Preparation of a Novel CO2-Responsive Polymer/Multiwall Carbon Nanotube Composite
Abstract
1. Introduction
2. Materials and Methods
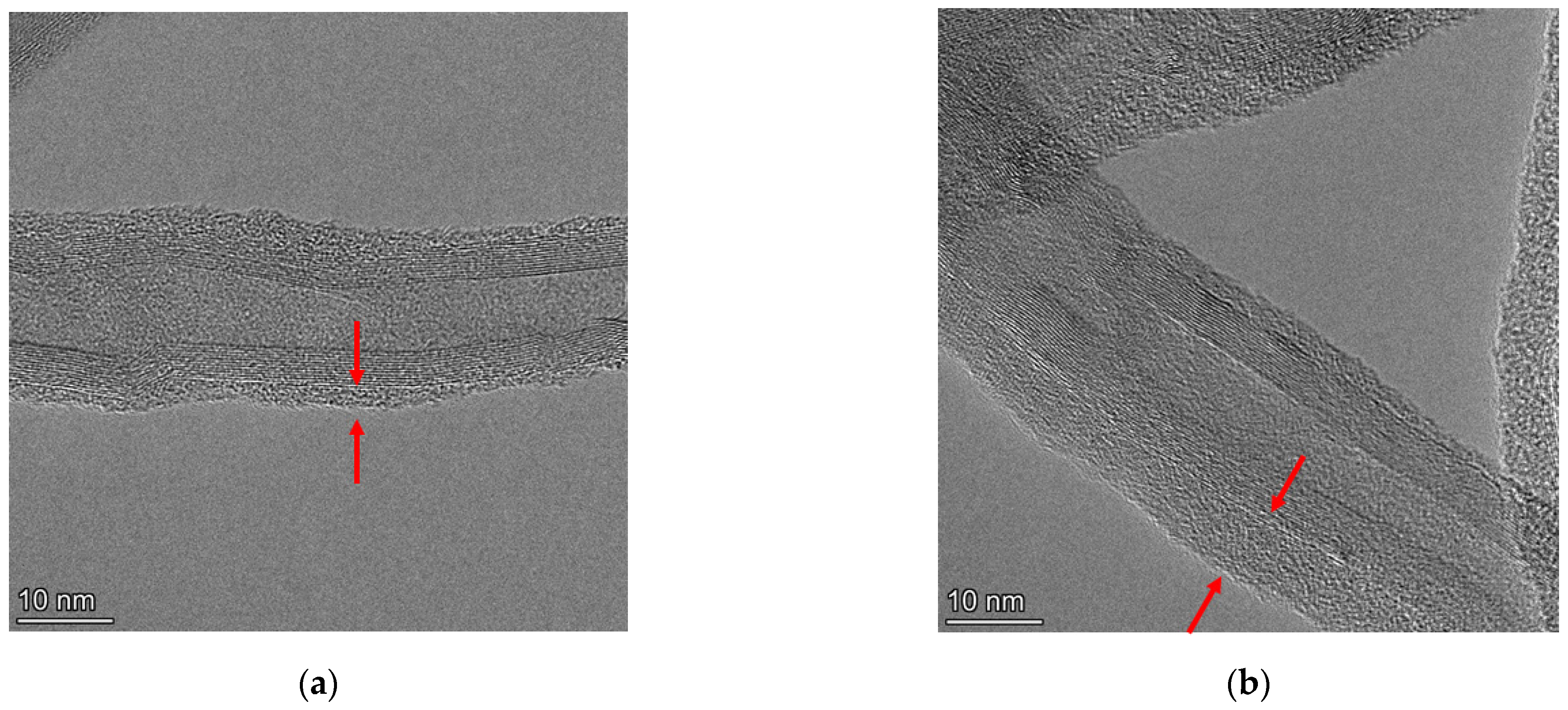
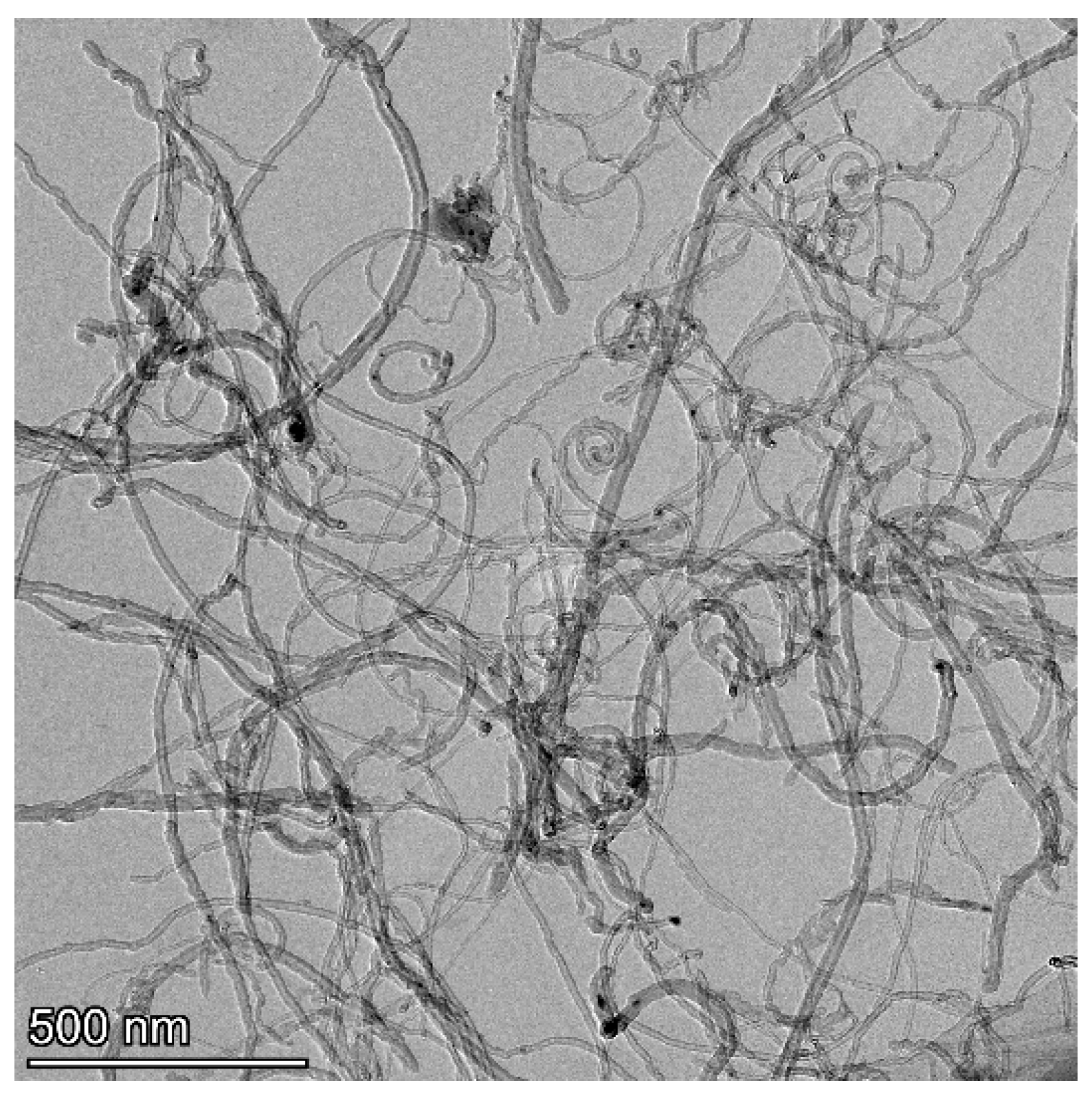
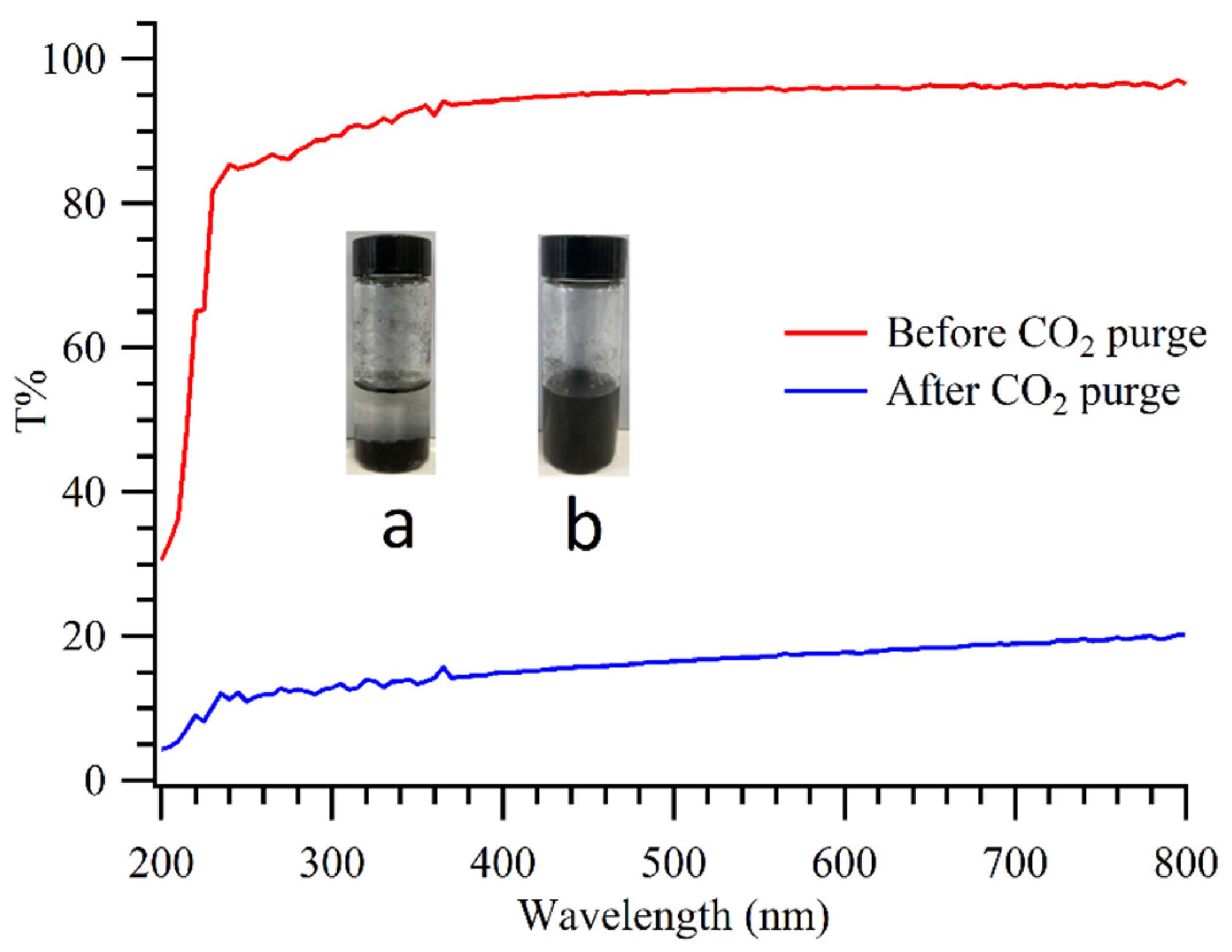
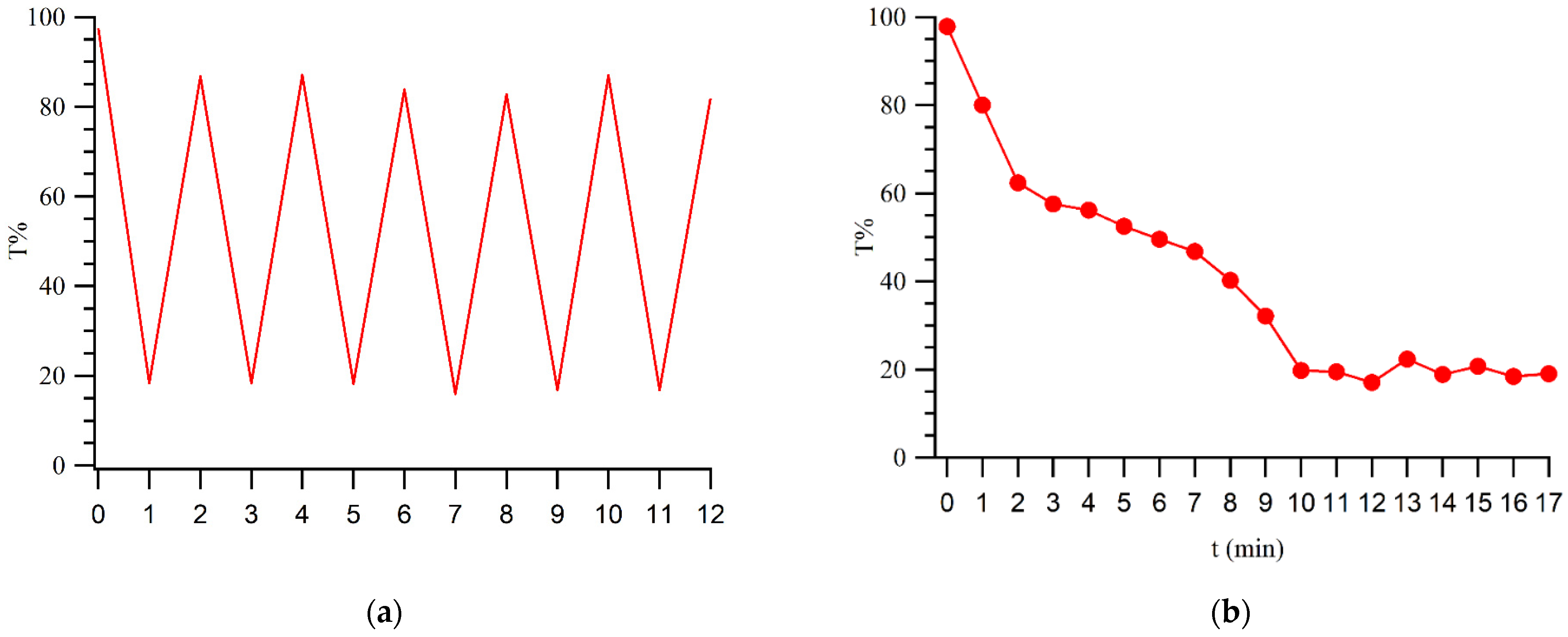
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jessop, P.G.; Mercer, S.M.; Heldebrant, D.J. CO2-triggered switchable solvents, surfactants, and other materials. Energy Environ. Sci. 2012, 5, 7240–7253. [Google Scholar] [CrossRef]
- Darabi, A.; Jessop, P.G.; Cunningham, M.F. CO2-responsive polymeric materials: Synthesis, self-assembly, and functional applications. Chem. Soc. Rev. 2016, 45, 4391–4436. [Google Scholar] [CrossRef] [PubMed]
- Phan, L.; Chiu, D.; Heldebrant, D.J.; Huttenhower, H.; John, E.; Li, X.; Pollet, P.; Wang, R.; Eckert, C.A.; Liotta, C.L.; et al. Switchable solvents consisting of amidine/alcohol or guanidine/alcohol mixtures. Ind. Eng. Chem. Res. 2008, 47, 539–545. [Google Scholar] [CrossRef]
- Phan, L.; Andreatta, J.R.; Horvey, L.K.; Edie, C.F.; Luco, A.L.; Mirchandani, A.; Darensbourg, D.J.; Jessop, P.G. Switchable-polarity solvents prepared with a single liquid component. J. Org. Chem. 2008, 73, 127–132. [Google Scholar] [CrossRef]
- Pollet, P.; Eckertabc, C.A.; Liotta, C.L. Switchable solvents. Chem. Sci. 2011, 2, 609–614. [Google Scholar] [CrossRef]
- Phan, L.; Brown, H.; White, J.; Hodgson, A.; Jessop, P.G. Soybean oil extraction and separation using switchable or expanded solvents. Green Chem. 2009, 11, 53–59. [Google Scholar] [CrossRef]
- Samorì, C.; Torri, C.; Samorì, G.; Fabbri, D.; Galletti, P.; Guerrini, F.; Pistocchi, R.; Tagliavini, E. Extraction of hydrocarbons from microalga Botryococcus braunii with switchable solvents. Bioresour. Technol. 2010, 101, 3274–3279. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, Y.; Barashkov, N.N.; Irgibaeva, I.S.; Lam, J.W.Y.; Hu, R.; Birimzhanova, D.; Yu, Y.; Tang, B.Z. Fluorescent chemosensor for detection and quantitation of carbon dioxide gas. J. Am. Chem. Soc. 2010, 132, 13951–13953. [Google Scholar] [CrossRef]
- Heldebrant, D.J.; Yonker, C.R.; Jessop, P.G.; Phan, L. Reversible Uptake of COS, CS2, and SO2: Ionic Liquids with O-Alkylxanthate, O-Alkylthiocarbonyl, and O-Alkylsulfite Anions. Chem. A Eur. J. 2009, 15, 7619–7627. [Google Scholar] [CrossRef]
- Kim, M.; Park, J.W. Reversible, solid state capture of carbon dioxide by hydroxylated amidines. Chem. Commun. 2010, 46, 2507–2509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kutnyakov, I.; Koech, P.K.; Zwoster, A.; Howard, C.; Zheng, F.; Freeman, C.J.; Heldebrant, D.J. CO2-binding-organic-liquids-enhanced CO2 capture using polarity-swing-assisted regeneration. Energy Procedia 2013, 37, 285–291. [Google Scholar] [CrossRef]
- Blasucci, V.; Hart, R.; Mestre, V.L.; Hahne, D.J.; Burlager, M.; Huttenhower, H.; Thio, B.J.R.; Pollet, P.; Liotta, C.L.; Eckert, C.A. Single component, reversible ionic liquids for energy applications. Fuel 2010, 89, 1315–1319. [Google Scholar] [CrossRef]
- Wang, C.; Mahurin, S.M.; Luo, H.; Baker, G.A.; Li, H.; Dai, S. Reversible and robust CO2 capture by equimolar task-specific ionic liquid–Superbase mixtures. Green Chem. 2010, 12, 870–887. [Google Scholar] [CrossRef]
- Wang, C.; Luo, X.; Luo, H.; Jiang, D.; Li, H.; Dai, S. Tuning the Basicity of Ionic Liquids for Equimolar CO2 Capture. Angew. Chem. 2011, 123, 5020–5024. [Google Scholar] [CrossRef]
- Jessop, P.G.; Kozycz, L.; Rahami, Z.G.; Schoenmakers, D.; Boyd, A.R.; Wechsler, D.; Holland, A.M. Tertiary amine solvents having switchable hydrophilicity. Green Chem. 2011, 13, 619–623. [Google Scholar] [CrossRef]
- Kohno, Y.; Arai, H.; Ohno, H. Dual stimuli-responsive phase transition of an ionic liquid/water mixture. Chem. Commun. 2011, 47, 4772–4774. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jessop, P.G.; Cunningham, M.; Eckert, C.A.; Liotta, C.L. Switchable surfactants. Science 2006, 313, 958–960. [Google Scholar] [CrossRef]
- Darwish, T.A.; Evans, R.A.; James, M.; Hanley, T.L. Spiropyran-amidine: A molecular canary for visual detection of carbon dioxide gas. Chem. A Eur. J. 2011, 17, 11399–11404. [Google Scholar] [CrossRef]
- Desset, S.L.; Cole-Hamilton, D.J. Carbon dioxide induced phase switching for homogeneous-catalyst recycling. Angew. Chem. Int. Ed. 2009, 48, 1472–1474. [Google Scholar] [CrossRef]
- Moore, N.A.L. Method for Shear Coagulation of Latex Resins. U.S. Patent 4,623,678, 18 November 1986. [Google Scholar]
- Smith, A.E.; Xu, X.; Abell, T.U.; Kirkland, S.E.; Hensarling, R.M.; McCormick, C.L. Tuning nanostructure morphology and gold nanoparticle “locking” of multi-responsive amphiphilic diblock copolymers. Macromolecules 2009, 42, 2958–2964. [Google Scholar] [CrossRef]
- Han, D.; Tong, X.; Boissière, O.; Zhao, Y. General strategy for making CO2-switchable polymers. ACS Macro Lett. 2012, 1, 57–61. [Google Scholar] [CrossRef]
- Lee, A.S.; Bütün, V.; Vamvakaki, M.; Armes, S.P.; Pople, J.A.; Gast, A.P. Structure of pH-dependent block copolymer micelles: Charge and ionic strength dependence. Macromolecules 2002, 35, 8540–8551. [Google Scholar] [CrossRef]
- Lutz, J.F. Thermo-switchable materials prepared using the OEGMA-platform. Adv. Mater. 2011, 23, 2237–2243. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- He, J.; Tong, X.; Zhao, Y. Photoresponsive nanogels based on photocontrollable cross-links. Macromolecules 2009, 42, 4845–4852. [Google Scholar] [CrossRef]
- Sumio, L. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar]
- Collins, P.G.; Zettl, A.; Bando, H.; Thess, A.; Smalley, R.E. Nanotube nanodevice. Science 1997, 278, 100–103. [Google Scholar] [CrossRef]
- Dai, H.J.; Kong, J.; Franklin, N.R.; Zhou, C.W.; Chapline, M.G.; Peng, S.; Cho, K.J. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar]
- Baughman, R.H.; Zakhidov, A.A.; De Heer, W.A. Carbon Nanotubes—The Route Toward Applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Tour, J.M. Materials science: Nanotube composites. Nature 2007, 447, 1066–1068. [Google Scholar] [CrossRef]
- Qu, L.; Peng, Q.; Dai, L.; Spinks, G.M.; Wallace, G.G.; Baughman, R.H. Carbon nanotube electroactive polymer materials: Opportunities and challenges. MRS Bull. 2008, 33, 215–224. [Google Scholar] [CrossRef][Green Version]
- Zhao, H.; Qiu, S.; Wu, L.; Zhang, L.; Chen, H.; Gao, C. Improving the performance of polyamide reverse osmosis membrane by incorporation of modified multi-walled carbon nanotubes. J. Memb. Sci. 2014, 450, 249–256. [Google Scholar] [CrossRef]
- Vatanpour, V.; Esmaeili, M.; Farahani, M.H.D.A. Fouling reduction and retention increment of polyethersulfone nanofiltration membranes embedded by amine-functionalized multi-walled carbon nanotubes. J. Memb. Sci. 2014, 466, 70–81. [Google Scholar] [CrossRef]
- Haeshin, L.; Shara, M.D.; William, M.M.; Phillip, B.M. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–431. [Google Scholar]
- Ye, Q.; Zhou, F.; Liu, W. Bioinspired catecholic chemistry for surface modification. Chem. Soc. Rev. 2011, 40, 4244–4258. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Lee, Y.; Hwang, J.W.; Hong, S.; Kim, C.; Park, T.G.; Lee, H.; Hong, S.H. High-strength carbon nanotube fibers fabricated by infiltration and curing of mussel-inspired catecholamine polymer. Adv. Mater. 2011, 23, 1971–1975. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Na, Y.S.; Choi, S.; Song, I.T.; Kim, W.Y.; Lee, H. Non-covalent self-assembly and covalent polymerization co-contribute to polydopamine formation. Adv. Funct. Mater. 2012, 22, 4711–4717. [Google Scholar] [CrossRef]
- Carson, L.; Kelly-Brown, C.; Stewart, M.; Oki, A.; Regisford, G.; Luo, Z.; Bakhmutov, V.I. Synthesis and characterization of chitosan–carbon nanotube composites. Mater. Lett. 2009, 63, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Hafner, D.; Jordan, R. Substrate-independent Cu(0)-mediated controlled radical polymerization: Grafting of block copolymer brushes from poly(dopamine) modified surfaces. Polym. Chem. 2020, 11, 2129–2136. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, S.; Xu, H.; Wang, Z.; Zhang, X.; Ngo, T.H.; Smet, M. Reversible dispersion of single-walled carbon nanotubes based on a CO2-responsive dispersant. Langmuir 2010, 26, 16667–16671. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Chen, X.; Han, D.; Zhao, Z.; Lu, W. Preparation of a Novel CO2-Responsive Polymer/Multiwall Carbon Nanotube Composite. Processes 2021, 9, 1638. https://doi.org/10.3390/pr9091638
Ma Y, Chen X, Han D, Zhao Z, Lu W. Preparation of a Novel CO2-Responsive Polymer/Multiwall Carbon Nanotube Composite. Processes. 2021; 9(9):1638. https://doi.org/10.3390/pr9091638
Chicago/Turabian StyleMa, Yonggang, Xin Chen, Dehui Han, Zhe Zhao, and Wenting Lu. 2021. "Preparation of a Novel CO2-Responsive Polymer/Multiwall Carbon Nanotube Composite" Processes 9, no. 9: 1638. https://doi.org/10.3390/pr9091638
APA StyleMa, Y., Chen, X., Han, D., Zhao, Z., & Lu, W. (2021). Preparation of a Novel CO2-Responsive Polymer/Multiwall Carbon Nanotube Composite. Processes, 9(9), 1638. https://doi.org/10.3390/pr9091638




