193], n (%) | 122 (63/2) | 85/117 (72.6) | 37/76 (48.7) | p = 0.001, χ2 = 11.4, OR = 2.8, 95%CI: 1.5–5.1 |
| Increased urea [N = 170], n (%) | 9 (5.3) | 4/105 (3.8) | 5/65 (7.7) | p = 0.236 |
| Increased creatinine [N = 163], n (%) | 2 (1.2) | 1/101 (1.0) | 1/62 (1.6) | p = 0.413 |
| Increased AST [N = 156], n (%) | 7 (4.5) | 4/99 (4.0) | 3/57 (5.3) | p = 0.722 |
| Increased ALT [N = 156], n (%) | 3 (1.9) | 2/99 (2.0) | 1/57 (1.8) | p = 0.907 |
| Antibiotic | Susceptible n (%) | Intermediate n (%) | Resistant n (%) |
|---|---|---|---|
| Ampicillin [n = 207] | 53 (25.6) | 22 (10.6) | 132 (63.8) |
| Amoxicillin/clavulanate [n = 207] | 77 (37.2) | 37 (17.9) | 93 (44.9) |
| Ceftriaxone [n = 207] | 180 (94.2) | 0 | 27 (13.0) |
| Cefuroxime [n = 207] | 164 (79.2) | 5 (2.4) | 38 (18.4) |
| Ceftazidime [n = 110] | 86 (78.2) | 3 (2.7) | 21 (19.1) |
| Ciprofloxacin [n = 198] | 171 (86.4) | 7 (3.5) | 20 (10.1) |
| Colistin [n = 72] | 72 (100) | 0 | 0 |
| Gentamicin [n = 207] | 187 (90.3) | 5 (2.5) | 15 (7.2) |
| Trimethoprim/sulfamethoxazole [n = 207] | 149 (72.0) | 2 (1.0) | 56 (27.0) |
| Meropenem [n = 16] | 16 (100) | 0 | 0 |
| Imipenem [n = 16] | 16 (100) | 0 | 0 |
| Antibiotic | Susceptible n (%) | Intermediate n (%) | Resistant n (%) |
|---|---|---|---|
| Ampicillin [n = 26] | 16 (61.5) | 0 | 10 (38.5) |
| Amoxicillin/clavulanate [n = 26] | 21 (80.8) | 4 (15.4) | 1 (3.8) |
| Ceftriaxone [n = 26] | 25 (96.2) | 0 | 1 (3.8) |
| Cefuroxime [n = 26] | 24 (92.3) | 0 | 2 (7.7) |
| Ceftazidime [n = 7] | 7 (100) | 0 | 0 |
| Ciprofloxacin [n = 23] | 23 (88.5) | 2 (7.7) | 1 (3.8) |
| Colistin [n = 7] | 7 (100) | 0 | 0 |
| Gentamicin [n = 26] | 24 (92.3) | 0 | 2 (7.7) |
| Trimethoprim/sulfamethoxazole [n = 26] | 17 (65.4) | 0 | 9 (34.6) |
| Meropenem [n = 7] | 7 (100) | 0 | 0 |
| Imipenem [n = 7] | 7 (100) | 0 | 0 |
| Antibiotic | Susceptible n (%) | Intermediate n (%) | Resistant n (%) |
|---|---|---|---|
| Ampicillin [n = 17] | 1 (5.9) | 0 | 16 (94.1) |
| Amoxicillin/clavulanate [n = 17] | 3 (17.6) | 3 (17.6) | 11 (64.7) |
| Ceftriaxone [n = 17] | 10 (58.8) | 0 | 7 (41.2) |
| Cefuroxime [n = 17] | 10 (58.8) | 0 | 7 (41.2) |
| Ceftazidime [n = 7] | 3 (42.9) | 1 (14.2) | 3 (42.9) |
| Ciprofloxacin [n = 17] | 13 (76.5) | 1 (5.9) | 3 (17.6) |
| Colistin [n = 7] | 7 (100) | 0 | 0 |
| Gentamicin [n = 17] | 11 (64.7) | 0 | 6 (35.3) |
| Trimethoprim/sulfamethoxazole [n = 17] | 14 (82.4) | 0 | 3 (17.6) |
| Meropenem [n = 7] | 6 (85.7) | 0 | 1 (14.3) |
| Imipenem [n = 7] | 5 (71.4) | 0 | 2 (28.6) |
© GERMS 2021.
Share and Cite
Miron, V.D.; Filimon, C.; Cabel, T.; Mihăescu, R.I.; Bar, G.; Leu, D.; Craiu, M. Urinary Tract Infections in Children: Clinical and Antimicrobial Resistance Data from Bucharest Area, Romania. GERMS 2021, 11, 583-591. https://doi.org/10.18683/germs.2021.1293
Miron VD, Filimon C, Cabel T, Mihăescu RI, Bar G, Leu D, Craiu M. Urinary Tract Infections in Children: Clinical and Antimicrobial Resistance Data from Bucharest Area, Romania. GERMS. 2021; 11(4):583-591. https://doi.org/10.18683/germs.2021.1293
Chicago/Turabian StyleMiron, Victor Daniel, Claudiu Filimon, Teodor Cabel, Roxana Ioana Mihăescu, Gabriela Bar, Denisa Leu, and Mihai Craiu. 2021. "Urinary Tract Infections in Children: Clinical and Antimicrobial Resistance Data from Bucharest Area, Romania" GERMS 11, no. 4: 583-591. https://doi.org/10.18683/germs.2021.1293
APA StyleMiron, V. D., Filimon, C., Cabel, T., Mihăescu, R. I., Bar, G., Leu, D., & Craiu, M. (2021). Urinary Tract Infections in Children: Clinical and Antimicrobial Resistance Data from Bucharest Area, Romania. GERMS, 11(4), 583-591. https://doi.org/10.18683/germs.2021.1293




