Health Benefits and Pharmacological Properties of Hinokitiol
Abstract
1. Introduction
2. Source of Hinokitiol
3. Pharmacological Properties of Hinokitiol
3.1. Antibacterial Effects and Mechanisms
3.2. Antifungal Effects and Mechanisms
3.3. Antiviral Effects and Mechanisms
3.4. Antiparasitic Effects
3.5. Antioxidant Effects and Mechanisms
3.6. Antidiabetic Effect
3.7. Anti-Inflammatory Effect
3.8. Hepatoprotective Effect
3.9. Neuroprotection Effects
3.10. Antiperiodontal Bone
3.11. Thrombus Inhibition and Antityrosinase Effect
3.11.1. Thrombus Inhibition
3.11.2. Antityrosinase Effect
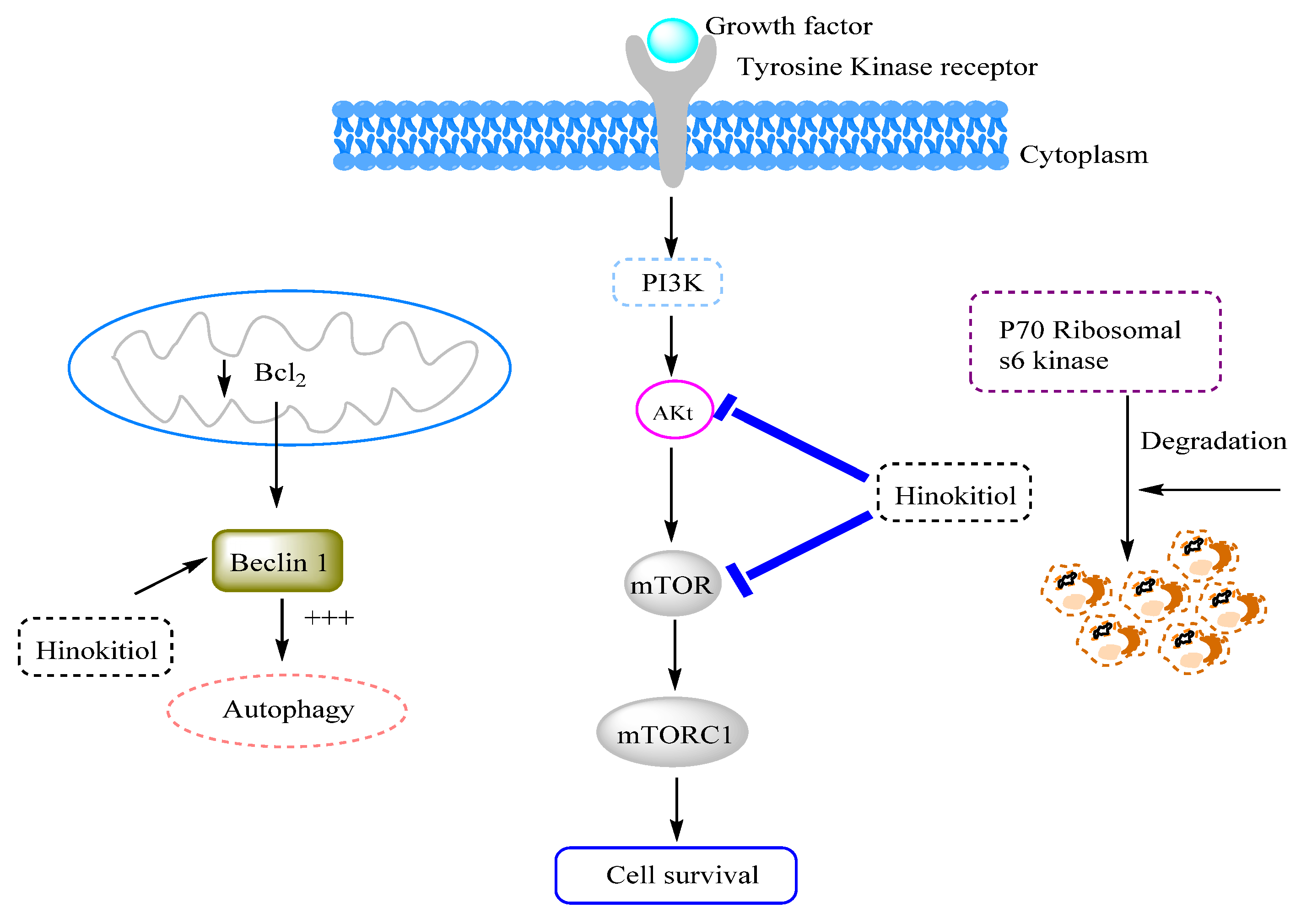
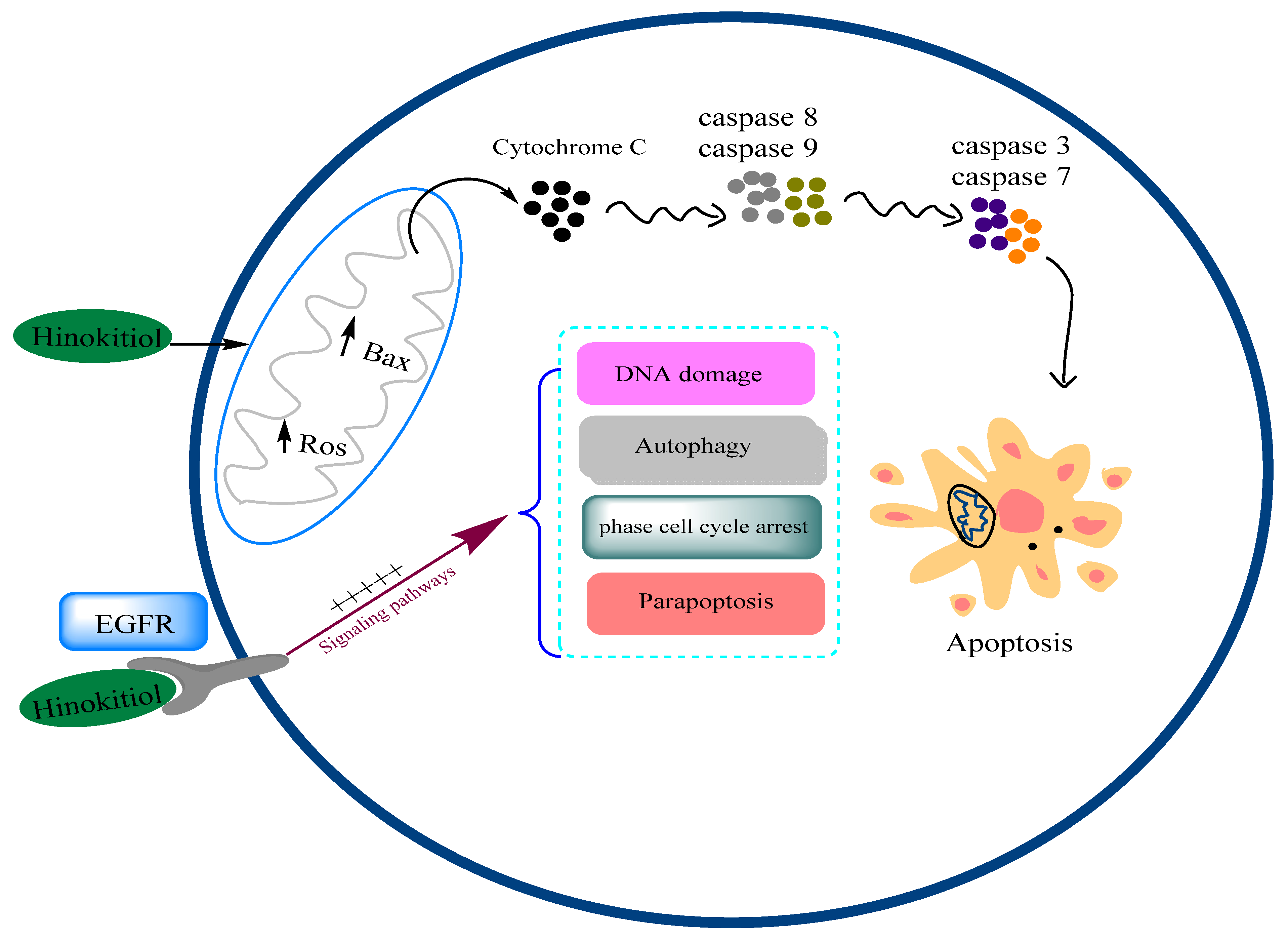
3.12. Anticancer Effects and Mechanisms
3.12.1. Effects on Breast Cancer
3.12.2. Effects on Lung Cancer
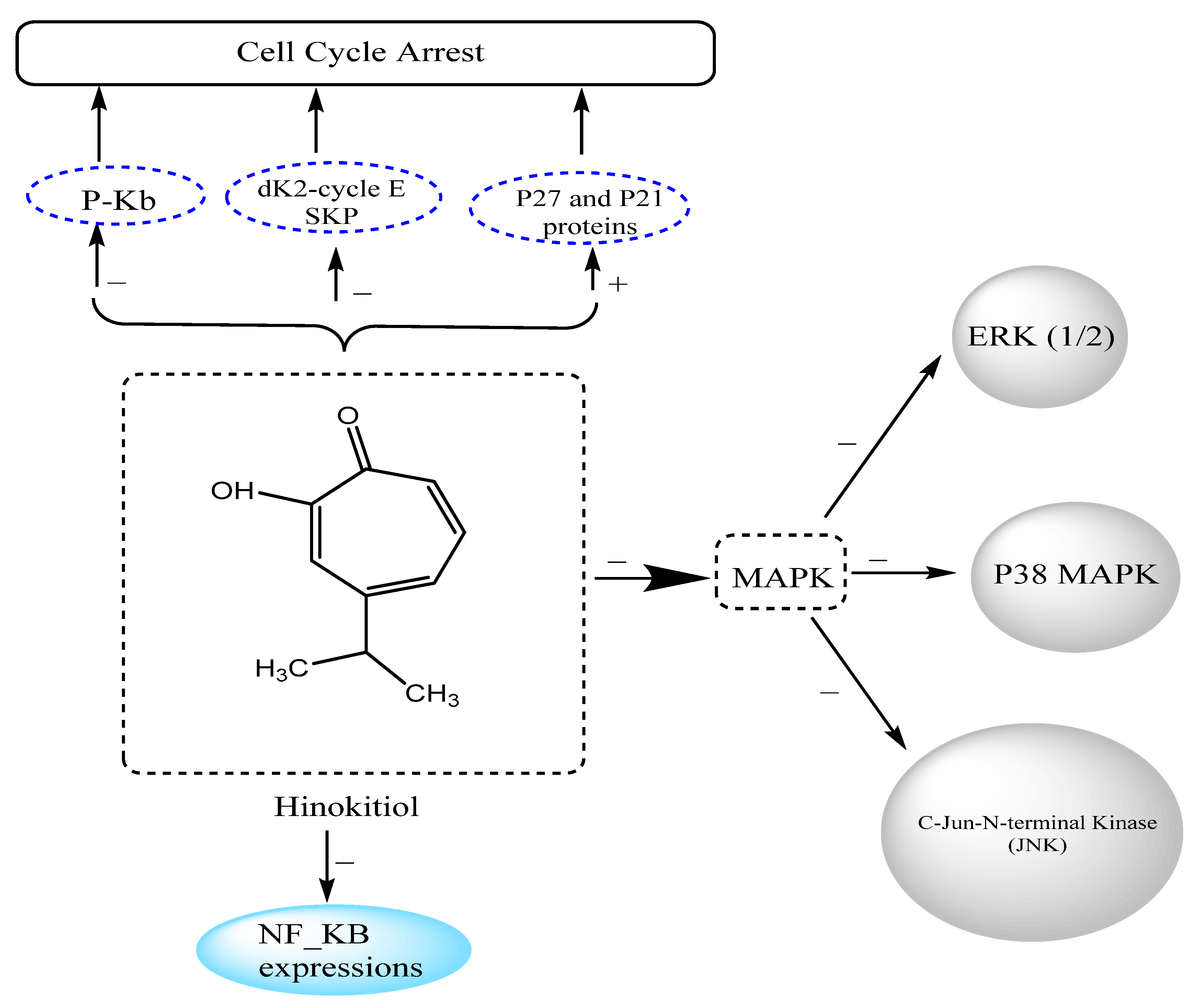
3.12.3. Effects on Melanoma Cancer
3.12.4. Other Types of Cancer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jayakumar, T.; Liu, C.-H.; Wu, G.-Y.; Lee, T.-Y.; Manubolu, M.; Hsieh, C.-Y.; Yang, C.-H.; Sheu, J.-R. Hinokitiol Inhibits Migration of A549 Lung Cancer Cells via Suppression of MMPs and Induction of Antioxidant Enzymes and Apoptosis. Int. J. Mol. Sci. 2018, 19, 939. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.-C.; Chen, R.-F.; Chen, Y.-F.; Lin, C.-H. Hinokitiol Suppresses Growth of B16 Melanoma by Activating ERK/MKP3/Proteosome Pathway to Downregulate Survivin Expression. Toxicol. Appl. Pharmacol. 2019, 366, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Lu, S.-H.; Chang, C.-C.; Thomas, P.A.; Jayakumar, T.; Sheu, J.-R. Hinokitiol, a Tropolone Derivative, Inhibits Mouse Melanoma (B16-F10) Cell Migration and in Vivo Tumor Formation. Eur. J. Pharmacol. 2015, 746, 148–157. [Google Scholar] [CrossRef]
- Nagao, Y.; Sata, M. Effect of Oral Care Gel on the Quality of Life for Oral Lichen Planus in Patients with Chronic HCV Infection. Virol. J. 2011, 8, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gilbard, J.P.; Douyon, Y.; Huson, R.B. Time-Kill Assay Results for a Linalool-Hinokitiol-Based Eyelid Cleanser for Lid Hygiene. Cornea 2010, 29, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-H.; Chang, K.-W.; Hsia, S.-M.; Yu, C.-C.; Fuh, L.-J.; Chi, T.-Y.; Shieh, T.-M. In Vitro Antimicrobial and Anticancer Potential of Hinokitiol against Oral Pathogens and Oral Cancer Cell Lines. Microbiol. Res. 2013, 168, 254–262. [Google Scholar] [CrossRef]
- Domon, H.; Hiyoshi, T.; Maekawa, T.; Yonezawa, D.; Tamura, H.; Kawabata, S.; Yanagihara, K.; Kimura, O.; Kunitomo, E.; Terao, Y. Antibacterial Activity of Hinokitiol against Both Antibiotic-Resistant and-Susceptible Pathogenic Bacteria That Predominate in the Oral Cavity and Upper Airways. Microbiol. Immunol. 2019, 63, 213–222. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Chen, T.; Xu, Y.; Tian, S. Antifungal Effects of Hinokitiol on Development of Botrytis Cinerea in Vitro and in Vivo. Postharvest Biol. Technol. 2020, 159, 111038. [Google Scholar] [CrossRef]
- Lee, J.-H.; Moon, J.-H.; Lee, Y.-J.; Park, S.-Y. SIRT1, a Class III Histone Deacetylase, Regulates LPS-Induced Inflammation in Human Keratinocytes and Mediates the Anti-Inflammatory Effects of Hinokitiol. J. Investig. Dermatol. 2017, 137, 1257–1266. [Google Scholar] [CrossRef]
- Ye, J.; Xu, Y.F.; Lou, L.X.; Jin, K.; Miao, Q.; Ye, X.; Xi, Y. Anti-Inflammatory Effects of Hinokitiol on Human Corneal Epithelial Cells: An in Vitro Study. Eye 2015, 29, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; He, J.; Ye, X.; Zhu, J.; Hu, X.; Shen, M.; Ma, Y.; Mao, Z.; Song, H.; Chen, F. β-Thujaplicin Induces Autophagic Cell Death, Apoptosis, and Cell Cycle Arrest through ROS-Mediated Akt and P38/ERK MAPK Signaling in Human Hepatocellular Carcinoma. Cell Death Dis. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.-S.; Wang, M.-J.; Jayakumar, T.; Chou, D.-S.; Ko, C.-Y.; Hsu, M.-J.; Hsieh, C.-Y. Antiproliferative Activity of Hinokitiol, a Tropolone Derivative, Is Mediated via the Inductions of p-JNK and p-PLCγ1 Signaling in PDGF-BB-Stimulated Vascular Smooth Muscle Cells. Molecules 2015, 20, 8198–8212. [Google Scholar] [CrossRef]
- Koufaki, M.; Theodorou, E.; Alexi, X.; Nikoloudaki, F.; Alexis, M.N. Synthesis of Tropolone Derivatives and Evaluation of Their in Vitro Neuroprotective Activity. Eur. J. Med. Chem. 2010, 45, 1107–1112. [Google Scholar] [CrossRef]
- Krenn, B.M.; Gaudernak, E.; Holzer, B.; Lanke, K.; Van Kuppeveld, F.J.M.; Seipelt, J. Antiviral Activity of the Zinc Ionophores Pyrithione and Hinokitiol against Picornavirus Infections. J. Virol. 2009, 83, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Fujisaki, R.; Kamei, K.; Yamamura, M.; Nishiya, H.; Inouye, S.; Takahashi, M.; Abe, S. In Vitro and in Vivo Anti-Plasmodial Activity of Essential Oils, Including Hinokitiol. Southeast Asian J. Trop. Med. Public Health 2012, 43, 270–279. [Google Scholar] [PubMed]
- Lu, W.-J.; Lin, K.-H.; Tseng, M.-F.; Yuan, K.-C.; Huang, H.-C.; Sheu, J.-R.; Chen, R.-J. New Therapeutic Strategy of Hinokitiol in Haemorrhagic Shock-Induced Liver Injury. J. Cell. Mol. Med. 2019, 23, 1723–1734. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-M.; Wang, B.-Y.; Lee, C.-H.; Lee, H.-T.; Li, J.-J.; Hong, G.-C.; Hung, Y.-C.; Chien, P.-J.; Chang, C.-Y.; Hsu, L.-S. Hinokitiol Up-Regulates MiR-494-3p to Suppress BMI1 Expression and Inhibits Self-Renewal of Breast Cancer Stem/Progenitor Cells. Oncotarget 2017, 8, 76057. [Google Scholar] [CrossRef]
- Wu, Y.-J.; Hsu, W.-J.; Wu, L.-H.; Liou, H.-P.; Pangilinan, C.R.; Tyan, Y.-C.; Lee, C.-H. Hinokitiol Reduces Tumor Metastasis by Inhibiting Heparanase via Extracellular Signal-Regulated Kinase and Protein Kinase B Pathway. Int. J. Med. Sci. 2020, 17, 403. [Google Scholar] [CrossRef]
- Hiyoshi, T.; Domon, H.; Maekawa, T.; Yonezawa, D.; Kunitomo, E.; Tabeta, K.; Terao, Y. Protective Effect of Hinokitiol against Periodontal Bone Loss in Ligature-Induced Experimental Periodontitis in Mice. Arch. Oral Biol. 2020, 112, 104679. [Google Scholar] [CrossRef]
- Lin, K.H.; Kuo, J.R.; Lu, W.J.; Chung, C.L.; Chou, D.S.; Huang, S.Y.; Lee, H.C.; Sheu, J.R. Hinokitiol Inhibits Platelet Activation Ex Vivo and Thrombus Formation in Vivo. Biochem. Pharmacol. 2013, 85, 1478–1485. [Google Scholar] [CrossRef]
- Shih, M.F.; Chen, L.Y.; Tsai, P.J.; Cherng, J.Y. In Vitro and in Vivo Therapeutics of β-Thujaplicin on LPS-Induced Inflammation in Macrophages and Septic Shock in Mice. Int. J. Immunopathol. Pharmacol. 2012, 25, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Nozoe, T. Über die farbstoffe im holzteile des “hinokl”-baumes. i. hinokitin und hinokitiol (Vorläufige Mitteilung). Bull. Chem. Soc. Jpn. 1936, 11, 295–298. [Google Scholar] [CrossRef]
- Morita, Y.; Matsumura, E.; Okabe, T.; Fukui, T.; Ohe, T.; Ishida, N.; Inamori, Y. Biological Activity of β-Dolabrin, γ-Thujaplicin, and 4-Acetyltropolone, Hinokitiol-Related Compounds. Biol. Pharm. Bull. 2004, 27, 1666–1669. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Ueda, T.; Juránek, I.; Yamamoto, S.; Katoh, T.; Node, M.; Suzuki, T. Hinokitiol, a Selective Inhibitor of the Platelet-Type Isozyme of Arachidonate 12-Lipoxygenase. Biochem. Biophys. Res. Commun. 2000, 275, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Nozoe, T.; Takase, K.; Ogata, M. β-Dolabrin: A New Natural Tropolone. Chem. Ind. 1957, 1070. [Google Scholar]
- Nozoe, T.; Seto, S.; Kikuchi, K.; Takeda, H. On the Synthesis of γ-Thujaplicin p-Isopropyltropolone. Proc. Jpn. Acad. 1951, 27, 146–148. [Google Scholar] [CrossRef]
- Suzuki, R.; Inoue, Y.; Murata, I.; Nomura, H.; Isshiki, Y.; Hashimoto, M.; Kudo, Y.; Kitagishi, H.; Kondo, S.; Kanamoto, I. Preparation, Characterization, and Study of the Antimicrobial Activity of a Hinokitiol-Copper (II)/γ-Cyclodextrin Ternary Complex. J. Mol. Struct. 2019, 1194, 19–27. [Google Scholar] [CrossRef]
- Rebia, R.A.; Tanaka, T. Natural Antibacterial Reagents (Centella, Propolis, and Hinokitiol) Loaded into Poly [(R)-3-Hydroxybutyrate-Co-(R)-3-Hydroxyhexanoate] Composite Nanofibers for Biomedical Applications. Nanomaterials 2019, 9, 1665. [Google Scholar] [CrossRef]
- Inamori, Y.; Muro, C.; Sajima, E.; Katagiri, M.; Okamoto, Y.; Tanaka, H.; Sakagami, Y.; Tsujibo, H. Biological Activity of Purpurogallin. Biosci. Biotechnol. Biochem. 1997, 61, 890–892. [Google Scholar] [CrossRef]
- Chang, K.-C.; Lin, D.-J.; Wu, Y.-R.; Chang, C.-W.; Chen, C.-H.; Ko, C.-L.; Chen, W.-C. Characterization of Genipin-Crosslinked Gelatin/Hyaluronic Acid-Based Hydrogel Membranes and Loaded with Hinokitiol: In Vitro Evaluation of Antibacterial Activity and Biocompatibility. Mater. Sci. Eng. C 2019, 105, 110074. [Google Scholar] [CrossRef]
- Chang, K.-C.; Chen, W.-C.; Chen, C.-H.; Ko, C.-L.; Liu, S.-M.; Chen, J.-C. Chemical Cross-Linking on Gelatin-Hyaluronan Loaded with Hinokitiol for the Preparation of Guided Tissue Regeneration Hydrogel Membranes with Antibacterial and Biocompatible Properties. Mater. Sci. Eng. C 2021, 119, 111576. [Google Scholar] [CrossRef]
- Huang, M.-H.; Shen, Y.-F.; Hsu, T.-T.; Huang, T.-H.; Shie, M.-Y. Physical Characteristics, Antimicrobial and Odontogenesis Potentials of Calcium Silicate Cement Containing Hinokitiol. Mater. Sci. Eng. C 2016, 65, 1–8. [Google Scholar] [CrossRef]
- Inoue, Y.; Suzuki, R.; Murata, I.; Nomura, H.; Isshiki, Y.; Kanamoto, I. Evaluation of Antibacterial Activity Expression of the Hinokitiol/Cyclodextrin Complex against Bacteria. ACS Omega 2020, 5, 27180–27187. [Google Scholar] [CrossRef] [PubMed]
- Ishii, J.; Omura, H.; Mitsui, T.; Eguchi, N.; Ueno, T.; Goto, H.; Ito, H. Effects of a Combination of Hinokitiol (β-Thujaplicin) and an Organic Acid Mixture on Ruminal Fermentation in Heifers Fed a High-Grain Diet. Anim. Sci. J. 2012, 83, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Matsumura, E.; Okabe, T.; Fukui, T.; Shibata, M.; Sugiura, M.; Ohe, T.; Tsujibo, H.; Ishida, N.; Inamori, Y. Biological Activity of α-Thujaplicin, the Isomer of Hinokitiol. Biol. Pharm. Bull. 2004, 27, 899–902. [Google Scholar] [CrossRef]
- Zeighampour, F.; Alihosseini, F.; Morshed, M.; Rahimi, A.A. Comparison of Prolonged Antibacterial Activity and Release Profile of Propolis-Incorporated PVA Nanofibrous Mat, Microfibrous Mat, and Film. J. Appl. Polym. Sci. 2018, 135, 45794. [Google Scholar] [CrossRef]
- Morita, Y.; Sakagami, Y.; Okabe, T.; Ohe, T.; Inamori, Y.; Ishida, N. The Mechanism of the Bactericidal Activity of Hinokitiol. Biocontrol Sci. 2007, 12, 101–110. [Google Scholar] [CrossRef]
- Trust, T.J.; Coombs, R.W. Antibacterial Activity of β-Thujaplicin. Can. J. Microbiol. 1973, 19, 1341–1346. [Google Scholar] [CrossRef]
- Mori, T.; Hirose, H.; Hanjavanit, C.; Hatai, K. Antifungal Activities of Plant Extracts against Some Aquatic Fungi. Biocontrol Sci. 2002, 7, 187–191. [Google Scholar] [CrossRef]
- Saniewska, A.; Saniewski, M. The Inhibitory Effect of Tropolone and Hinokitiol on the Mycelium Growth of Phoma Narcissi in Vitro. Acta Agrobot. 2007, 60, 107–112. [Google Scholar] [CrossRef][Green Version]
- Komaki, N.; Watanabe, T.; Ogasawara, A.; Sato, N.; Mikami, T.; Matsumoto, T. Antifungal Mechanism of Hinokitiol against Candida Albicans. Biol. Pharm. Bull. 2008, 31, 735–737. [Google Scholar] [CrossRef]
- Saniewska, A.; Jarecka, M. The Inhibitory Effect of Tropolone and Hinokitiol on the Growth and Development of Fusarium Oxysporum f. Sp. Tulipae. Phytopathol Pol 2008, 50, 33–41. [Google Scholar]
- Hu, J.; Shen, Y.; Pang, S.; Gao, Y.; Xiao, G.; Li, S.; Xu, Y. Application of Hinokitiol Potassium Salt for Wood Preservative. J. Environ. Sci. 2013, 25, S32–S35. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, M.; Lu, J.; Duan, X.; Chen, J.; Liu, Y.; Chang, W.; Lou, H. Hinokitiol Chelates Intracellular Iron to Retard Fungal Growth by Disturbing Mitochondrial Respiration. J. Adv. Res. 2021, in press. [Google Scholar] [CrossRef]
- Morita, Y.; Matsumura, E.; Okabe, T.; Shibata, M.; Sugiura, M.; Ohe, T.; Tsujibo, H.; Ishida, N.; Inamori, Y. Biological Activity of Tropolone. Biol. Pharm. Bull. 2003, 26, 1487–1490. [Google Scholar] [CrossRef] [PubMed]
- Uchide, N.; Ohyama, K.; Bessho, T.; Yuan, B.; Yamakawa, T. Effect of Antioxidants on Apoptosis Induced by Influenza Virus Infection: Inhibition of Viral Gene Replication and Transcription with Pyrrolidine Dithiocarbamate. Antiviral Res. 2002, 56, 207–217. [Google Scholar] [CrossRef]
- Korant, B.D.; Kauer, J.C.; Butterworth, B.E. Zinc Ions Inhibit Replication of Rhinoviruses. Nature 1974, 248, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Suara, R.O.; Crowe, J.E., Jr. Effect of Zinc Salts on Respiratory Syncytial Virus Replication. Antimicrob. Agents Chemother. 2004, 48, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Beaufay, C.; Bero, J.; Quetin-Leclercq, J. Antimalarial terpenic compounds isolated from plants used in traditional medicine (2010–July 2016). In Natural Antimicrobial Agents; Springer: Berlin/Heidelberg, Germany, 2018; pp. 247–268. [Google Scholar]
- Barnard, J.F.; Vander Jagt, D.L.; Honek, J.F. Small Molecule Probes of Glyoxalase I and Glyoxalase II. Biochim. Biophys. Acta BBA-Protein Struct. Mol. Enzymol. 1994, 1208, 127–135. [Google Scholar] [CrossRef]
- Ishiyama, A.; Iwatsuki, M.; Yamamoto, T.; Miura, H.; Ōmura, S.; Otoguro, K. Antimalarial Tropones and Their Plasmodium Falciparum Glyoxalase I (Pf GLOI) Inhibitory Activity. J. Antibiot. 2014, 67, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Chisty, M.M.; Nargis, M.; Inaba, T.; Ishita, K.; Osanai, A.; Kamiya, H. Transmission Electron Microscopy of Schistosoma Mansoni Cercariae Treated with Hinokitiol (β-Thujaplicin), a Compound for Potential Skin Application against Cercarial Penetration. Tohoku J. Exp. Med. 2004, 202, 63–67. [Google Scholar] [CrossRef][Green Version]
- Rivas, F.; Medeiros, A.; Arce, E.R.; Comini, M.; Ribeiro, C.M.; Pavan, F.R.; Gambino, D. New Heterobimetallic Ferrocenyl Derivatives: Evaluation of Their Potential as Prospective Agents against Trypanosomatid Parasites and Mycobacterium Tuberculosis. J. Inorg. Biochem. 2018, 187, 73–84. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, L.; Xiong, W.; Song, M.; Du, H.; Wang, Y.; Ming, K.; Wu, Y.; Wang, D.; Hu, Y. Anti-Duck Virus Hepatitis Mechanisms of Bush Sophora Root Polysaccharide and Its Sulfate Verified by Intervention Experiments. Virus Res. 2015, 204, 58–67. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Wang, F.; Yang, X.; Yao, F.; Ming, K.; Yuan, W.; Zeng, L.; Liu, J. Antiviral Effect of Baicalin Phospholipid Complex against Duck Hepatitis A Virus Type 1. Poult. Sci. 2018, 97, 2722–2732. [Google Scholar] [CrossRef]
- Okumura, S.; Hoshino, M.; Joshita, K.; Nishinomiya, T.; Murata, M. Hinokitiol Inhibits Polyphenol Oxidase and Enzymatic Browning. Food Sci. Technol. Res. 2011, 17, 251–256. [Google Scholar] [CrossRef]
- Yamane, M.; Adachi, Y.; Yoshikawa, Y.; Sakurai, H. A New Anti-Diabetic Zn (II)–Hinokitiol (β-Thujaplicin) Complex with Zn (O4) Coordination Mode. Chem. Lett. 2005, 34, 1694–1695. [Google Scholar] [CrossRef]
- Naito, Y.; Yoshikawa, Y.; Yasui, H. Cellular Mechanism of Zinc–Hinokitiol Complexes in Diabetes Mellitus. Bull. Chem. Soc. Jpn. 2011, 84, 298–305. [Google Scholar] [CrossRef]
- Byeon, S.E.; Lee, Y.G.; Kim, J.-C.; Han, J.G.; Lee, H.Y.; Cho, J.Y. Hinokitiol, a Natural Tropolone Derivative, Inhibits TNF-α Production in LPS-Activated Macrophages via Suppression of NF-ΚB. Planta Med. 2008, 74, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, X.; Yang, K.; Chen, W.; Jiang, L.; Bao, J.; Wu, L.; Xiong, Y. Hinokitiol Reduces Matrix Metalloproteinase Expression by Inhibiting Wnt/β-Catenin Signaling in Vitro and in Vivo. Int. Immunopharmacol. 2014, 23, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-H.; Lin, D.-J.; Chang, K.-W.; Hsia, S.-M.; Ko, S.-Y.; Lee, S.-Y.; Hsue, S.-S.; Wang, T.-H.; Chen, Y.-L.; Shieh, T.-M. Evaluation Physical Characteristics and Comparison Antimicrobial and Anti-Inflammation Potentials of Dental Root Canal Sealers Containing Hinokitiol in Vitro. PLoS ONE 2014, 9, e94941. [Google Scholar]
- Ford, J.; Jiang, M.; Milner, J.O. Cancer-Specific Functions of SIRT1 Enable Human Epithelial Cancer Cell Growth and Survival. Cancer Res. 2005, 65, 10457–10463. [Google Scholar] [CrossRef]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.-L. Small Molecule Activators of Sirtuins Extend Saccharomyces Cerevisiae Lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Jayakumar, T.; Hsu, W.-H.; Yen, T.-L.; Luo, J.-Y.; Kuo, Y.-C.; Fong, T.-H.; Sheu, J.-R. Hinokitiol, a Natural Tropolone Derivative, Offers Neuroprotection from Thromboembolic Stroke in Vivo. Evid. Based Complement. Alternat. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Varier, K.M.; Sumathi, T. Hinokitiol Offers Neuroprotection against 6-OHDA-Induced Toxicity in SH-SY5Y Neuroblastoma Cells by Downregulating MRNA Expression of MAO/α-Synuclein/LRRK2/PARK7/PINK1/PTEN Genes. Neurotox. Res. 2019, 35, 945–954. [Google Scholar] [CrossRef]
- Moon, J.-H.; Lee, J.-H.; Lee, Y.-J.; Park, S.-Y. Hinokitiol Protects Primary Neuron Cells against Prion Peptide-Induced Toxicity via Autophagy Flux Regulated by Hypoxia Inducing Factor-1. Oncotarget 2016, 7, 29944. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sajadi Hezaveh, Z.; Shidfar, F. Hydrophilic Phytochelators in Iron Overload Condition. J. Nutr. Food Secur. 2019, 4, 142–151. [Google Scholar]
- Wu, Y.-H.; Taya, Y.; Kuraji, R.; Ito, H.; Soeno, Y.; Numabe, Y. Dynamic Microstructural Changes in Alveolar Bone in Ligature-Induced Experimental Periodontitis. Odontology 2019, 108, 339–349. [Google Scholar] [CrossRef]
- Van Dyke, T.E.; Serhan, C.N. Resolution of Inflammation: A New Paradigm for the Pathogenesis of Periodontal Diseases. J. Dent. Res. 2003, 82, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Liu, H.Q. Improving the hemocompatibility of stents. In Hemocompatibility of Biomaterials for Clinical Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 379–394. [Google Scholar]
- Kalathottukaren, M.T.; Kizhakkedathu, J.N. Mechanisms of blood coagulation in response to biomaterials: Extrinsic factors. In Hemocompatibility of Biomaterials for Clinical Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 29–49. [Google Scholar]
- Jayakumar, T.; Yang, C.-H.; Geraldine, P.; Yen, T.-L.; Sheu, J.-R. The Pharmacodynamics of Antiplatelet Compounds in Thrombosis Treatment. Expert Opin. Drug Metab. Toxicol. 2016, 12, 615–632. [Google Scholar] [CrossRef]
- Ito, S.; Nakanishi, Y.; Valenzuela, R.K.; Brilliant, M.H.; Kolbe, L.; Wakamatsu, K. Usefulness of Alkaline Hydrogen Peroxide Oxidation to Analyze Eumelanin and Pheomelanin in Various Tissue Samples: Application to Chemical Analysis of Human Hair Melanins. Pigment Cell Melanoma Res. 2011, 24, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Yoshimori, A.; Oyama, T.; Takahashi, S.; Abe, H.; Kamiya, T.; Abe, T.; Tanuma, S. Structure–Activity Relationships of the Thujaplicins for Inhibition of Human Tyrosinase. Bioorg. Med. Chem. 2014, 22, 6193–6200. [Google Scholar] [CrossRef]
- Tsao, Y.-T.; Huang, Y.-F.; Kuo, C.-Y.; Lin, Y.-C.; Chiang, W.-C.; Wang, W.-K.; Hsu, C.-W.; Lee, C.-H. Hinokitiol Inhibits Melanogenesis via AKT/MTOR Signaling in B16F10 Mouse Melanoma Cells. Int. J. Mol. Sci. 2016, 17, 248. [Google Scholar] [CrossRef] [PubMed]
- Casañola-Martín, G.M.; Marrero-Ponce, Y.; Khan, M.T.H.; Ather, A.; Sultan, S.; Torrens, F.; Rotondo, R. TOMOCOMD-CARDD Descriptors-Based Virtual Screening of Tyrosinase Inhibitors: Evaluation of Different Classification Model Combinations Using Bond-Based Linear Indices. Bioorg. Med. Chem. 2007, 15, 1483–1503. [Google Scholar] [CrossRef]
- Sakuma, K.; Ogawa, M.; Sugibayashi, K.; Yamada, K.; Yamamoto, K. Relationship between Tyrosinase Inhibitory Action and Oxidation-Reduction Potential of Cosmetic Whitening Ingredients and Phenol Derivatives. Arch. Pharm. Res. 1999, 22, 335–339. [Google Scholar] [CrossRef]
- Wang, W.-K.; Lin, S.-T.; Chang, W.-W.; Liu, L.-W.; Li, T.Y.-T.; Kuo, C.-Y.; Hsieh, J.-L.; Lee, C.-H. Hinokitiol Induces Autophagy in Murine Breast and Colorectal Cancer Cells. Environ. Toxicol. 2016, 31, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Tu, D.-G.; Yu, Y.; Lee, C.-H.; Kuo, Y.-L.; Lu, Y.-C.; Tu, C.-W.; Chang, W.-W. Hinokitiol Inhibits Vasculogenic Mimicry Activity of Breast Cancer Stem/Progenitor Cells through Proteasome-Mediated Degradation of Epidermal Growth Factor Receptor. Oncol. Lett. 2016, 11, 2934–2940. [Google Scholar] [CrossRef]
- Shen, Y.-F.; Ho, C.-C.; Shie, M.-Y.; Wang, K.; Fang, H.-Y. Hinokitiol-Loaded Mesoporous Calcium Silicate Nanoparticles Induce Apoptotic Cell Death through Regulation of the Function of MDR1 in Lung Adenocarcinoma Cells. Materials 2016, 9, 306. [Google Scholar] [CrossRef]
- Li, L.-H.; Wu, P.; Lee, J.-Y.; Li, P.-R.; Hsieh, W.-Y.; Ho, C.-C.; Ho, C.-L.; Chen, W.-J.; Wang, C.-C.; Yen, M.-Y. Hinokitiol Induces DNA Damage and Autophagy Followed by Cell Cycle Arrest and Senescence in Gefitinib-Resistant Lung Adenocarcinoma Cells. PLoS ONE 2014, 9, e104203. [Google Scholar]
- Chen, X.; Zhang, X.; Chen, J.; Yang, Q.; Yang, L.; Xu, D.; Zhang, P.; Wang, X.; Liu, J. Hinokitiol Copper Complex Inhibits Proteasomal Deubiquitination and Induces Paraptosis-like Cell Death in Human Cancer Cells. Eur. J. Pharmacol. 2017, 815, 147–155. [Google Scholar] [CrossRef]
- Khalili, M.; Radosevich, J.A. Paraptosis. In Apoptosis and Beyond: Many Ways Cells Die; Wiley: Hoboken, NJ, USA, 2018; pp. 343–366. [Google Scholar]
- Gray-Schopfer, V.C.; Karasarides, M.; Hayward, R.; Marais, R. Tumor Necrosis Factor-α Blocks Apoptosis in Melanoma Cells When BRAF Signaling Is Inhibited. Cancer Res. 2007, 67, 122–129. [Google Scholar] [CrossRef]
- Liu, S.; Yamauchi, H. P27-Associated G1 Arrest Induced by Hinokitiol in Human Malignant Melanoma Cells Is Mediated via down-Regulation of PRb, Skp2 Ubiquitin Ligase, and Impairment of Cdk2 Function. Cancer Lett. 2009, 286, 240–249. [Google Scholar] [CrossRef]
- Huang, C.-H.; Jayakumar, T.; Chang, C.-C.; Fong, T.-H.; Lu, S.-H.; Thomas, P.A.; Choy, C.-S.; Sheu, J.-R. Hinokitiol Exerts Anticancer Activity through Downregulation of MMPs 9/2 and Enhancement of Catalase and SOD Enzymes: In Vivo Augmentation of Lung Histoarchitecture. Molecules 2015, 20, 17720–17734. [Google Scholar] [CrossRef]
- Vlodavsky, I.; Ilan, N.; Naggi, A.; Casu, B. Heparanase: Structure, Biological Functions, and Inhibition by Heparin-Derived Mimetics of Heparan Sulfate. Curr. Pharm. Des. 2007, 13, 2057–2073. [Google Scholar] [CrossRef]
- Liu, S.; Yamauchi, H. Hinokitiol, a Metal Chelator Derived from Natural Plants, Suppresses Cell Growth and Disrupts Androgen Receptor Signaling in Prostate Carcinoma Cell Lines. Biochem. Biophys. Res. Commun. 2006, 351, 26–32. [Google Scholar] [CrossRef]
- Seo, J.S.; Choi, Y.H.; Moon, J.W.; Kim, H.S.; Park, S.-H. Hinokitiol Induces DNA Demethylation via DNMT1 and UHRF1 Inhibition in Colon Cancer Cells. BMC Cell Biol. 2017, 18, 1–11. [Google Scholar] [CrossRef]
- Matsumura, E.; Morita, Y.; Date, T.; Tsujibo, H.; Yasuda, M.; Okabe, T.; Ishida, N.; Inamori, Y. Cytotoxicity of the Hinokitiol-Related Compounds, γ-Thujaplicin and β-Dolabrin. Biol. Pharm. Bull. 2001, 24, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.B.; Jun, J.H. Can Hinokitiol Kill Cancer Cells? Alternative Therapeutic Anticancer Agent via Autophagy and Apoptosis. Korean J. Clin. Lab. Sci. 2019, 51, 221–234. [Google Scholar] [CrossRef]
- Shih, Y.-H.; Chang, K.-W.; Yu, C.-C.; Kao, M.-C.; Chen, M.Y.; Wang, T.-H.; Chi, T.-Y.; Chen, Y.-L.; Shieh, T.-M. Hinokitiol Suppressed Pan-Histone Expression and Cell Growth in Oral Squamous Cell Carcinoma Cells. J. Funct. Foods 2015, 15, 452–463. [Google Scholar] [CrossRef]
- Wang, C.-C.; Chen, B.-K.; Chen, P.-H.; Chen, L.-C. Hinokitiol Induces Cell Death and Inhibits Epidermal Growth Factor-Induced Cell Migration and Signaling Pathways in Human Cervical Adenocarcinoma. Taiwan. J. Obstet. Gynecol. 2020, 59, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.-C.; Liao, Y.-W.; Chen, P.-N.; Lu, K.-H.; Yu, C.-C.; Hsieh, P.-L. Hinokitiol Suppresses Cancer Stemness and Oncogenicity in Glioma Stem Cells by Nrf2 Regulation. Cancer Chemother. Pharmacol. 2017, 80, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Ido, Y.; Muto, N.; Inada, A.; Kohroki, J.; Mano, M.; Odani, T.; Itoh, N.; Yamamoto, K.; Tanaka, K. Induction of Apoptosis by Hinokitiol, a Potent Iron Chelator, in Teratocarcinoma F9 Cells Is Mediated through the Activation of Caspase-3. Cell Prolif. 1999, 32, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-Y.; Liu, C.-P.; Shiao, W.-C.; Jayakumar, T.; Li, Y.-S.; Chang, N.-C.; Huang, S.-Y.; Hsieh, C.-Y. Inhibitory Effect of PDGF-BB and Serum-Stimulated Responses in Vascular Smooth Muscle Cell Proliferation by Hinokitiol via up-Regulation of P21 and P53. Arch. Med. Sci. AMS 2018, 14, 579. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-B.; Seo, E.-J.; Lee, J.-Y.; Jun, J.H. Synergistic Anticancer Effects of Curcumin and Hinokitiol on Gefitinib Resistant Non-Small Cell Lung Cancer Cells. Nat. Prod. Commun. 2018, 13, 1934578X1801301223. [Google Scholar] [CrossRef]

| Cancer Type | Cell Lines | Key Results | References |
|---|---|---|---|
| Breast cancer | Breast and colorectal cancer cells | Induced autophagy in cancer cell lines by downregulating phosphoprotein kinase B (PAKT)/p-mTOR signaling pathway | [94] |
| Breast cancer | MDA-MB-231 human breast cancer cells | IC50 = 46.6 ± 7.5 µM | [80] |
| Breast cancer | AS-B244 human breast cancer cells | IC50 = 33.6 ± 8.8 µM | [80] |
| Breast cancer | Breast (4T1) cancer cells | Inhibited cell growth, migration, and metastasis through inhibiting heparanase expression | [18] |
| Breast cancer | Breast cancer stem/progenitor cells (BCSCs) | Activated miR-494-3p, which decreased BMI1 expression and suppressed self-renewal of BCSCs | [17] |
| Glioma cancer | U87MG glioma cells | IC50 = 316.5 ± 35.5 µM | [95] |
| Glioma cancer | T98G glioma cells | IC50 = 152.5 ± 25.3 µM | [95] |
| Teratoma cancer | Teratocarcinoma F9 cells | Triggered cell apoptosis by activating caspase-3 | [96] |
| Oral cancer | HSC3, SAS, and SCC4 oral squamous carcinoma cell lines | Resulted in a growth rate of between 15% and 45% | [6] |
| Oral cancer | Vascular smooth muscle cell (VSMC) | Inhibited (1–10 µM) DNA synthesis and proliferation of VSMC via platelet-derived growth factor-BB (PDGF-BB)-induced phosphorylation of ERK1/2, Akt, PI3K or JAK2 and upregulating of p21 and p53 proteins | [97] |
| Oral cancer | Oral squamous carcinoma cells | Promoted cell cycle arrest in G1 or G1/S phase and caused cell apoptosis Significantly inhibited pan-histone mRNA expression (at 6.25–12.5 µM) | [93] |
| Lung cancer | HCC827 non-small cell lung cancer cell lines | IC50 = 75.0 ± 4.2 μM | [98] |
| Lung cancer | HCC827-GRKU non-small cell lung cancer cell lines | IC50 = 74.0 ± 2.1 μM | [98] |
| Lung cancer | HCC827 non-small cell lung cancer (NSCLC) cells | IC50 = 37.63 ± 5.41 μM Induced apoptosis and autophagy in NCLSC | [92] |
| Lung cancer | Human A549 and K562 cell lines | Attenuated the 19S proteasomal DUBs, triggering paraptosis-like cell death | [83] |
| Lung cancer | H1975 lung adenocarcinoma cells | IC50 = 1.57 mM | [82] |
| Lung cancer | PC9-IR lung adenocarcinoma cells | IC50 = 1.87 mM | [82] |
| Lung cancer | A549 human lung adenocarcinoma cells | Markedly inhibited cell migration 1–5 μM Induced a significant change in the expression of p53 and Bax proteins, accompanied by downregulation of caspase-9 and -3 and metalloproteinases (MMPs) -2/-9 | [1] |
| Lung cancer | A549 lung adenocarcinoma Cells | Induced apoptosis by enhancing the production of ROS and the expression levels of proteins caspase-3/-9 | [81] |
| Melanoma cancer | FEM human melanoma cells | Inhibited cell growth and DNA synthesis by blocking G1–S-phase transition accompanied by increased levels of p27 and p21 protein and decreased expression of Cdk2, cyclin E, and phosphorylated Rb | [86] |
| Melanoma cancer | B16-F10 melanoma cells | Downregulated the expression levels and activity of MMPs-2 and -9 in B16-F10 melanoma cell lines and inhibited ROS generation by increasing the activity antioxidant enzymes CAT and SOD | [87] |
| Melanoma cancer | B16-F10 melanoma cells | Significantly suppressed colony formation and tumor viability in a time and concentration-dependent manner Reduced survivin protein expressions and increased survivin ubiquitination Caused ERK phosphorylation accompanied by an increase in the expression levels of tumor suppressor MKP-3 | [2] |
| Melanoma cancer | B16-F10 melanoma cells | Autophagy regulation of melanoma cell hyperpigmentation by inhibition of microphthalmia-associated transcription factor (MITF) and tyrosinase | [76] |
| Melanoma cancer | B16-F10 mouse melanoma | Antimetastatic effects and inhibition of cell viability on cancer cells Downregulated heparanase expression by targeting extracellular signal-regulated kinase (ERK) and protein kinase B (AKT) signaling pathways | [18] |
| Melanoma cancer | B16-F10 melanoma cells | Suppressed cell migration and metastasis through downregulation of matrix metalloproteinase-1 (MMP-1) expression and inhibition of the phosphorylation of mitogen-activated protein kinase (MAPK) signaling factors (ERK 1/2, p38 MAPK, and c-Jun N-terminal kinases (JNK)) | [3] |
| Vascular smooth muscle tumors | Vascular smooth muscle (VSM) cells | Inhibited platelet-derived growth factor PDGF-BB- stimulated proliferation of VSM cells, attenuating JNK1/2 phosphorylation and phospholipase C (PLC)-γ1 Modulated the levels of proliferating cell nuclear antigen (PCNA), promoting thus, cell cycle arrest in the G0/G1 phase | [12] |
| Cervical cancer | HeLa cells | IC50 = 38.58 ± 6.72 μM | [92] |
| Cervical cancer | HeLa cervical carcinoma cells | Inhibited the growth of tumor Induced cell cycle arrest in the G1 phase Upregulated levels of p53 and p21, with a concomitant reduction in expression of cell cycle regulatory proteins (cyclin D and cyclin E) Significantly increased the expression levels of autophagy regulators, including beclin 1 and microtubule-associated protein 1 light chain 3 (LC3-II), in a dose-dependent manner | [8] |
| Gastric cancer | KATO-III human stomach cancer | Inhibited tumor growth (54%) at 0.32 mg/mL | [91] |
| Ehrlich ascites cancer | Ehrlich–Lettre ascites cacinoma | Inhibited tumor growth (58%) at 0.32 mg/mL | [91] |
| Colon cancer | HCT-116 colon cancer cells | Caused DNA demethylation by suppressing DNMT1 and UHRF1 expression in colon cancer cell lines | [90] |
| Prostate cancer | Prostate carcinoma cells | Inhibited the cell growth Induced the disruption of androgen receptor (AR) signaling in prostate carcinoma cells | [89] |
| Liver cancer | Human hepatocellularcarcinoma | Triggered cell autophagy mediated by ROS- induced downregulation of Akt-mTOR Induced cell apoptosis by targeting mitochondrial-dependent pathway | [11] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Hachlafi, N.; Lakhdar, F.; Khouchlaa, A.; Bakrim, S.; El Omari, N.; Balahbib, A.; Shariati, M.A.; Zengin, G.; Fikri-Benbrahim, K.; Orlando, G.; et al. Health Benefits and Pharmacological Properties of Hinokitiol. Processes 2021, 9, 1680. https://doi.org/10.3390/pr9091680
El Hachlafi N, Lakhdar F, Khouchlaa A, Bakrim S, El Omari N, Balahbib A, Shariati MA, Zengin G, Fikri-Benbrahim K, Orlando G, et al. Health Benefits and Pharmacological Properties of Hinokitiol. Processes. 2021; 9(9):1680. https://doi.org/10.3390/pr9091680
Chicago/Turabian StyleEl Hachlafi, Naoufal, Fatima Lakhdar, Aya Khouchlaa, Saad Bakrim, Nasreddine El Omari, Abdelaali Balahbib, Mohammad Ali Shariati, Gokhan Zengin, Kawtar Fikri-Benbrahim, Giustino Orlando, and et al. 2021. "Health Benefits and Pharmacological Properties of Hinokitiol" Processes 9, no. 9: 1680. https://doi.org/10.3390/pr9091680
APA StyleEl Hachlafi, N., Lakhdar, F., Khouchlaa, A., Bakrim, S., El Omari, N., Balahbib, A., Shariati, M. A., Zengin, G., Fikri-Benbrahim, K., Orlando, G., Ferrante, C., Meninghi, L., & Bouyahya, A. (2021). Health Benefits and Pharmacological Properties of Hinokitiol. Processes, 9(9), 1680. https://doi.org/10.3390/pr9091680












