Multidrug-Resistant Community-Acquired Urinary Tract Infections in a Northern Region of Morocco: Epidemiology and Risk Factors
Abstract
Introduction
Methods
Statistical methods
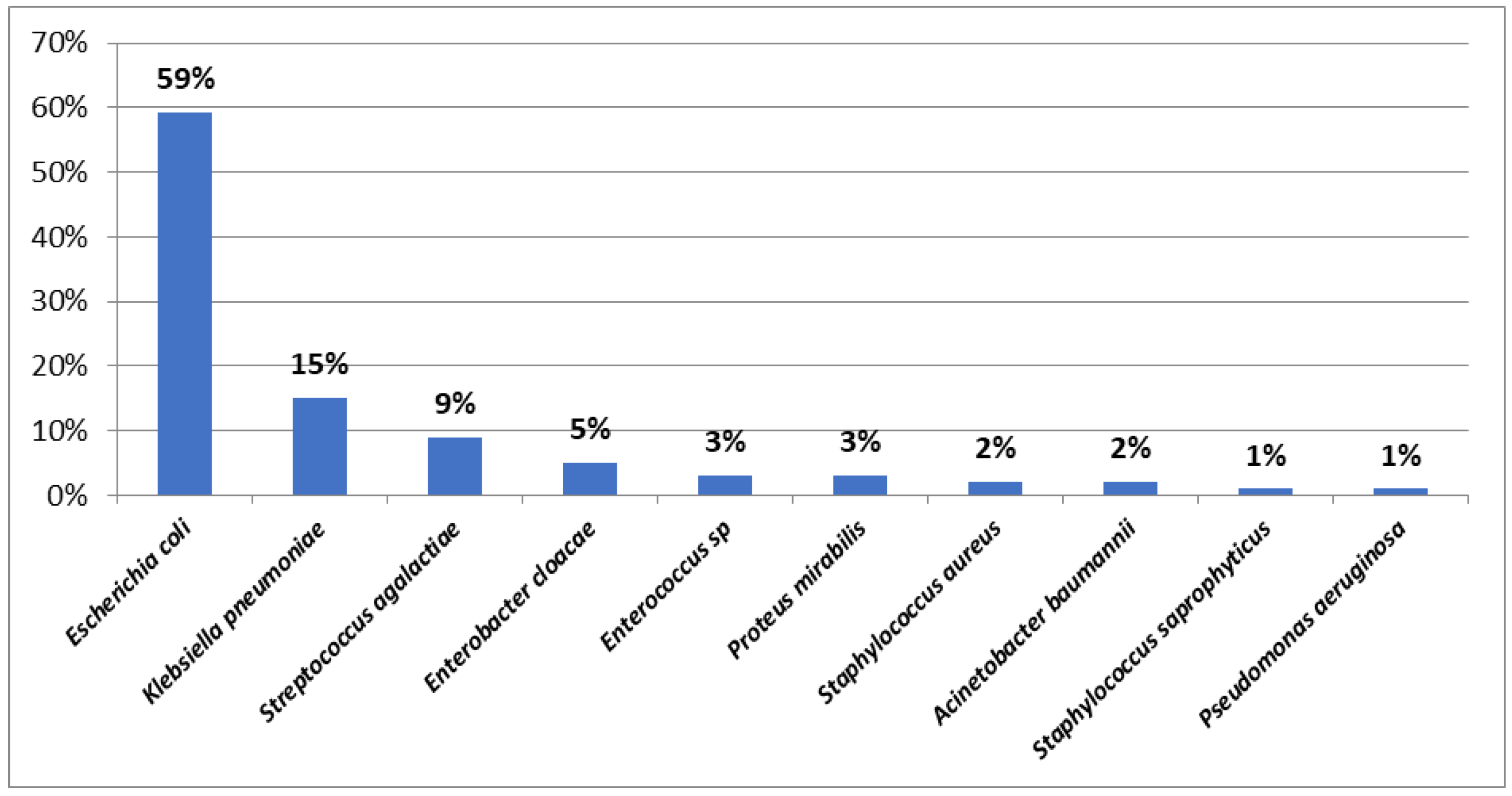
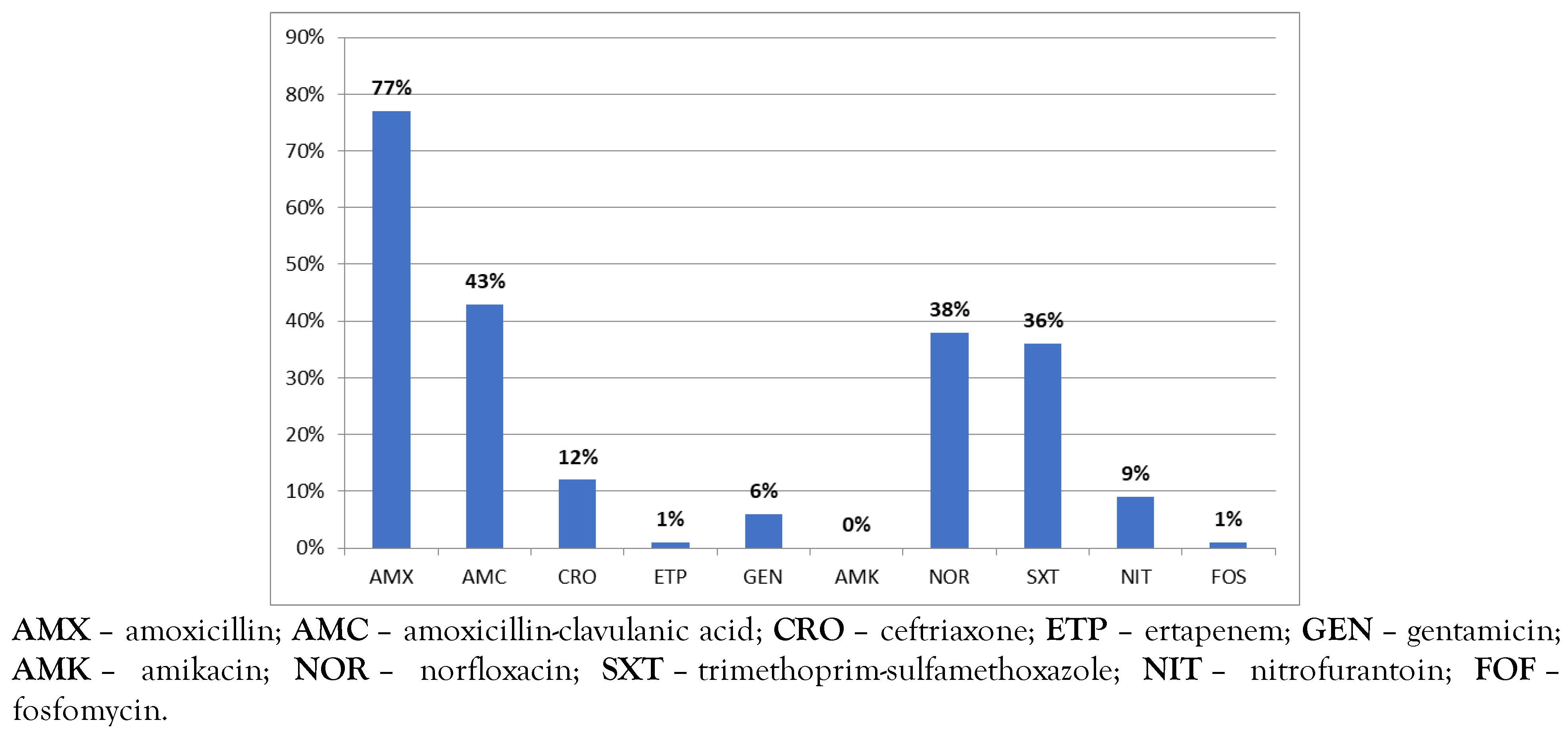
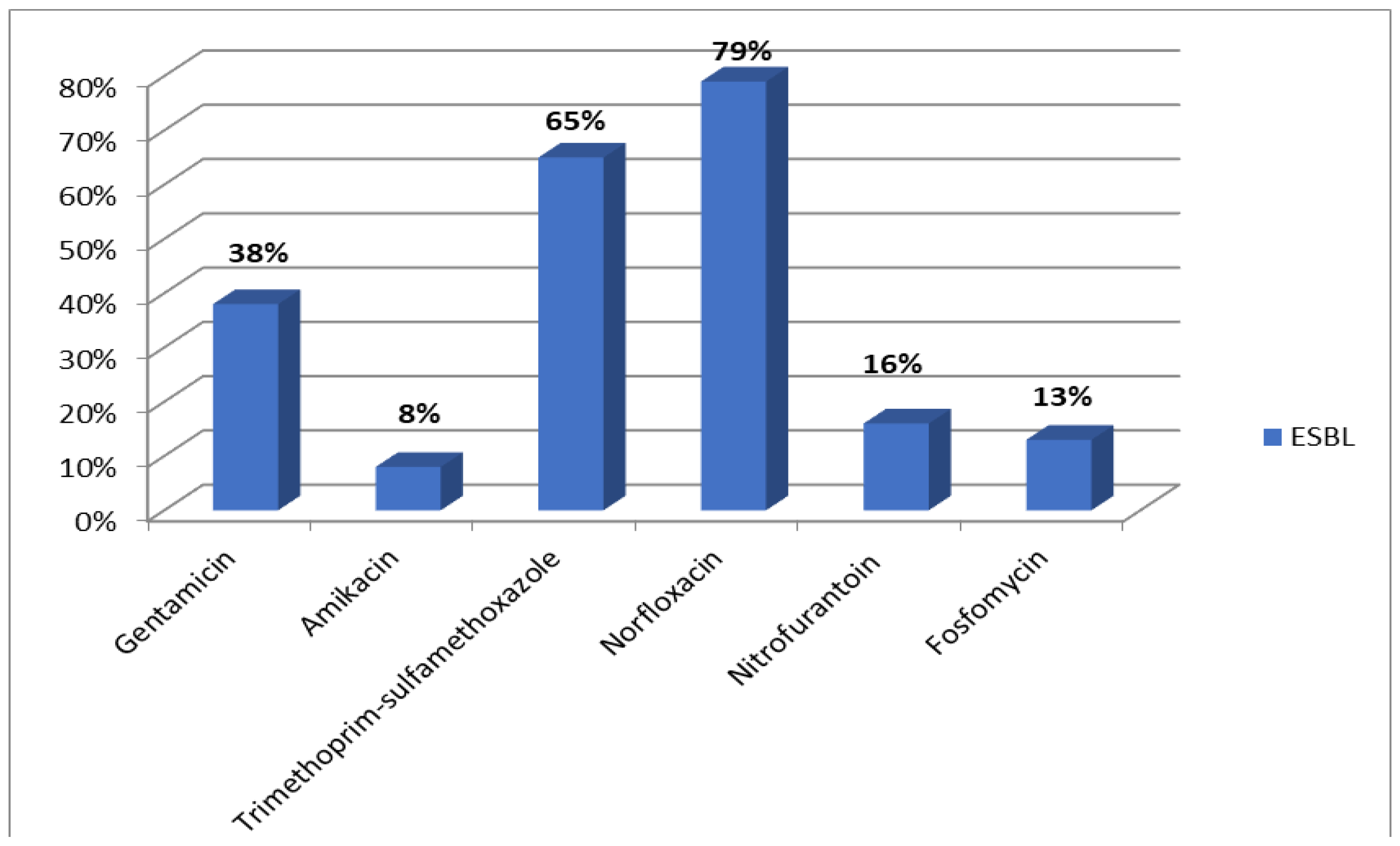
Results
Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, D.S.; Lee, S.J.; Choe, H.S. Community-acquired urinary tract infection by Escherichia coli in the era of antibiotic resistance. Biomed Res Int. 2018, 2018, 7656752. [Google Scholar] [CrossRef]
- Guermazi-Toumi, S.; Boujlel, S.; Assoudi, M.; Issaoui, R.; Tlili, S.; Hlaiem, M.E. Susceptibility profiles of bacteria causing urinary tract infections in Southern Tunisia. J Glob Antimicrob Resist. 2018, 12, 48–52. [Google Scholar] [CrossRef]
- Benhiba, I.; Bouzakraoui, T.; Zahidi, J.; et al. Epidemiology and antibiotic resistance Enterobacteriaceae urinary of adults infections in Marrakech CHU and commitment therapy. Uro’Andro. 2015, 1, 166–171. [Google Scholar]
- Sbiti, M.; Lahmadi, K.; Louzi, L. Epidemiological profile of uropathogenic enterobacteria producing extended spectrum beta-lactamases. Pan Afr Med J. 2017, 28, 29. [Google Scholar] [CrossRef] [PubMed]
- Society of Infectious Pathology of French Language (SPILF). Diagnosis and antibiotic therapy of community-acquired bacterial urinary tract infections in adults. Update. 2015. Available online: https://www.infectiologie.com/UserFiles/File/spilf/rec os/infections-urinaires-305-spilf-argumentaire.pdf (accessed on 08 January 2021).
- Société Française de Microbiologie. CASFM/EUCAST V2. Société Française de Microbiologie Ed; 2019. Available online: https://www.sfm-microbiologie.org/2019/05/06/casfm-eucast-2019-v2/ (accessed on 08 January 2021).
- BIO-RAD. Détection rapide des souches d’entérobactéries productrices de carbapenemases. Available online: www.bio-rad.com/webroot/web/pdf/inserts/CDG/fr/68260_88 1159_FR.pdf (accessed on 22 January 2021).
- Vakilzadeh, M.M.; Heidari, A.; Mehri, A.; et al. Antimicrobial resistance among community-acquired uropathogens in Mashhad, Iran. J Environ Public Health. 2020, 2020, 3439497. [Google Scholar] [CrossRef] [PubMed]
- Hameed, T.; Al Nafeesah, A.; Chishti, S.; Al Shaalan, M.; Al Fakeeh, K. Community-acquired urinary tract infections in children: Resistance patterns of uropathogens in a tertiary care center in Saudi Arabia. Int J Pediatr Adolesc Med. 2019, 6, 51–54. [Google Scholar] [CrossRef]
- Blomberg, B.; Jureen, R.; Manji, K.P.; et al. High rate of fatal cases of pediatric septicemia caused by gram-negative bacteria with extended-spectrum beta-lactamases in Dar es Salaam, Tanzania. J Clin Microbiol. 2005, 43, 745–749. [Google Scholar] [CrossRef]
- Mshana, S.E.; Falgenhauer, L.; Mirambo, M.M.; et al. Predictors of blaCTX-M-15 in varieties of Escherichia coli genotypes from humans in community settings in Mwanza, Tanzania. BMC Infect Dis. 2016, 16, 187. [Google Scholar] [CrossRef]
- Mshana, S.E.; Imirzalioglu, C.; Hain, T.; Domann, E.; Lyamuya, E.F.; Chakraborty, T. Multiple ST clonal complexes, with a predominance of ST131, of Escherichia coli harbouring blaCTX-M-15 in a tertiary hospital in Tanzania. Clin Microbiol Infect. 2011, 17, 1279–1282. [Google Scholar] [CrossRef]
- Tagajdid, M.R.; Boumhil, L.; Iken, M.; Adnaoui, M.; Benouda, A. Resistance to fluoroquinolones and third generation cephalosporin of Escherichia coli isolated from urines. Med Mal Infect. 2010, 40, 70–73. [Google Scholar] [CrossRef]
- El Bouamri, M.C.; Arsalane, L.; Kamouni, Y.; Berraha, M.; Zouhair, S. Recent evolution of the epidemiological profile of extended-spectrum β-lactamase producing uropathogenic enterobacteria in Marrakech, Morocco. Prog Urol. 2014, 24, 451–455. [Google Scholar] [CrossRef]
- Abreu, A.G.; Marques, S.G.; Monteiro-Neto, V.; Gonçalves, A.G. Extended-spectrum β-lactamase-producing Enterobacteriaceae in community-acquired urinary tract infections in São Luís, Brazil. Braz J Microbiol. 2013, 44, 469–471. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodríguez-Baño, J.; Alcalá, J.; Cisneros, J.M.; et al. Escherichia coli producing SHV-type extended-spectrum beta-lactamase is a significant cause of community-acquired infection. J Antimicrob Chemother. 2009, 63, 781–784. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guyomard-Rabenirina, S.; Malespine, J.; Ducat, C.; et al. Temporal trends and risks factors for antimicrobial resistant Enterobacteriaceae urinary isolates from outpatients in Guadeloupe. BMC Microbiol. 2016, 16, 121. [Google Scholar] [CrossRef] [PubMed]
- Neuhauser, M.M.; Weinstein, R.A.; Rydman, R.; Danziger, L.H.; Karam, G.; Quinn, J.P. Antibiotic resistance among gram-negative bacilli in US intensive care units: Implications for fluoroquinolone use. JAMA 2003, 289, 885–888. [Google Scholar] [CrossRef]
- Ling, T.K.; Xiong, J.; Yu, Y.; Lee, C.C.; Ye, H.; Hawkey, P.M. Multicenter antimicrobial susceptibility survey of gram-negative bacteria isolated from patients with community-acquired infections in the People’s Republic of China. Antimicrob Agents Chemother. 2006, 50, 374–378. [Google Scholar] [CrossRef]
- Conseil scientifique de l’ONERBA. Bacterial resistance to antibiotics. Data from the National Observatory of Bacterial Resistance Epidemiology (ONERBA). Med Mal Infect. 2005, 35, 155–169. [Google Scholar] [CrossRef]
- Cizman, M.; Orazem, A.; Krizan-Hergouth, V.; Kolman, J. Correlation between increased consumption of fluoroquinolones in outpatients and resistance of Escherichia coli from urinary tract infections. J Antimicrob Chemother. 2001, 47, 502. [Google Scholar] [CrossRef]
- El Bouamri, M.C.; Arsalane, L.; Kamouni, Y.; et al. Current antibiotic resistance profile of uropathogenic Escherichia coli strains and therapeutic consequences. Prog Urol. 2014, 24, 1058–1062. [Google Scholar] [CrossRef]
- Guessennd, N.; Bremont, S.; Gbonon, V.; et al. Qnr-type quinolone resistance in extended-spectrum beta-lactamase producing enterobacteria in Abidjan, Ivory Coast. Pathol Biol (Paris). 2008, 56, 439–446. [Google Scholar] [CrossRef]
- Larramendy, S.; Deglaire, V.; Dusollier, P.; et al. Risk factors of extended-spectrum beta-lactamases-producing Escherichia coli community acquired urinary tract infections: A systematic review. Infect Drug Resist. 2020, 13, 3945–3955. [Google Scholar] [CrossRef]
| Total N = 373 | Case N = 50 | Control N = 323 | p | Odds ratio | 95%CI | |
|---|---|---|---|---|---|---|
| Male sex | 110 | 34 | 76 | 0.001 | 6.2 | 2.2–10.1 |
| Female sex | 240 | 16 | 224 | 0.09 | 1.1 | 0.9–2.2 |
| Age >65 years | 168 | 32 | 136 | 0.007 | 8.1 | 5.4–20.8 |
| Urban origin | 331 | 44 | 287 | 0.003 | 5.42 | 1.5–14.1 |
| Recurrent urinary tract infections | 43 | 10 | 33 | 0.681 | 2.1 | 0.5–8.4 |
| Previous hospitalization within 3 months | 57 | 17 | 40 | 0.001 | 9.51 | 6.02–17.3 |
| Antibiotic treatment within 6 months | 50 | 18 | 32 | 0.001 | 7.82 | 3.1–24.6 |
| Diabetes | 109 | 9 | 100 | 0.841 | 0.9 | 0.4–1.9 |
| Neoplasm | 20 | 8 | 12 | 0.752 | 0.66 | 0.2–1.7 |
| Pregnancy | 36 | 1 | 35 | 0.987 | 0.7 | 0.2–1.2 |
| Renal insufficiency | 21 | 2 | 19 | 0.564 | 1.1 | 0.6–2.1 |
| p | Odds ratio | 95%CI | |
|---|---|---|---|
| Male sex | 0.08 | 3.88 | 0.82–6.22 |
| Female sex | 0.62 | 0.84 | 0.47–1.68 |
| Age >65 years | 0.048 | 8.4 | 2.1–42 |
| Urban origin | 0.29 | 2.66 | 0.91–3.08 |
| Recurrent urinary tract infections | 0.14 | 1.01 | 1.00–1.03 |
| Previous hospitalization within 3 months | 0.003 | 13.4 | 3.3–140.2 |
| Antibiotic treatment within 6 months | 0.012 | 9.2 | 4.1–60.1 |
| Diabetes | 0.28 | 2.4 | 0.5–4.4 |
| Neoplasm | 0.32 | 0.4 | 0.1–2.8 |
| Pregnancy | 0.066 | 2.2 | 0.9–5.2 |
| Renal insufficiency | 0.41 | 1.9 | 0.4–2.2 |
© 2021 by the authors.
Share and Cite
Benaissa, E.; Belouad, E.; Mechal, Y.; Benlahlou, Y.; Chadli, M.; Maleb, A.; Elouennass, M. Multidrug-Resistant Community-Acquired Urinary Tract Infections in a Northern Region of Morocco: Epidemiology and Risk Factors. Germs 2021, 11, 562-569. https://doi.org/10.18683/germs.2021.1291
Benaissa E, Belouad E, Mechal Y, Benlahlou Y, Chadli M, Maleb A, Elouennass M. Multidrug-Resistant Community-Acquired Urinary Tract Infections in a Northern Region of Morocco: Epidemiology and Risk Factors. Germs. 2021; 11(4):562-569. https://doi.org/10.18683/germs.2021.1291
Chicago/Turabian StyleBenaissa, Elmostafa, Elmehdi Belouad, Youness Mechal, Yassine Benlahlou, Mariama Chadli, Adil Maleb, and Mostafa Elouennass. 2021. "Multidrug-Resistant Community-Acquired Urinary Tract Infections in a Northern Region of Morocco: Epidemiology and Risk Factors" Germs 11, no. 4: 562-569. https://doi.org/10.18683/germs.2021.1291
APA StyleBenaissa, E., Belouad, E., Mechal, Y., Benlahlou, Y., Chadli, M., Maleb, A., & Elouennass, M. (2021). Multidrug-Resistant Community-Acquired Urinary Tract Infections in a Northern Region of Morocco: Epidemiology and Risk Factors. Germs, 11(4), 562-569. https://doi.org/10.18683/germs.2021.1291




