Periodontal Disease in Seropositive Rheumatoid Arthritis: Scoping Review of the Epidemiological Evidence
Abstract
Introduction
RA and PD
Seropositivity in RA
ACPA Positivity and PD
Direct Bacterial Insult and Local Inflammation Generating Auto-Antigens
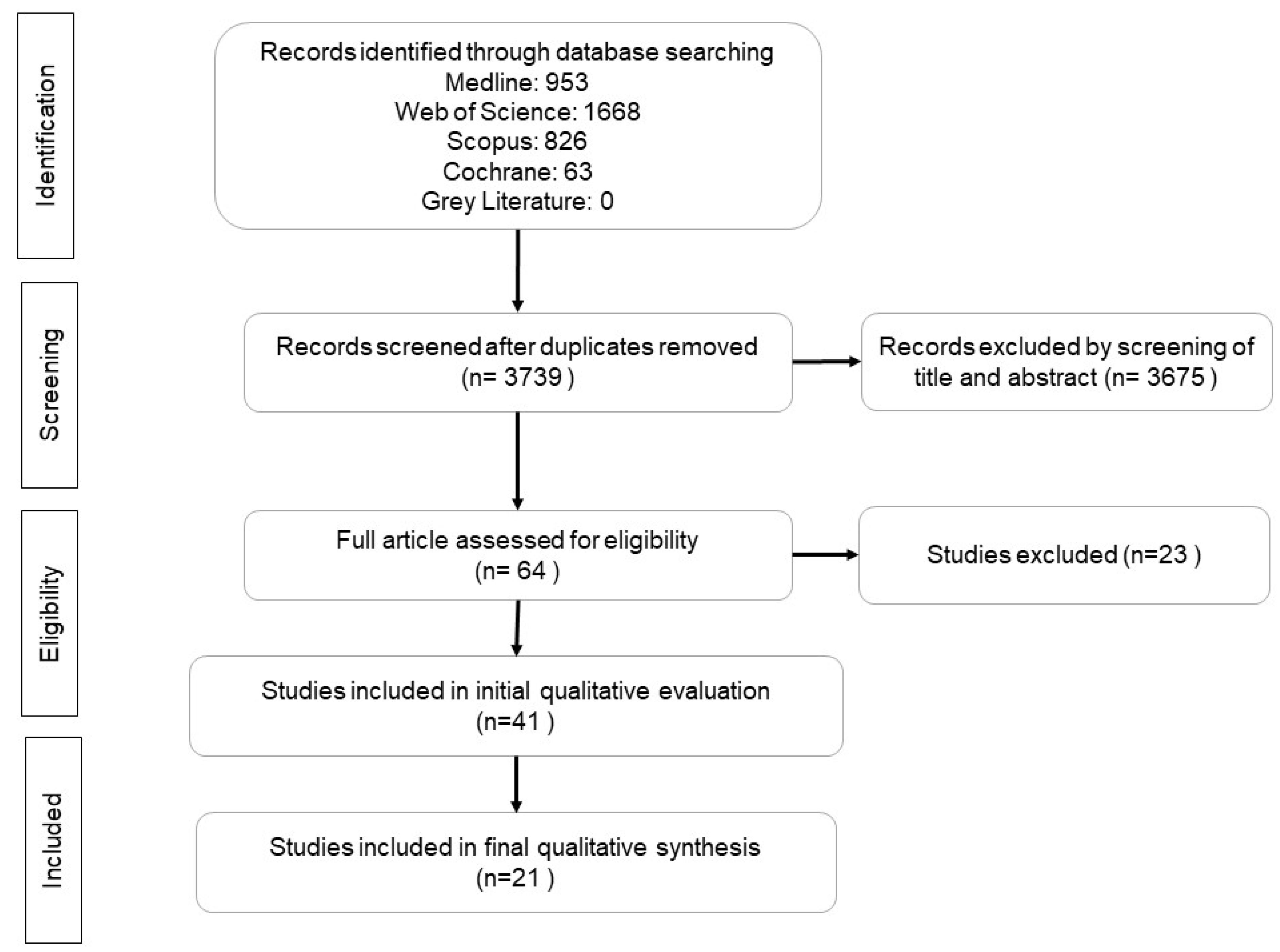
Search Criteria
Data Extraction and Analysis
Review Results
Study selection and Characteristics
Prevalence, Severity and Associations Between PD and ACPA Seropositivity
Discussion
| Authors, Year of Publication | Setting/Location | Aims of the Study | Sample Numbers | Control Type | Type of Study | PD Diagnostic Classification | RA Diagnostic Classification | |
|---|---|---|---|---|---|---|---|---|
| RA | Control | |||||||
| Havemose-Poulsen, 2006 [55] | Copenhagen, Denmark | To compare periodontal and hematological characteristics of patients with localized aggressive periodontitis (LAgP), generalized aggressive periodontitis (GAgP), juvenile idiopathic arthritis (JIA) or RA to those of control individuals. | LAgP = 18, GAgP = 27, JIA = 10, RA = 23 | 25 | Healthy controls | Case control | AAP 1999 (Armitage et al., 1999) | ACR 1987; JIA Classification 1994 (Arnett et al., 1988; Petty et al., 1994) |
| Dissick, 2010 [61] | United States veterans | To evaluate the prevalence and severity of periodontitis in a population of United States veterans with RA, making comparisons to a group of patients with non-inflammatory arthritis. | 69 | 35 | Osteoarthritis | Case control | AAP 2003 (Armitage et al, 2003) | ACR 1987 (Arnett et al., 1988) |
| de Smit, 2012 [62] | Groningen, Netherlands | To further explore whether the association between periodontitis and RA is dependent on P. gingivalis, by comparing host immune responses in RA patients with and without periodontitis in relation to presence of cultivable P gingivalis in subgingival plaque | 95 | 44, 36 | Non-RA controls, healthy controls | Case control | DPSI (van der Velden, 2009) | ACR 1987 (Arnett et al., 1988) |
| Potikuri 2012 [46] | Hyderabad, India | To find the strength of association between PD and RA in non-smoking, disease modifying antirheumatic drug-naive RA patients | 91 | 93 | Healthy controls | Case control | Mean pocket depth is ≥3 mm | ACR 1987 (Arnett et al., 1988) |
| Mikuls 2015 [47] | United States veterans | To examine the degree to which shared risk factors explain the relationship of PD with RA and to examine associations of PD and Porphyromonas gingivalis with disease features. | 287 | 330 | Osteoarthritis | Case control | Machtei et al., 1992 | ACR 1987 (Arnett et al., 1988) |
| Gonzales, 2015 [48] | United States veterans | (1) To examine alveolar bone loss in (ACPA)-positive rheumatoid arthritis (RA) patients versus osteoarthritis controls and (2) To examine the association of alveolar bone loss with RA disease activity and ACPA concentrations, including multiple antigen-specific ACPA. | 287 | 330 | Osteoarthritis | Case control | Machtei et al., 1992 | ACR 1987 (Arnett et al., 1988) |
| Terao, 2015 [43] | Kyoto, Japan | To analyze the associations between PD status and RA-related autoantibodies especially ACPA | 9575 subjects who did not have connective tissue diseases | NR | Communit y-based prospective cohort study | CPI (WHO) | NR | |
| Choi, 2016 [49] | Seoul, Korea | To investigate the association between severity of periodontitis and clinical manifestation of RA. | 264 | 88 | NR | Case control | AAP 2003 (Armitage et al, 2003) | ACR 1987 (Arnett et al., 1988) |
| Erikkson, 2016 [44] | Sweden | To investigate the prevalence of periodontitis in the Swedish Epidemiological Investigation of RA (EIRA), a well-characterized population-based RA case-control cohort. | 2,740 | 3,942 | Healthy controls | Population-based case-control | AAP 1999 (Armitage et al, 1999) | ACR 1987 (Arnett et al., 1988) |
| Erikkson, 2018 [50] | Sweden | To investigate the effects of smoking on the risk of periodontitis in seropositive and seronegative (ACPA/ RF) subsets of RA | 2,327 | NR | NR | Population-based case-control | AAP 1999 (Armitage et al, 1999) | ACR 1987 (Arnett et al., 1988) |
| Wan Mohamad, 2018 [56] | Malaysia | To assess the presence of periodontal disease and whether the association with ACPA occurs among RA patients in local population. | 44 | NR | NR | Cross-sectional | The severity of periodontal status was defined as mild (1–2 mm CAL) or moderate to severe ( ≥ 3 mm CAL). | ACR 2010 (Aletaha et al., 2010) |
| Erikkson, 2019 [45] | Sweden | To investigate the severity of periodontitis in Swedish RA patients in relation to autoantibody status (ACPA and RF), inflammatory mediators, RA disease activity and medication as well as the microbiota in saliva and subgingival plaque. | 40 | NR | NR | Cross-sectional | CDC/AAP 2007 (Page et al., 2007) | ACR 2010 (Aletaha et al., 2010) |
| Rodríguez-Lozano, 2019 [57] | Tenerife, Spain | To investigate the link between RA and periodontitis, to assess whether RA disease activity is associated with periodontitis severity and to determine the degree to which this association is affected by shared risk factors. | 187 | 157 | Osteoarthritis/Soft tissue rheumatic disease | Case control | Tonetti et al., 2005 | ACR 2010 (Aletaha et al., 2010) |
| Zhao, 2019 [51] | Nantong, China | To assess the prevalence and severity of PD in Chinese RA patients and analyze to what extent confounders may affect the association potentially. | 128 | 109 | Healthy controls | Case control | No periodontitis; mild periodontitis ≤30% bone loss and minimal or no BOP; moderate periodontitis ≤50% bone loss, BOP, and tooth mobility <2; severe periodontitis ≥50% bone loss, marked BOP, and tooth mobility ≥2. | ACR 2010 (Aletaha et al., 2010) |
| González-Febles, 2020 [52] | Spain | To investigate the link between periodontitis and its severity with the presence and levels of ACPA in RA patients, and the possible impact of shared risk factors as tobacco habit on the presence and levels of ACPA. | 164 | NR | NR | Cross-sectional | Tonetti et al., 2018 | ACR 2010 (Aletaha et al., 2010) |
| Nguyen, 2020 [53] | Ho Chi Minh City, Vietnam | To survey periodontal status of Vietnamese patients with RA and investigate the association between periodontitis and RA in these patients. | 150 | 150 | Osteoarthritis | Case control | CDC/AAP 2012 (Eke et al., 2012) | ACR 2010 (Aletaha et al., 2010) |
| Rahajoe, 2020 [60] | Yogyakarta, Indonesia | To assess RA associated autoantibodies, especially the IgA isotypes of ACPA and RF in the gingival crevicular fluid of RA patients and in healthy controls with or without PD. | 72 | 151 | Healthy controls | Case control | CDC/AAP 2012 (Eke et al., 2012) | ACR 2010 (Aletaha et al., 2010) |
| Reichert, 2020 [54] | Martin-Luther University Halle-Wittenberg, Germany | to investigate a putative relationship between the periodontal status and/or the detection of five periodontal pathogenic bacteria in subgingival plaque with autoantibody levels against CEP-1 and/or CCP in patients with RA. | 107 | NR | NR | Cross-sectional | CDC/AAP 2012 (Eke et al., 2012 | ACR 2010 (Aletaha et al., 2010) |
| Renvert, 2020 [58] | Karlskrona, Sweden | To assess if a diagnosis of periodontitis is more common in individuals (≥61 years) with RA than among age-stratified individuals from the normal population without a diagnosis of RA. | 126 | 249 | Healthy controls | Case control | Gingivitis: BOP at ≥20% of sites. Periodontitis: BOP at >20% of sites, presence of >2 non-adjacent sites with (PPD) ≥5 mm, bone loss at ≥2 sites, furcation involvement. | ACR 1987 (Arnett et al., 1988) and ACR 2010 (Aletaha et al, 2010) |
| Stefanov, 2020 [67] | Bulgaria | To investigate the relationship between serum ACPAs and the clinical parameters of periodontitis, as well as to define their predictive value for assessing severity and activity of the periodontal disease in patients with concomitant periodontitis and RA. | 60 | NR | NR | Cross-sectional | Tonetti et al., 2018 | ACR 2010 (Aletaha et al., 2010) |
| Svard, 2020 [59] | Karlskrona, Sweden | To investigate the presence and levels of IgA ACPA in saliva and serum of elderly RA patients, in relation to clinically verified periodontitis and smoking. | 132 | NR | NR | Cross-sectional | Gingivitis: BOP at ≥20% of sites. Periodontitis: BOP at >20% of sites, presence of >2 non-adjacent sites with PPD ≥ 5 mm, bone loss at ≥2 sites, furcation involvement. | ACR 1987 (Arnett et al., 1988) and ACR 2010 (Aletaha et al, 2010) |
| Authors, Year of Publication | PD Clinical Parameters | RA Clinical Parameters | Prevalence/Severity of PD and ACPA Seropositivity | Association Between ACPA And PD Parameters | Author’s Conclusion Regarding ACPA Seropositivity and PD |
|---|---|---|---|---|---|
| Havemose-Poulsen, 2006 [55] | Plaque, BOP, PPD, CAL, ABL | Erythrocyte fraction, leukocytes and differential counts, ESR, CRP, RF, ACPA | NR | ACPA levels did not correlate with any of the PD variables | NR |
| Dissick, 2010 [61] | BOP, erythema/edema, purulence, tooth mobility, ABL | Multidimensional health assessment questionnaire, CRP, RF, ACPA, DAS28, the presence of radiographic erosions on hand or foot films | Among patients who were ACPA seropositive, 56% had moderate to severe periodontitis, 31% had mild periodontitis, and 14% had no periodontitis. Patients who were ACPA negative had 22% (moderate to severe), 22% (mild), and 56% (none) periodontitis (p = 0.01 for the difference). | NR | The presence of periodontitis in patients with RA was associated with ACPA seropositivity. Moderate to severe periodontitis was more frequently observed among RA patients who were seropositive for ACPA. |
| de Smit, 2012 [62] | Dutch periodontal screening index (DPSI) including BOP, PPD, CAL | DAS28, RA disease duration, subgingival plaque samples analysis for presence of Porphyromonas gingivalis | Between RA patients with no, moderate, or severe periodontitis, no differences were seen in ACPA levels | NR | NR |
| Potikuri 2012 [46] | PPD, screening questionnaire for the presence of gingival swelling, gingival bleeding, tooth sensitivity, tooth mobility and past history of tooth loss due to PD | RA disease duration, early morning stiffness, TJC28, SJC28, joint deformities and patient global assessment of disease severity on a visual analogue scale, DAS 28-ESR | NR | 1. Mean pocket depth was significantly higher in RA with ACPA positive patients compared with RA with ACPA negative (3.94 ± 1.13 mm vs. 3.40 ± 1.25 mm; p = 0.04). The mean pocket depth correlated positively with titers of ACPA (r = 0.24; p = 0.02). 2. ACPA titers were significantly higher in the PD group (753.05 ± 1088.27 vs. 145.15 ± 613.16 IU/mL; p = 0.001). | PD in RA is associated with high titers of ACPAs. |
| Mikuls 2015 [47] | BOP, PPD, REC, supragingival plaque (serving as an indicator of oral hygiene, missing teeth, subgingival plaques specimen analysis | ACPA, RF, hs-CRP, HLA-DRB1 status analysis, TJC28, SJC28, VAS global well-being scores, DAS-28-CRP, posterior-anterior hand and wrist radiographs | PD was more common in ACPA positive RA (37%; ORunadj 1.65; 95% CI 1.15, 2.36; p = 0.006) compared to controls (26%). | 1. After multivariable adjustment, including adjustments for smoking and HLA-DRB1 SE, PD remained significantly more frequent among ACPA positive RA cases (OR 1.59; 95%CI 1.01–2.49; p = 0.043) than controls, an association that was attenuated and not significant when all RA cases including ACPA negative patients were evaluated (OR 1.36; 95%CI 0.89–2.06; p = 0.153). 2. Relative to controls, ACPA positive cases (p = 0.005) demonstrated a higher percentage of sites with probing depths ≥ 5 mm with a non-significant trend towards a higher proportion of sites with attachment loss ≥ 5 mm among ACPA positive cases (p = 0.060). | PD demonstrated an independent relationship with established seropositive RA. Associations of PD with established seropositive RA were independent of all covariates examined including evidence of Porphyromonas gingivalis infection. |
| Gonzales, 2015 [48] | BOP, PPD, REC, supragingival plaque (serving as an indicator of oral hygiene, missing teeth | ACPA, RF, hs-CRP, HLA-DRB1 status analysis, TJC28, SJC28, VAS global well-being scores, DAS-28-CRP, posterior-anterior hand and wrist radiographs | NR | 1. ACPA-positive RA cases had a statistically significantly higher mean percentage of sites with alveolar bone loss greater than 20% compared to osteoarthritis controls (p = 0.03). 2. Alveolar bone loss was associated with higher ACPA (p = 0.007). 3. Following multivariate adjustment, high alveolar bone loss remained significantly associated with higher values of the continuous variables ACPA concentration (p = 0.004). | There is an association between alveolar bone loss and ACPA concentrations and RA disease activity measures. |
| Terao, 2015 [43] | Missing teeth, CPI, CAL | ACPA, RF | NR | 1. Significant or suggestive positive associations between increasing positivity of ACPA and all of the PD parameters (missing teeth, CPI, CAL) conditioned with covariates (p = 0.024, 0.0042 and 0.037). 2. CPI was shown to be associated with increasing ACPA positivity and levels when CPI was more than 2. | The significant associations between PD parameters and positivity and levels of ACPA in the healthy population support the fundamental involvement of PD with ACPA production. |
| Choi, 2016 [49] | BOP, GI, PPD, CAL | ACPA, RF, TJC68, SJC68, disease duration, ESR, CRP | NR | 1. BOP was correlated with ACPA (r = 0.183, p = 0.009). 2. GI was correlated with ACPA (r = 0.203, p = 0.004). | ACPA are more abundant in patients with more severe periodontal inflammation. |
| Erikkson, 2016 [44] | Prevalence of periodontitis investigated using a self-administered questionnaire. Treatment codes for PD retrieved through the National Dental Health Registry. | Treatment codes for RA retrieved through linking the National Dental Health Registry | 70% of ACPA +ve and 69% of ACPA −ve participants had PD | The prevalence of periodontal treatment codes did not differ between ACPA-positive and ACPA-negative RA (p > 0.05). | No differences in PD parameters based on ACPA among RA subjects. |
| Erikkson, 2018 [50] | Prevalence of periodontitis investigated using a self-administered questionnaire. Treatment codes for PD retrieved through the National Dental Health Registry. | Treatment codes for RA retrieved through linking the National Dental Health Registry | 52.6% of ACPA +ve and 53.8% of ACPA −ve participants had PD | 1. In ACPA-positive RA, smoking was associated with a significantly (p < 0.05) higher prevalence of periodontitis, mainly in current smokers (OR = 1.9, 95% CI 1.5–2.5). 2. The OR for periodontitis increased among patients double positive for ACPA and RF antibodies, with OR of 3.3 (95% CI 1.8–6.2) observed in current smoking men compared with never smokers. | The highest risk of periodontitis in patients with established RA was observed among seropositive current smokers, especially those that are double positive for ACPA and RF antibodies. |
| Wan Mohamad, 2018 [56] | PS, GS, PPD, CAL | ACPA | 33.3% of ACPA +ve and 55.7% of ACPA −ve participants had moderate to severe PD (p = 0.27) | No significant association between ACPA levels and the severity of periodontal disease based on CAL. | The association with periodontal status was not evident in this study. |
| Erikkson, 2019 [45] | BOP, PI, PPD, CAL, stimulated salivary flow, number of missing and mobile teeth, subgingival plaque sample | ACPA, RF, DAS-28, DAS28-CRP, CRP, VAS global well-being scores, HAQ | 86% of ACPA +ve and 14% of ACPA −ve participants had PD | ACPA positivity was significantly (p = 0.032) more frequent in patients with moderate/severe periodontitis (86%), compared to the group with no/mild disease (50%). | Patients with ACPA-positive RA have more severe forms of periodontitis, irrespective of DMARD therapy or the presence of subgingival Porphyromonas gingivalis. |
| Rodríguez-Lozano, 2019 [57] | BOP, PI, PPD, CAL, REC, tooth loss | ACPA, RF, DAS28-CRP, CRP, clinical disease activity index, simplified disease activity index, VAS global well-being scores | NR | No association was observed between the immunological characteristics of RA (presence of ACPA) and the presence of periodontitis. | NR |
| Zhao, 2019 [51] | BOP, PI, GI, PPD, CAL | ACPA, RF, DAS28-ESR, HAQ, CRP, ESR, radiographs | 59 cases were ACPA positive in PD group and 8 cases were positive in non-PD group (67.82% vs. 19.51%; p < 0.001). | Multivariate logistic regression showed higher ACPA positivity were the variables related to periodontitis in RA patients (OR 8.963; 95% CI: 2.171–36.998, p < 0.002). | PD is related to ACPA positivity. |
| González-Febles, 2020 [52] | PI, BOP, PPD, CAL, number of missing and mobile teeth | ACPA, RF, DAS28-CRP, CRP, SDAI, VAS global well-being scores | 100% of ACPA +ve and 98% of ACPA −ve participants had PD | 1. ACPA positive patients showed significantly higher CAL (4.16 ± 1.43 vs. 3.72 ± 0.85, p = 0.015), higher numbers (16.93 ± 19.63 vs. 11.64 ± 11.02, p = 0.029) and percentages of pockets ≥5 mm (0.14 ± 0.16 vs. 0.09 ± 0.09, p = 0.014), and higher mean PI (31.0 ± 19.7 vs. 22.4 ± 13.3, p < 0.001), compared to their ACPA negative counterparts. 2. When ACPA were stratified by level, an ordinal logistic regression model showed a direct association between these levels and the mean CAL with an OR of 1.593 (95% CI 1.017–2.482, p = 0.043) in patients with high ACPA titers versus non-ACPA patients. A significant association was also demonstrated for patients with high ACPA titers and the number of pockets ≥5mm and the mean PI with adjusted ORs of 1.031 (95%CI 1.003–1.062, p = 0.031) and 1.060 (95% CI 1.027–1.093, p < 0.001), respectively. | There is a link between ACPA titers and periodontitis, in which worsening periodontal conditions, in terms of mean CAL, mean PI, and number of pockets >5 mm, were statistically associated with both ACPA positivity and higher levels of ACPA titers. |
| Nguyen, 2020 [53] | PI, GI, PPD, CAL, number of missing and mobile teeth | ACPA, RF, DAS28-CRP, CRP, VAS global well-being scores, TJC28, SJC28 | 74.7% of ACPA +ve and 54.2% of ACPA −ve participants had PD (p = 0.16). | 1. There was a positive correlation between ACPAs levels, ACPAs categories and severity of periodontitis (p < 0.05). 2. There was a weak correlation between the level of periodontal status and serum ACPAs (Spearman’s r 0.32, p < 0.001). | Periodontal status was proportional to ACPAs positivity. |
| Rahajoe, 2020 [60] | BOP, PPD, CAL, PISA, GCF sample | ACPA, RF, DAS28-ESR, ESR, disease duration | NR | ACPA levels in the RA patients’ serum were not related to the presence of PD. | NR |
| Reichert, 2020 [54] | BOP, PPD, CAL, PISA, PESA, subgingival plaque specimens | ACPA, anti-CEP-1 antibodies, human leukocyte antigen (HLA) typing | 67.2% of ACPA +ve and 60% of ACPA −ve participants had moderate or severe PD (p = 0.257). | Weak but significant association between periodontitis assessed as PESA and the prevalence of ACPA positivity (95% CI 1.000–1.005, p = 0.040). | Periodontitis is if any only a minor risk factor for ACPA positivity. |
| Renvert, 2020 [58] | BOP, PPD, CAL, ABL, number of missing, caries and mobile teeth | Disease duration, VAS pain score, ESR, CRP, DAS28-ESR, ACPA, RF | NR | No association between ACPA positivity and periodontitis was found (p = 0.92). | NR |
| Stefanov, 2020 [67] | Hygiene index, papilla bleeding index, BOP, PPD, CAL, REC, presence of furcation lesions, PISA | ACPA, DAS28-CRP, CRP | NR | No statistically significant difference in serum levels of ACPA depending on the severity of periodontal parameters. Serum ACPA levels showed a weak positive association only with the number of teeth lost (r = 0.260, p = 0.045). | Serum ACPA is not a biomarker that can well differentiate severe from mild periodontal lesions in patients with concomitant periodontitis and RA. The predictive value of ACPA for assessing the severity of periodontal disease in patients with RA is low. |
| Svard, 2020 [59] | BOP, PS, PPD, CAL, ABL, number of missing, caries and mobile teeth | Disease duration, VAS pain score, ESR, CRP, DAS28-ESR, DAS28-CRP, ACPA, RF, SJC (28), TJC (28) | IgG ACPA in serum was found in 66% of RA patients with periodontitis, and in 69% of RA patients without periodontitis (p = 0.849). IgA ACPA in serum was found in 35% of RA patients with periodontitis, and in 43% of RA patients without periodontitis (p = 0.461). | No difference between levels of ACPA in RA patients with or without periodontitis. | No correlation was observed between periodontitis and saliva IgA ACPA or between periodontitis and IgA or IgG ACPA in serum. The findings do not support the hypothesis that periodontitis leads to increased formation of IgA ACPA in saliva or serum. |
Conclusions
Funding
Authors’ Contributions Statement
Conflicts of Interest
References
- Young, A.; Koduri, G. Extra-articular manifestations and complications of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007, 21, 907–927. [Google Scholar] [CrossRef]
- Jung, G.U.; Han, J.Y.; Hwang, K.G.; Park, C.J.; Stathopoulou, P.G.; Fiorellini, J.P. Effects of conventional synthetic disease-modifying antirheumatic drugs on response to periodontal treatment in patients with rheumatoid arthritis. Biomed Res Int. 2018, 2018, 1465402. [Google Scholar] [CrossRef]
- Chen, H.H.; Huang, N.; Chen, Y.M.; et al. Association between a history of periodontitis and the risk of rheumatoid arthritis: A nationwide, population-based, case-control study. Ann Rheum Dis. 2013, 72, 1206–1211. [Google Scholar] [CrossRef]
- Schmickler, J.; Rupprecht, A.; Patschan, S.; et al. Cross-sectional evaluation of periodontal status and microbiologic and rheumatoid parameters in a large cohort of patients with rheumatoid arthritis. J Periodontol. 2017, 88, 368–379. [Google Scholar] [CrossRef]
- Joseph, R.; Rajappan, S.; Nath, S.G.; Paul, B.J. Association between chronic periodontitis and rheumatoid arthritis: A hospital-based case-control study. Rheumatol Int. 2013, 33, 103–109. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Ferreira, R.; de Brito Silva, R.; Magno, M.B.; et al. Does periodontitis represent a risk factor for rheumatoid arthritis? A systematic review and meta-analysis. Ther Adv Musculoskelet Dis. 2019, 11, 1759720X19858514. [Google Scholar] [CrossRef]
- Suhaimi, N.; Kamaruzaman; Natasha Taib, H.; Wan Mohamad, W.M.; Wan Ghazali, W.S. Assessment of Periodontal Status in patients with Rheumatoid Arthritis. J Int Dent Med Res. 2016, 9, 108–112. [Google Scholar]
- Khantisopon, N.; Louthrenoo, W.; Kasitanon, N.; et al. Periodontal disease in Thai patients with rheumatoid arthritis. Int J Rheum Dis. 2014, 17, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Monsarrat, P.; Vergnes, J.N.; Blaizot, A.; et al. Oral health status in outpatients with rheumatoid arthritis: The OSARA study. Oral Health Dent Manag. 2014, 13, 113–119. [Google Scholar] [PubMed]
- Pischon, N.; Pischon, T.; Kröger, J.; et al. Association among rheumatoid arthritis, oral hygiene, and periodontitis. J Periodontol. 2008, 79, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Hashimoto, S.; Muto, A.; Dewake, N.; Shimazaki, Y. Influence of plaque control on the relationship between rheumatoid arthritis and periodontal health status among Japanese rheumatoid arthritis patients. J Periodontol. 2018, 89, 1033–1042. [Google Scholar] [CrossRef]
- Fuggle, N.R.; Smith, T.O.; Kaul, A.; Sofat, N. Hand to mouth: A systematic review and meta-analysis of the association between rheumatoid arthritis and periodontitis. Front Immunol. 2016, 7, 80. [Google Scholar] [CrossRef]
- Kaur, S.; White, S.; Bartold, P.M. Periodontal disease and rheumatoid arthritis: A systematic review. J Dent Res. 2013, 92, 399–408. [Google Scholar] [CrossRef]
- Araújo, V.M.; Melo, I.M.; Lima, V. Relationship between periodontitis and rheumatoid arthritis: Review of the literature. Mediators Inflamm. 2015, 2015, 259074. [Google Scholar] [CrossRef]
- Synderman, R.; McCarty, G.A. Host-Parasite Interactions in Periodontal Disease; American Society for Microbiology: Washington, DC, USA, 1982; pp. 354–362. [Google Scholar]
- Abrão, A.L.; Santana, C.M.; Bezerra, A.C.; et al. What rheumatologists should know about orofacial manifestations of autoimmune rheumatic diseases. Rev Bras Reumatol Engl Ed. 2016, 56, 441–450. [Google Scholar] [CrossRef][Green Version]
- Li, R.; Tian, C.; Postlethwaite, A.; et al. Rheumatoid arthritis and periodontal disease: What are the similarities and differences? Int J Rheum Dis. 2018, 20, 1887–1901. [Google Scholar] [CrossRef]
- Lundberg, K.; Wegner, N.; Yucel-Lindberg, T.; Venables, P.J. Periodontitis in RA-the citrullinated enolase connection. Nat Rev Rheumatol. 2010, 6, 727–730. [Google Scholar] [CrossRef]
- Farquharson, D.; Butcher, J.P.; Culshaw, S. Periodontitis, Porphyromonas, and the pathogenesis of rheumatoid arthritis. Mucosal Immunol. 2012, 5, 112–120. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology / European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010, 69, 1892. [Google Scholar] [CrossRef]
- Gomez, E.L.; Gun, S.C.; Somnath, S.D.; et al. The prevalence of rheumatoid factor isotypes and anti-cyclic citrullinated peptides in Malaysian rheumatoid arthritis patients. Int J Rheum Dis. 2011, 14, 12–17. [Google Scholar] [CrossRef]
- Arkema, E.V.; Goldstein, B.L.; Robinson, W.; et al. Anti-citrullinated peptide autoantibodies, human leukocyte antigen shared epitope and risk of future rheumatoid arthritis: A nested case-control study. Arthritis Res Ther. 2013, 15, R159. [Google Scholar] [CrossRef]
- Vander Cruyssen, B.; Peene, I.; Cantaert, T.; et al. Anti-citrullinated protein/peptide antibodies (ACPA) in rheumatoid arthritis: Specificity and relation with rheumatoid factor. Autoimmun Rev. 2005, 4, 468–474. [Google Scholar] [CrossRef]
- Rosenstein, E.D.; Kushner, L.J.; Kramer, N. Rheumatoid arthritis and periodontal disease: A rheumatologist’s perspective. Curr Oral Health Reports. 2015, 2, 9–19. [Google Scholar] [CrossRef]
- Koziel, J.; Mydel, P.; Potempa, J. The link between periodontal disease and rheumatoid arthritis: An updated review. Curr Rheumatol Rep. 2014, 16, 408. [Google Scholar] [CrossRef]
- Quirke, A.M.; Lugli, E.B.; Wegner, N.; et al. Heightened immune response to autocitrullinated Porphyromonas gingivalis peptidylarginine deiminase: A potential mechanism for breaching immunologic tolerance in rheumatoid arthritis. Ann Rheum Dis. 2014, 73, 263–269. [Google Scholar] [CrossRef]
- Golub, L.M.; Payne, J.B.; Reinhardt, R.A.; Nieman, G. Can systemic diseases co-induce (not just exacerbate) periodontitis? A hypothetical “two-hit” model. J Dent Res. 2006, 85, 102–105. [Google Scholar] [CrossRef]
- Hitchon, C.A.; Chandad, F.; Ferucci, E.D.; et al. Antibodies to Porphyromonas gingivalis are associated with anticitrullinated protein antibodies in patients with rheumatoid arthritis and their relatives. J Rheumatol. 2010, 37, 1105–1112. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef]
- Reichert, S.; Haffner, M.; Keyßer, G.; et al. Detection of oral bacterial DNA in synovial fluid. J Clin Periodontol. 2013, 40, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Potempa, J.; Mydel, P.; Koziel, J. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat Rev Rheumatol. 2017, 13, 606–620. [Google Scholar] [CrossRef]
- Konig, M.F.; Abusleme, L.; Reinholdt, J.; et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci Transl Med. 2016, 8, 369ra176. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Armitage, G.C. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999, 4, 1–6. [Google Scholar] [CrossRef]
- GC, Research, Science and Therapy Committee of the American Academy of Periodontology. Diagnosis of periodontal diseases. J Periodontol. 2003, 74, 1237–1247. [Google Scholar] [CrossRef]
- Van der Velden, U. The Dutch periodontal screening index validation and its application in The Netherlands. J Clin Periodontol. 2009, 36, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Machtei, E.E.; Christersson, L.A.; Grossi, S.G.; Dunford, R.; Zambon, J.J.; Genco, R.J. Clinical criteria for the definition of “established periodontitis”. J Periodontol. 1992, 63, 206–214. [Google Scholar] [CrossRef]
- Page, R.C.; Eke, P.I. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007, 78, 1387–1399. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Claffey, N.; European Workshop in Periodontology group, C. Advances in the progression of periodontitis and proposal of definitions of a periodontitis case and disease progression for use in risk factor research. Group C consensus report of the 5th European Workshop in Periodontology. J Clin Periodontol. 2005, 32, 210–213. [Google Scholar] [CrossRef]
- Eke, P.I.; Page, R.C.; Wei, L.; Thornton-Evans, G.; Genco, R.J. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012, 83, 1449–1454. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef] [PubMed]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef]
- Terao, C.; Asai, K.; Hashimoto, M.; et al. Significant association of periodontal disease with anti-citrullinated peptide antibody in a Japanese healthy population—The Nagahama study. J Autoimmun. 2015, 59, 85–90. [Google Scholar] [CrossRef]
- Eriksson, K.; Nise, L.; Kats, A.; et al. Prevalence of periodontitis in patients with established rheumatoid arthritis: A swedish population based case-control study. PLoS ONE. 2016, 11, e0155956. [Google Scholar] [CrossRef]
- Eriksson, K.; Fei, G.; Lundmark, A.; et al. Periodontal health and oral microbiota in patients with rheumatoid arthritis. J Clin Med. 2019, 8, 630. [Google Scholar] [CrossRef]
- Potikuri, D.; Dannana, K.C.; Kanchinadam, S.; et al. Periodontal disease is significantly higher in non-smoking treatment-naive rheumatoid arthritis patients: Results from a case-control study. Ann Rheum Dis. 2012, 71, 1541–1544. [Google Scholar] [CrossRef] [PubMed]
- Mikuls, T.R.; Payne, J.B.; Yu, F.; et al. Periodontitis and Porphyromonas gingivalis in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014, 66, 1090–1100. [Google Scholar] [CrossRef]
- Gonzalez, S.M.; Payne, J.B.; Yu, F.; et al. Alveolar bone loss is associated with circulating anti-citrullinated protein antibody (ACPA) in patients with rheumatoid arthritis. J Periodontol. 2015, 86, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.A.; Kim, J.; Kim, Y.M.; et al. Periodontitis is associated with rheumatoid arthritis: A study with longstanding rheumatoid arthritis patients in Korea. Korean J Intern Med. 2016, 31, 977–986. [Google Scholar] [CrossRef]
- Eriksson, K.; Nise, L.; Alfredsson, L.; et al. Seropositivity combined with smoking is associated with increased prevalence of periodontitis in patients with rheumatoid arthritis. Ann Rheum Dis. 2018, 77, 1236–1238. [Google Scholar] [CrossRef]
- Zhao, R.; Gu, C.; Zhang, Q.; et al. Periodontal disease in Chinese patients with rheumatoid arthritis: A case-control study. Oral Dis. 2019, 25, 2003–2009. [Google Scholar] [CrossRef]
- González-Febles, J.; Rodríguez-Lozano, B.; Sánchez-Piedra, C.; et al. Association between periodontitis and anti-citrullinated protein antibodies in rheumatoid arthritis patients: A cross-sectional study. Arthritis Res Ther. 2020, 22, 27. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Nguyen, T.T.; Huynh, N.C.; Le, T.A.; Hoang, H.T. Relationship between periodontitis and rheumatoid arthritis in Vietnamese patients. Acta Odontol Scand. 2020, 78, 522–528. [Google Scholar] [CrossRef]
- Reichert, S.; Schlumberger, W.; Dähnrich, C.; et al. Association of levels of antibodies against citrullinated cyclic peptides and citrullinated α-enolase in chronic and aggressive periodontitis as a risk factor of rheumatoid arthritis: A case control study. J Transl Med. 2015, 13, 283. [Google Scholar] [CrossRef]
- Havemose-Poulsen, A.; Westergaard, J.; Stoltze, K.; et al. Periodontal and hematological characteristics associated with aggressive periodontitis, juvenile idiopathic arthritis, and rheumatoid arthritis. J Periodontol. 2006, 77, 280–288. [Google Scholar] [CrossRef]
- Mohamad, W.M.W.; Jia, S.K.; Ghazali, W.S.W.; Taib, H. Anti-cyclic citrullinated peptide antibody and periodontal status in rheumatoid arthritis patients. Pak J Med Sci. 2018, 34, 907–912. [Google Scholar] [CrossRef]
- Rodríguez-Lozano, B.; González-Febles, J.; Garnier-Rodríguez, J.L.; et al. Association between severity of periodontitis and clinical activity in rheumatoid arthritis patients: A case—Control study. Arthritis Res Ther. 2019, 21, 7. [Google Scholar] [CrossRef]
- Renvert, S.; Berglund, J.S.; Persson, G.R.; Söderlin, M.K. The association between rheumatoid arthritis and periodontal disease in a population-based cross-sectional case-control study. BMC Rheumatol. 2020, 20, 31. [Google Scholar] [CrossRef]
- Svärd, A.; Renvert, S.; Sanmartin Berglund, J.; Persson, R.G.; Söderlin, M. Antibodies to citrullinated peptides in serum and saliva in patients with rheumatoid arthritis and their association to periodontitis. Clin Exp Rheumatol. 2020, 38, 699–704. [Google Scholar] [PubMed]
- Rahajoe, P.S.; de Smit, M.; Schuurmans, G.; et al. Increased IgA anti-citrullinated protein antibodies in the periodontal inflammatory exudate of healthy individuals compared to rheumatoid arthritis patients. J Clin Periodontol. 2020, 47, 552–560. [Google Scholar] [CrossRef]
- Dissick, A.; Redman, R.S.; Jones, M.; et al. Association of periodontitis with rheumatoid arthritis: A pilot study. J Periodontol. 2010, 81, 223–230. [Google Scholar] [CrossRef] [PubMed]
- de Smit, M.; Westra, J.; Vissink, A.; Doornbos-van der Meer, B.; Brouwer, E.; van Winkelhoff, A.J. Periodontitis in established rheumatoid arthritis patients: A cross-sectional clinical, microbiological and serological study. Arthritis Res Ther. 2012, 14, R222. [Google Scholar] [CrossRef]
- Äyräväinen, L.; Leirisalo-Repo, M.; Kuuliala, A.; et al. Periodontitis in early and chronic rheumatoid arthritis: A prospective follow-up study in Finnish population. BMJ Open. 2017, 7, e011916. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, S.K.; Hashim, N.T.; Elsalamabi, E.M.; Gismalla, B.G. Periodontal status of rheumatoid arthritis patients in Khartoum State. BMC Res Notes. 2011, 4, 460. [Google Scholar] [CrossRef]
- Kobayashi, T.; Murasawa, A.; Komatsu, Y.; et al. Serum cytokine and periodontal profiles in relation to disease activity of rheumatoid arthritis in Japanese adults. J Periodontol. 2010, 81, 650–657. [Google Scholar] [CrossRef]
- Mercado, F.B.; Marshall, R.I.; Klestov, A.C.; Bartold, P.M. Relationship between rheumatoid arthritis and periodontitis. J Periodontol. 2001, 72, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Stefanov, L.; Bolyarova-Konova, T.; Kolarov, Z.; Pavlova, P.; Ivanova, M. Serum anti-CCP antibodies in periodontitis associated with rheumatoid arthritis—Relative value for the severity of periodontitis. Rheumatol (Bulgaria). 2020, 28, 11–18. [Google Scholar] [CrossRef]
© GERMS 2021.
Share and Cite
Nik-Azis, N.-M.; Mohd, N.; Baharin, B.; Said, M.S.M.; Fadzilah, F.M.; Haflah, N.H.M. Periodontal Disease in Seropositive Rheumatoid Arthritis: Scoping Review of the Epidemiological Evidence. Germs 2021, 11, 266-286. https://doi.org/10.18683/germs.2021.1263
Nik-Azis N-M, Mohd N, Baharin B, Said MSM, Fadzilah FM, Haflah NHM. Periodontal Disease in Seropositive Rheumatoid Arthritis: Scoping Review of the Epidemiological Evidence. Germs. 2021; 11(2):266-286. https://doi.org/10.18683/germs.2021.1263
Chicago/Turabian StyleNik-Azis, Nik-Madihah, Nurulhuda Mohd, Badiah Baharin, Mohd Shahrir Mohamed Said, Fazalina Mohd Fadzilah, and Nor Hazla Mohamed Haflah. 2021. "Periodontal Disease in Seropositive Rheumatoid Arthritis: Scoping Review of the Epidemiological Evidence" Germs 11, no. 2: 266-286. https://doi.org/10.18683/germs.2021.1263
APA StyleNik-Azis, N.-M., Mohd, N., Baharin, B., Said, M. S. M., Fadzilah, F. M., & Haflah, N. H. M. (2021). Periodontal Disease in Seropositive Rheumatoid Arthritis: Scoping Review of the Epidemiological Evidence. Germs, 11(2), 266-286. https://doi.org/10.18683/germs.2021.1263




