Preparation of a Novel CO2-Responsive Polymer/Multiwall Carbon Nanotube Composite
Abstract
:1. Introduction
2. Materials and Methods
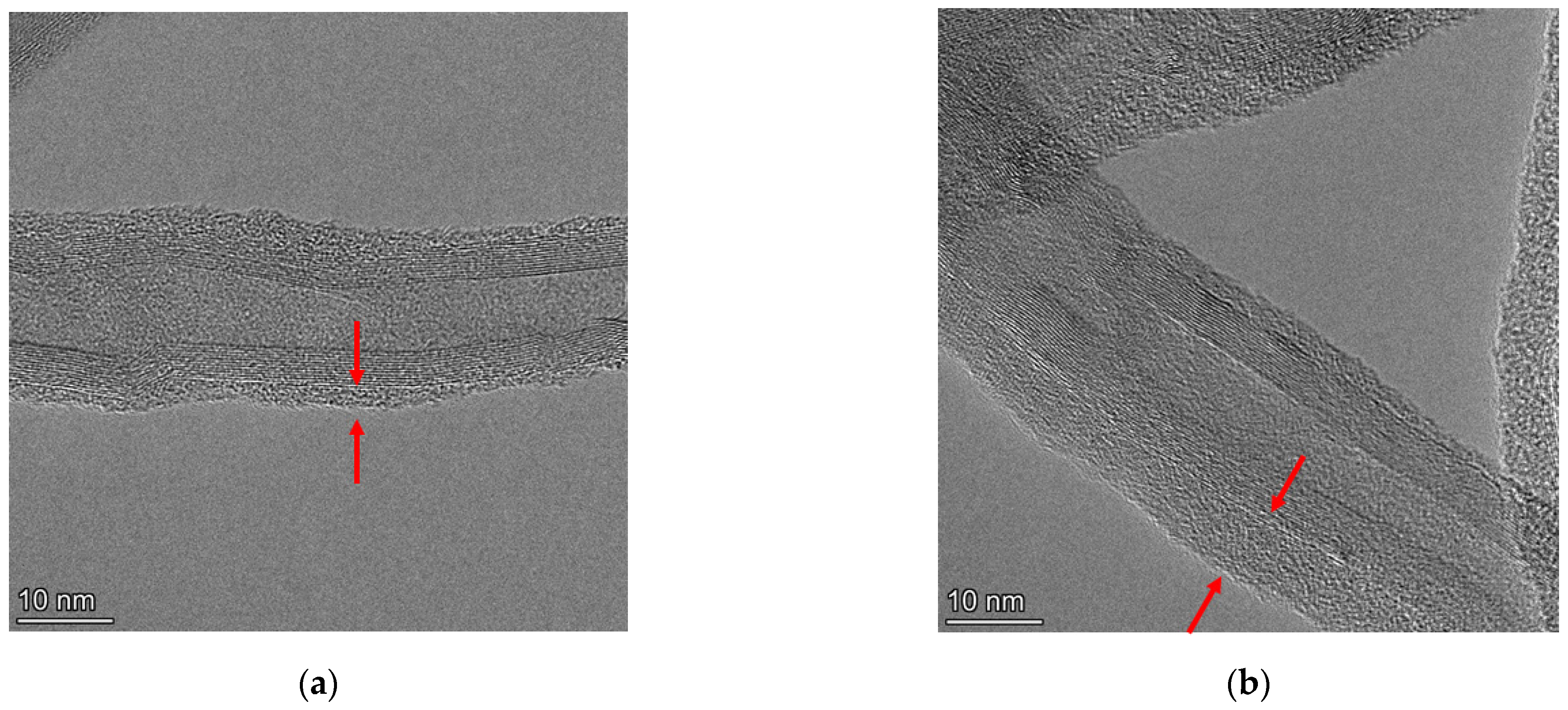
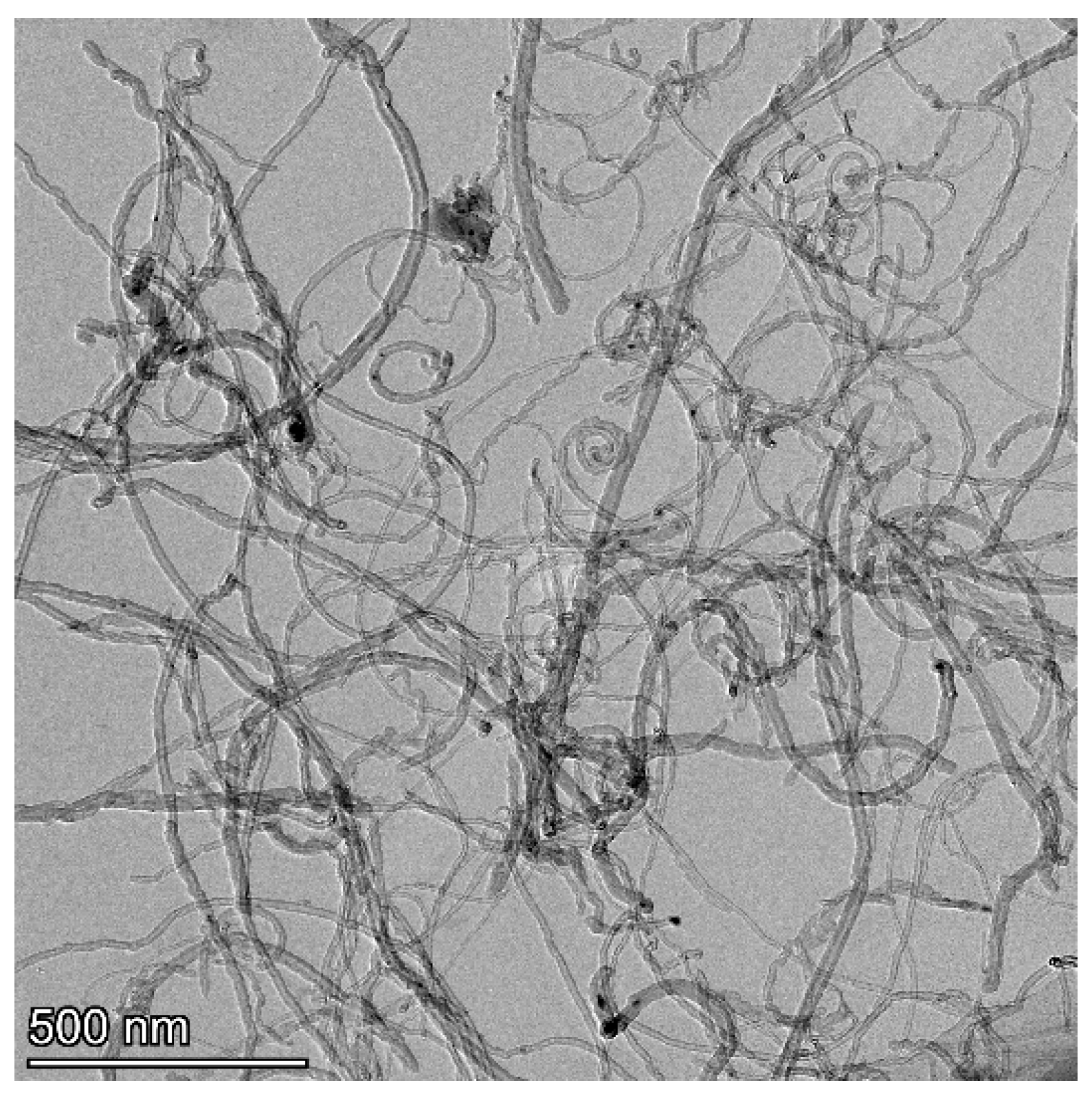
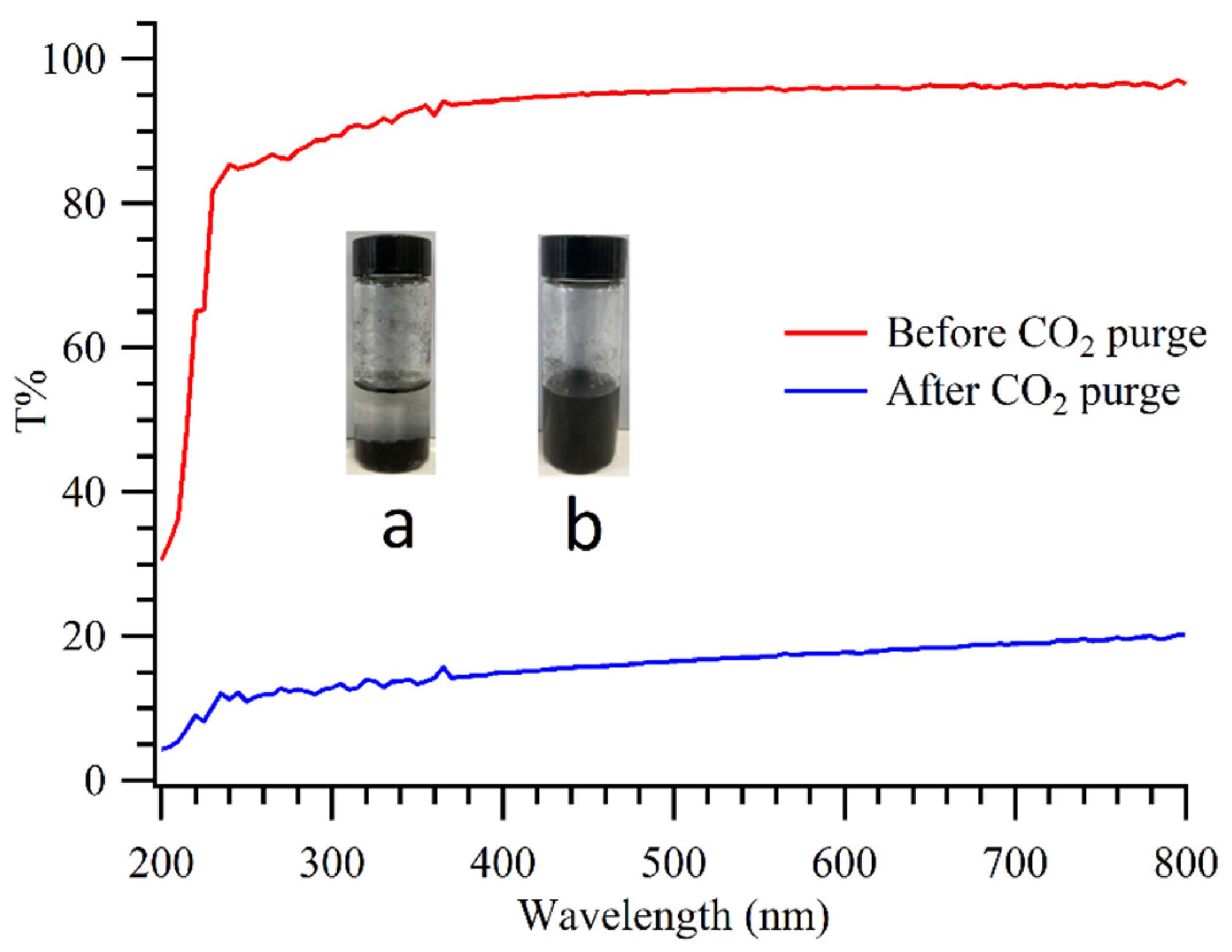
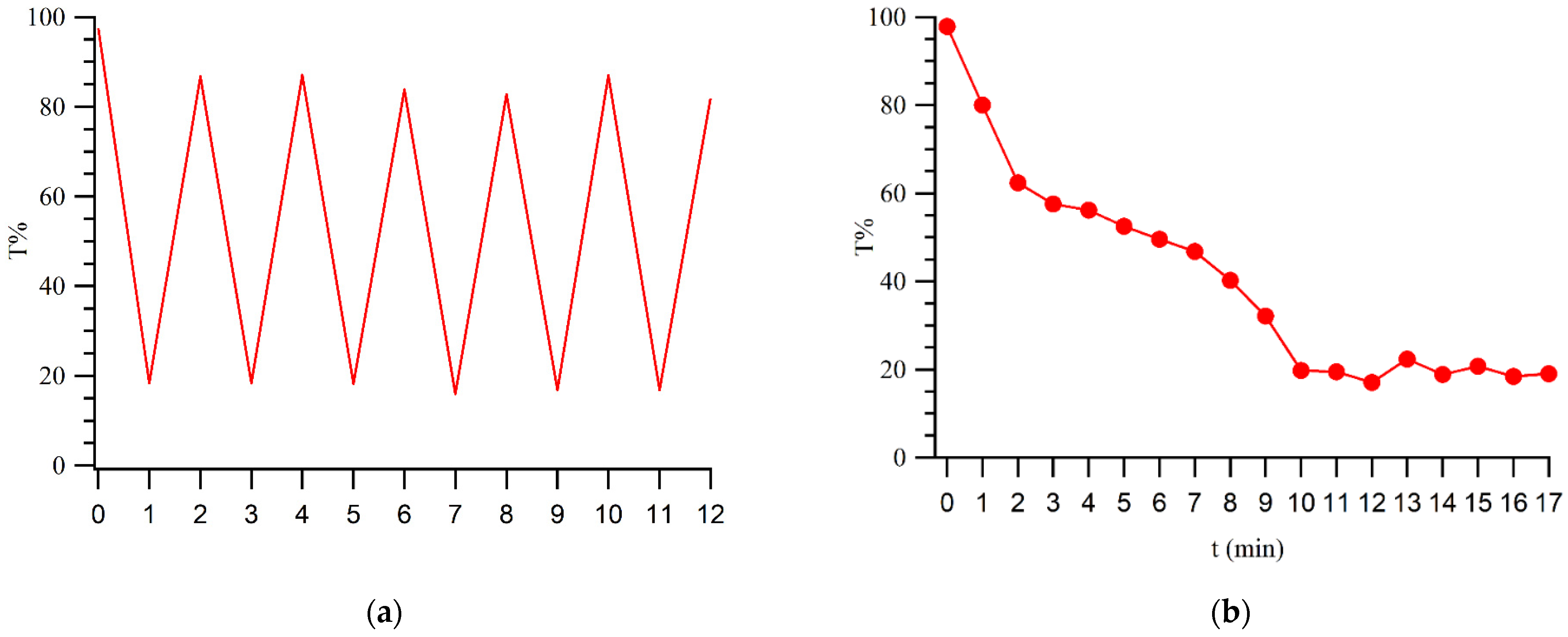
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jessop, P.G.; Mercer, S.M.; Heldebrant, D.J. CO2-triggered switchable solvents, surfactants, and other materials. Energy Environ. Sci. 2012, 5, 7240–7253. [Google Scholar] [CrossRef]
- Darabi, A.; Jessop, P.G.; Cunningham, M.F. CO2-responsive polymeric materials: Synthesis, self-assembly, and functional applications. Chem. Soc. Rev. 2016, 45, 4391–4436. [Google Scholar] [CrossRef] [PubMed]
- Phan, L.; Chiu, D.; Heldebrant, D.J.; Huttenhower, H.; John, E.; Li, X.; Pollet, P.; Wang, R.; Eckert, C.A.; Liotta, C.L.; et al. Switchable solvents consisting of amidine/alcohol or guanidine/alcohol mixtures. Ind. Eng. Chem. Res. 2008, 47, 539–545. [Google Scholar] [CrossRef]
- Phan, L.; Andreatta, J.R.; Horvey, L.K.; Edie, C.F.; Luco, A.L.; Mirchandani, A.; Darensbourg, D.J.; Jessop, P.G. Switchable-polarity solvents prepared with a single liquid component. J. Org. Chem. 2008, 73, 127–132. [Google Scholar] [CrossRef]
- Pollet, P.; Eckertabc, C.A.; Liotta, C.L. Switchable solvents. Chem. Sci. 2011, 2, 609–614. [Google Scholar] [CrossRef]
- Phan, L.; Brown, H.; White, J.; Hodgson, A.; Jessop, P.G. Soybean oil extraction and separation using switchable or expanded solvents. Green Chem. 2009, 11, 53–59. [Google Scholar] [CrossRef]
- Samorì, C.; Torri, C.; Samorì, G.; Fabbri, D.; Galletti, P.; Guerrini, F.; Pistocchi, R.; Tagliavini, E. Extraction of hydrocarbons from microalga Botryococcus braunii with switchable solvents. Bioresour. Technol. 2010, 101, 3274–3279. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, Y.; Barashkov, N.N.; Irgibaeva, I.S.; Lam, J.W.Y.; Hu, R.; Birimzhanova, D.; Yu, Y.; Tang, B.Z. Fluorescent chemosensor for detection and quantitation of carbon dioxide gas. J. Am. Chem. Soc. 2010, 132, 13951–13953. [Google Scholar] [CrossRef]
- Heldebrant, D.J.; Yonker, C.R.; Jessop, P.G.; Phan, L. Reversible Uptake of COS, CS2, and SO2: Ionic Liquids with O-Alkylxanthate, O-Alkylthiocarbonyl, and O-Alkylsulfite Anions. Chem. A Eur. J. 2009, 15, 7619–7627. [Google Scholar] [CrossRef]
- Kim, M.; Park, J.W. Reversible, solid state capture of carbon dioxide by hydroxylated amidines. Chem. Commun. 2010, 46, 2507–2509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kutnyakov, I.; Koech, P.K.; Zwoster, A.; Howard, C.; Zheng, F.; Freeman, C.J.; Heldebrant, D.J. CO2-binding-organic-liquids-enhanced CO2 capture using polarity-swing-assisted regeneration. Energy Procedia 2013, 37, 285–291. [Google Scholar] [CrossRef] [Green Version]
- Blasucci, V.; Hart, R.; Mestre, V.L.; Hahne, D.J.; Burlager, M.; Huttenhower, H.; Thio, B.J.R.; Pollet, P.; Liotta, C.L.; Eckert, C.A. Single component, reversible ionic liquids for energy applications. Fuel 2010, 89, 1315–1319. [Google Scholar] [CrossRef]
- Wang, C.; Mahurin, S.M.; Luo, H.; Baker, G.A.; Li, H.; Dai, S. Reversible and robust CO2 capture by equimolar task-specific ionic liquid–Superbase mixtures. Green Chem. 2010, 12, 870–887. [Google Scholar] [CrossRef]
- Wang, C.; Luo, X.; Luo, H.; Jiang, D.; Li, H.; Dai, S. Tuning the Basicity of Ionic Liquids for Equimolar CO2 Capture. Angew. Chem. 2011, 123, 5020–5024. [Google Scholar] [CrossRef]
- Jessop, P.G.; Kozycz, L.; Rahami, Z.G.; Schoenmakers, D.; Boyd, A.R.; Wechsler, D.; Holland, A.M. Tertiary amine solvents having switchable hydrophilicity. Green Chem. 2011, 13, 619–623. [Google Scholar] [CrossRef]
- Kohno, Y.; Arai, H.; Ohno, H. Dual stimuli-responsive phase transition of an ionic liquid/water mixture. Chem. Commun. 2011, 47, 4772–4774. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jessop, P.G.; Cunningham, M.; Eckert, C.A.; Liotta, C.L. Switchable surfactants. Science 2006, 313, 958–960. [Google Scholar] [CrossRef]
- Darwish, T.A.; Evans, R.A.; James, M.; Hanley, T.L. Spiropyran-amidine: A molecular canary for visual detection of carbon dioxide gas. Chem. A Eur. J. 2011, 17, 11399–11404. [Google Scholar] [CrossRef]
- Desset, S.L.; Cole-Hamilton, D.J. Carbon dioxide induced phase switching for homogeneous-catalyst recycling. Angew. Chem. Int. Ed. 2009, 48, 1472–1474. [Google Scholar] [CrossRef]
- Moore, N.A.L. Method for Shear Coagulation of Latex Resins. U.S. Patent 4,623,678, 18 November 1986. [Google Scholar]
- Smith, A.E.; Xu, X.; Abell, T.U.; Kirkland, S.E.; Hensarling, R.M.; McCormick, C.L. Tuning nanostructure morphology and gold nanoparticle “locking” of multi-responsive amphiphilic diblock copolymers. Macromolecules 2009, 42, 2958–2964. [Google Scholar] [CrossRef]
- Han, D.; Tong, X.; Boissière, O.; Zhao, Y. General strategy for making CO2-switchable polymers. ACS Macro Lett. 2012, 1, 57–61. [Google Scholar] [CrossRef]
- Lee, A.S.; Bütün, V.; Vamvakaki, M.; Armes, S.P.; Pople, J.A.; Gast, A.P. Structure of pH-dependent block copolymer micelles: Charge and ionic strength dependence. Macromolecules 2002, 35, 8540–8551. [Google Scholar] [CrossRef]
- Lutz, J.F. Thermo-switchable materials prepared using the OEGMA-platform. Adv. Mater. 2011, 23, 2237–2243. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- He, J.; Tong, X.; Zhao, Y. Photoresponsive nanogels based on photocontrollable cross-links. Macromolecules 2009, 42, 4845–4852. [Google Scholar] [CrossRef]
- Sumio, L. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar]
- Collins, P.G.; Zettl, A.; Bando, H.; Thess, A.; Smalley, R.E. Nanotube nanodevice. Science 1997, 278, 100–103. [Google Scholar] [CrossRef]
- Dai, H.J.; Kong, J.; Franklin, N.R.; Zhou, C.W.; Chapline, M.G.; Peng, S.; Cho, K.J. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar]
- Baughman, R.H.; Zakhidov, A.A.; De Heer, W.A. Carbon Nanotubes—The Route Toward Applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [Green Version]
- Ajayan, P.M.; Tour, J.M. Materials science: Nanotube composites. Nature 2007, 447, 1066–1068. [Google Scholar] [CrossRef]
- Qu, L.; Peng, Q.; Dai, L.; Spinks, G.M.; Wallace, G.G.; Baughman, R.H. Carbon nanotube electroactive polymer materials: Opportunities and challenges. MRS Bull. 2008, 33, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Qiu, S.; Wu, L.; Zhang, L.; Chen, H.; Gao, C. Improving the performance of polyamide reverse osmosis membrane by incorporation of modified multi-walled carbon nanotubes. J. Memb. Sci. 2014, 450, 249–256. [Google Scholar] [CrossRef]
- Vatanpour, V.; Esmaeili, M.; Farahani, M.H.D.A. Fouling reduction and retention increment of polyethersulfone nanofiltration membranes embedded by amine-functionalized multi-walled carbon nanotubes. J. Memb. Sci. 2014, 466, 70–81. [Google Scholar] [CrossRef]
- Haeshin, L.; Shara, M.D.; William, M.M.; Phillip, B.M. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–431. [Google Scholar]
- Ye, Q.; Zhou, F.; Liu, W. Bioinspired catecholic chemistry for surface modification. Chem. Soc. Rev. 2011, 40, 4244–4258. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Lee, Y.; Hwang, J.W.; Hong, S.; Kim, C.; Park, T.G.; Lee, H.; Hong, S.H. High-strength carbon nanotube fibers fabricated by infiltration and curing of mussel-inspired catecholamine polymer. Adv. Mater. 2011, 23, 1971–1975. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Na, Y.S.; Choi, S.; Song, I.T.; Kim, W.Y.; Lee, H. Non-covalent self-assembly and covalent polymerization co-contribute to polydopamine formation. Adv. Funct. Mater. 2012, 22, 4711–4717. [Google Scholar] [CrossRef]
- Carson, L.; Kelly-Brown, C.; Stewart, M.; Oki, A.; Regisford, G.; Luo, Z.; Bakhmutov, V.I. Synthesis and characterization of chitosan–carbon nanotube composites. Mater. Lett. 2009, 63, 617–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafner, D.; Jordan, R. Substrate-independent Cu(0)-mediated controlled radical polymerization: Grafting of block copolymer brushes from poly(dopamine) modified surfaces. Polym. Chem. 2020, 11, 2129–2136. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Chen, S.; Xu, H.; Wang, Z.; Zhang, X.; Ngo, T.H.; Smet, M. Reversible dispersion of single-walled carbon nanotubes based on a CO2-responsive dispersant. Langmuir 2010, 26, 16667–16671. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Chen, X.; Han, D.; Zhao, Z.; Lu, W. Preparation of a Novel CO2-Responsive Polymer/Multiwall Carbon Nanotube Composite. Processes 2021, 9, 1638. https://doi.org/10.3390/pr9091638
Ma Y, Chen X, Han D, Zhao Z, Lu W. Preparation of a Novel CO2-Responsive Polymer/Multiwall Carbon Nanotube Composite. Processes. 2021; 9(9):1638. https://doi.org/10.3390/pr9091638
Chicago/Turabian StyleMa, Yonggang, Xin Chen, Dehui Han, Zhe Zhao, and Wenting Lu. 2021. "Preparation of a Novel CO2-Responsive Polymer/Multiwall Carbon Nanotube Composite" Processes 9, no. 9: 1638. https://doi.org/10.3390/pr9091638
APA StyleMa, Y., Chen, X., Han, D., Zhao, Z., & Lu, W. (2021). Preparation of a Novel CO2-Responsive Polymer/Multiwall Carbon Nanotube Composite. Processes, 9(9), 1638. https://doi.org/10.3390/pr9091638




