Nanocellulose from Agricultural Wastes: Products and Applications—A Review
Abstract
:1. Introduction
2. Biomass Sources
3. Nanocellulose Products
3.1. Microcrystalline Cellulose (MCC)
3.2. Cellulose Nanocrystals (CNC)
3.3. Cellulose Nanofibers (CNF)
3.4. Bacterial Nanocellulose (BNC)
4. Conditioning, Pretreatments and Bleaching Processes
4.1. Biomass Conditioning
4.2. Pretreatments
4.2.1. Alkaline
4.2.2. Hot Water and Ionic Liquids
4.3. Bleaching Process
5. Applications and Future Perspectives
5.1. Food Packaging
5.2. Wastewater Treatment
5.3. Foodstuffs
5.4. Biomedical Applications
5.5. Wood Adhesives
5.6. Cosmetic
5.7. Electrical and Optical Materials
5.8. Textile
5.9. CO Capture
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy |
| AmimCl | 1-allyl-3-methylimidazolium chloride |
| BmimCl | 1-butyl-3-methylimidazolium chloride |
| BNC | Bacterial nanocellulose |
| CBH | Cellobiohydrolases |
| CNC | Cellulose nanocrystals |
| CNF | Cellulose nanofibers |
| DLS | Dynamic light scattering |
| DSC | Differential scanning calorimetry |
| DTA | Differential thermal analysis |
| HIUS | High intensity ultrasonication |
| IL | Ionic liquids |
| LCB | Lignocellulosic biomass |
| LHW | Liquid hot water |
| MCC | Microcrystalline cellulose |
| SANS | Small-angle neutron scattering |
| SEM | Scanning electron microscopy |
| TAED | Tetraacetylethylenediamine |
| TEM | Transmission electron microscopy |
| TEMPO | 2,2,6,6-tetramethylpiperidine-1-oxyl |
| TGA | Thermogravimetric analysis |
References
- Araújo, D.; Vilarinho, M.; Machado, A. Effect of combined dilute-alkaline and green pretreatments on corncob fractionation: Pretreated biomass characterization and regenerated cellulose film production. Ind. Crop. Prod. 2019, 141, 111785. [Google Scholar] [CrossRef]
- Collazo-Bigliardi, S.; Ortega-Toro, R.; Boix, A.C. Isolation and characterisation of microcrystalline cellulose and cellulose nanocrystals from coffee husk and comparative study with rice husk. Carbohydr. Polym. 2018, 191, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Trache, D.; Hussin, M.H.; Chuin, C.T.H.; Sabar, S.; Fazita, M.N.; Taiwo, O.F.; Hassan, T.; Haafiz, M.M. Microcrystalline cellulose: Isolation, characterization and bio-composites application—A review. Int. J. Biol. Macromol. 2016, 93, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Brodin, M.; Vallejos, M.; Opedal, M.T.; Area, M.C.; Chinga-Carrasco, G. Lignocellulosics as sustainable resources for production of bioplastics—A review. J. Clean. Prod. 2017, 162, 646–664. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: Challenges and opportunities. Bioresour. Technol. 2016, 199, 92–102. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Lei, F.; Li, P.; Jiang, J. Lignocellulosic biomass to biofuels and biochemicals: A comprehensive review with a focus on ethanol organosolv pretreatment technology. Biotechnol. Bioeng. 2018, 115, 2683–2702. [Google Scholar] [CrossRef]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef]
- Heise, K.; Kontturi, E.; Allahverdiyeva, Y.; Tammelin, T.; Linder, M.B.; Nonappa; Ikkala, O. Nanocellulose: Recent fundamental advances and emerging biological and biomimicking applications. Adv. Mater. 2020, 33, 2004349. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Ribeiro, R.S.A.; Pohlmann, B.C.; Calado, V.; Bojorge, N.; Pereira, N. Production of nanocellulose by enzymatic hydrolysis: Trends and challenges. Eng. Life Sci. 2019, 19, 279–291. [Google Scholar] [CrossRef] [Green Version]
- Squinca, P.; Bilatto, S.; Badino, A.C.; Farinas, C.S. Nanocellulose production in future biorefineries: An integrated approach using Tailor-made enzymes. ACS Sustain. Chem. Eng. 2020, 8, 2277–2286. [Google Scholar] [CrossRef]
- De Campos, A.; Correa, A.C.; Cannella, D.; de M Teixeira, E.; Marconcini, J.M.; Dufresne, A.; Mattoso, L.H.C.; Cassland, P.; Sanadi, A.R. Obtaining nanofibers from curauá and sugarcane bagasse fibers using enzymatic hydrolysis followed by sonication. Cellulose 2013, 20, 1491–1500. [Google Scholar] [CrossRef]
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose—Its barrier properties and applications in cellulosic materials: A review. Carbohydr. Polym. 2012, 90, 735–764. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Wang, H.; Li, D.; Hong, M.; Dou, J. Syntheses, structural characterization and in vitro cytotoxic activity of triorganotin(IV) complexes based on 1,7-dihydroxycarbonyl-1,7-dicarba-closo-dodecaborane ligand. J. Organomet. Chem. 2013, 740, 1–9. [Google Scholar] [CrossRef]
- Kapu, N.S.; Trajano, H.L. Review of hemicellulose hydrolysis in softwoods and bamboo. Biofuels Bioprod. Biorefin. 2014, 8, 857–870. [Google Scholar] [CrossRef]
- Hu, Y.; Hamed, O.; Salghi, R.; Abidi, N.; Jodeh, S.; Hattb, R. Extraction and characterization of cellulose from agricultual waste argan press cake. Cellul. Chem. Technol. 2017, 51, 263–272. [Google Scholar]
- Melikoglu, A.Y.; Bilek, S.E.; Cesur, S. Optimum alkaline treatment parameters for the extraction of cellulose and production of cellulose nanocrystals from apple pomace. Carbohydr. Polym. 2019, 215, 330–337. [Google Scholar] [CrossRef]
- Raghav, N.; Sharma, M.R.; Kennedy, J.F. Nanocellulose: A mini-review on types and use in drug delivery systems. Carbohydr. Polym. Technol. Appl. 2021, 2, 100031. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, Z.; Lin, X.; Ren, Z.; Li, B.; Zhang, Y. Preparation and characterization of microcrystalline cellulose (MCC) from tea waste. Carbohydr. Polym. 2018, 184, 164–170. [Google Scholar] [CrossRef]
- Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Vázquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2007, 15, 149–159. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, X.; Zhu, J.Y. Kinetics of strong acid hydrolysis of a bleached kraft pulp for producing cellulose nanocrystals (CNCs). Ind. Eng. Chem. Res. 2014, 53, 11007–11014. [Google Scholar] [CrossRef]
- Hou, W.; Ling, C.; Shi, S.; Yan, Z. Preparation and characterization of microcrystalline cellulose from waste cotton fabrics by using phosphotungstic acid. Int. J. Biol. Macromol. 2019, 123, 363–368. [Google Scholar] [CrossRef]
- Stupinska, H.; Iller, E.; Zimek, Z.; Wawro, D.; Ciechanska, D.; Kopania, E.; Palenik, J.; Milczarek, S.; Steplewski, W.; Krzyzanowska, G. An environment-friendly method to prepare microcrystalline cellulose. Fibres Text. East. Eur. 2007, 15, 167–172. [Google Scholar]
- Jacquet, N.; Vanderghem, C.; Danthine, S.; Quiévy, N.; Blecker, C.; Devaux, J.; Paquot, M. Influence of steam explosion on physicochemical properties and hydrolysis rate of pure cellulose fibers. Bioresour. Technol. 2012, 121, 221–227. [Google Scholar] [CrossRef]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 2006, 13, 171–180. [Google Scholar] [CrossRef]
- Bhat, A.H.; Dasan, Y.; Khan, I.; Soleimani, H.; Usmani, A. Application of nanocrystalline cellulose. In Cellulose-Reinforced Nanofibre Composites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 215–240. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, J.; Zhang, L. Structure and properties of the nanocomposite films of chitosan reinforced with cellulose whiskers. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 1069–1077. [Google Scholar] [CrossRef]
- Xu, K.; Liu, C.; Kang, K.; Zheng, Z.; Wang, S.; Tang, Z.; Yang, W. Isolation of nanocrystalline cellulose from rice straw and preparation of its biocomposites with chitosan: Physicochemical characterization and evaluation of interfacial compatibility. Compos. Sci. Technol. 2018, 154, 8–17. [Google Scholar] [CrossRef]
- Habibi, Y.; Goffin, A.L.; Schiltz, N.; Duquesne, E.; Dubois, P.; Dufresne, A. Bionanocomposites based on poly(ε-caprolactone)-grafted cellulose nanocrystals by ring-opening polymerization. J. Mater. Chem. 2008, 18, 5002. [Google Scholar] [CrossRef]
- Pääkkö, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O.; et al. Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef]
- Fall, A.B.; Lindström, S.B.; Sundman, O.; Ödberg, L.; Wargberg, L. Colloidal stability of aqueous nanofibrillated cellulose dispersions. Langmuir 2011, 27, 11332–11338. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Besbes, I.; Alila, S.; Boufi, S. Nanofibrillated cellulose from TEMPO-oxidized eucalyptus fibres: Effect of the carboxyl content. Carbohydr. Polym. 2011, 84, 975–983. [Google Scholar] [CrossRef]
- Mazela, B.; Perdoch, W.; Peplińska, B.; Zieliński, M. Influence of Chemical Pre-Treatments and Ultrasonication on the Dimensions and Appearance of Cellulose Fibers. Materials 2020, 13, 5274. [Google Scholar] [CrossRef]
- Mishra, S.P.; Manent, A.S.; Chabot, B.; Daneault, C. The Use of Sodium Chlorite in Post-Oxidation of TEMPO-Oxidized Pulp: Effect on Pulp Characteristics and Nanocellulose Yield. J. Wood Chem. Technol. 2012, 32, 137–148. [Google Scholar] [CrossRef]
- Jozala, A.F.; de Lencastre-Novaes, L.C.; Lopes, A.M.; de Carvalho Santos-Ebinuma, V.; Mazzola, P.G.; Pessoa, A., Jr.; Grotto, D.; Gerenutti, M.; Chaud, M.V. Bacterial nanocellulose production and application: A 10-year overview. Appl. Microbiol. Biotechnol. 2016, 100, 2063–2072. [Google Scholar] [CrossRef] [Green Version]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Kim, J.N.; Wee, Y.J.; Park, D.H.; Ryu, H.W. Bacterial cellulose production by Gluconacetobacter sp. PKY5 in a rotary biofilm contactor. Appl. Biochem. Biotechnol. 2007, 137–140, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Sajjad, W.; Khan, T.; Wahid, F. Production of bacterial cellulose from industrial wastes: A review. Cellulose 2019, 26, 2895–2911. [Google Scholar] [CrossRef]
- Richter, G.A.; Schur, M.O.; Rasch, R.H. Process of Conditioning Cellulose Fiber for Conversion into Cellulose Derivatives, and Product of Same. U.S. Patent 1,701,543, 12 February 1929. [Google Scholar]
- Bhutto, A.W.; Qureshi, K.; Harijan, K.; Abro, R.; Abbas, T.; Bazmi, A.A.; Karim, S.; Yu, G. Insight into progress in pre-treatment of lignocellulosic biomass. Energy 2017, 122, 724–745. [Google Scholar] [CrossRef]
- Seidl, P.R.; Goulart, A.K. Pretreatment processes for lignocellulosic biomass conversion to biofuels and bioproducts. Curr. Opin. Green Sustain. Chem. 2016, 2, 48–53. [Google Scholar] [CrossRef]
- Hassan, M.L.; Mathew, A.P.; Hassan, E.A.; El-Wakil, N.A.; Oksman, K. Nanofibers from bagasse and rice straw: Process optimization and properties. Wood Sci. Technol. 2010, 46, 193–205. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues—Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Stevens, M.A.; Zhu, Y.; Holmes, J.; Xu, H. Understanding of alkaline pretreatment parameters for corn stover enzymatic saccharification. Biotechnol. Biofuels 2013, 6, 8. [Google Scholar] [CrossRef]
- De Souza Fonseca, A.; Panthapulakkal, S.; Konar, S.K.; Sain, M.; Bufalinof, L.; Raabe, J.; de Andrade Miranda, I.P.; Martins, M.A.; Tonoli, G.H.D. Improving cellulose nanofibrillation of non-wood fiber using alkaline and bleaching pre-treatments. Ind. Crop. Prod. 2019, 131, 203–212. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Zhou, Y.; Li, Y. Liquid hot water and alkaline pretreatment of soybean straw for improving cellulose digestibility. Bioresour. Technol. 2011, 102, 6254–6259. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process. Technol. 2017, 160, 196–206. [Google Scholar] [CrossRef]
- Rabemanolontsoa, H.; Saka, S. Various pretreatments of lignocellulosics. Bioresour. Technol. 2016, 199, 83–91. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Yue, Y.; Han, J.; Han, G.; Aita, G.M.; Wu, Q. Cellulose fibers isolated from energycane bagasse using alkaline and sodium chlorite treatments: Structural, chemical and thermal properties. Ind. Crop. Prod. 2015, 76, 355–363. [Google Scholar] [CrossRef]
- Achaby, M.E.; Kassab, Z.; Barakat, A.; Aboulkas, A. Alfa fibers as viable sustainable source for cellulose nanocrystals extraction: Application for improving the tensile properties of biopolymer nanocomposite films. Ind. Crop. Prod. 2018, 112, 499–510. [Google Scholar] [CrossRef]
- Moya, A.J.; Peinado, S.; Mateo, S.; Fonseca, B.G.; Sánchez, S. Improving bioethanol production from olive pruning biomass by deacetylation step prior acid hydrolysis and fermentation processes. Bioresour. Technol. 2016, 220, 239–245. [Google Scholar] [CrossRef]
- Kim, Y.; Hendrickson, R.; Mosier, N.S.; Ladisch, M.R. Liquid hot water pretreatment of cellulosic biomass. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2009; pp. 93–102. [Google Scholar] [CrossRef]
- Babicka, M.; Woźniak, M.; Szentner, K.; Bartkowiak, M.; Peplińska, B.; Dwiecki, K.; Borysiak, S.; Ratajczak, I. Nanocellulose Production Using Ionic Liquids with Enzymatic Pretreatment. Materials 2021, 14, 3264. [Google Scholar] [CrossRef]
- Phanthong, P.; Karnjanakom, S.; Reubroycharoen, P.; Hao, X.; Abudula, A.; Guan, G. A facile one-step way for extraction of nanocellulose with high yield by ball milling with ionic liquid. Cellulose 2017, 24, 2083–2093. [Google Scholar] [CrossRef]
- Haron, G.A.S.; Mahmood, H.; Noh, M.H.; Alam, M.Z.; Moniruzzaman, M. Ionic liquids as a sustainable platform for nanocellulose processing from bioresources: Overview and current status. ACS Sustain. Chem. Eng. 2021, 9, 1008–1034. [Google Scholar] [CrossRef]
- Turner, M.B.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Production of bioactive cellulose films reconstituted from ionic liquids. Biomacromolecules 2004, 5, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Bell, T.J.; Handa, S.; Stoddart, B. O-Acetylation of cellulose and monosaccharides using a zinc based ionic liquid. Green Chem. 2005, 7, 705. [Google Scholar] [CrossRef] [Green Version]
- Achaby, M.E.; Miri, N.E.; Hannache, H.; Gmouh, S.; Youcef, H.B.; Aboulkas, A. Production of cellulose nanocrystals from vine shoots and their use for the development of nanocomposite materials. Int. J. Biol. Macromol. 2018, 117, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Kampeerapappun, P. Extraction and characterization of cellulose nanocrystals produced by acid hydrolysis from corn husk. J. Met. Mater. Miner. 2015, 25, 19–26. [Google Scholar] [CrossRef]
- Zhou, S.; Runge, T.; Karlen, S.D.; Ralph, J.; Gonzales-Vigil, E.; Mansfield, S.D. Chemical Pulping Advantages of Zip-lignin Hybrid Poplar. ChemSusChem 2017, 10, 3565–3573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.; Hse, C.Y.; Hoop, C.F.D.; Hu, T.; Qi, J.; Shupe, T.F. Isolation and characterization of cellulose nanofibers from bamboo using microwave liquefaction combined with chemical treatment and ultrasonication. Carbohydr. Polym. 2016, 151, 725–734. [Google Scholar] [CrossRef]
- Tibolla, H.; Pelissari, F.M.; Menegalli, F.C. Cellulose nanofibers produced from banana peel by chemical and enzymatic treatment. LWT Food Sci. Technol. 2014, 59, 1311–1318. [Google Scholar] [CrossRef]
- Kouadri, I.; Satha, H. Extraction and characterization of cellulose and cellulose nanofibers from Citrullus colocynthis seeds. Ind. Crop. Prod. 2018, 124, 787–796. [Google Scholar] [CrossRef]
- Rehman, N.; de Miranda, M.I.G.; Rosa, S.M.L.; Pimentel, D.M.; Nachtigall, S.M.B.; Bica, C.I.D. Cellulose and nanocellulose from maize straw: An insight on the crystal properties. J. Polym. Environ. 2013, 22, 252–259. [Google Scholar] [CrossRef]
- Khan, M.N.; Rehman, N.; Sharif, A.; Ahmed, E.; Farooqi, Z.H.; Din, M.I. Environmentally benign extraction of cellulose from dunchi fiber for nanocellulose fabrication. Int. J. Biol. Macromol. 2020, 153, 72–78. [Google Scholar] [CrossRef]
- Lamaming, J.; Hashim, R.; Sulaiman, O.; Leh, C.P.; Sugimoto, T.; Nordin, N.A. Cellulose nanocrystals isolated from oil palm trunk. Carbohydr. Polym. 2015, 127, 202–208. [Google Scholar] [CrossRef]
- Fillat, Ú.; Wicklein, B.; Martín-Sampedro, R.; Ibarra, D.; Ruiz-Hitzky, E.; Valencia, C.; Sarrión, A.; Castro, E.; Eugenio, M.E. Assessing cellulose nanofiber production from olive tree pruning residue. Carbohydr. Polym. 2018, 179, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Rambabu, N.; Panthapulakkal, S.; Sain, M.; Dalai, A. Production of nanocellulose fibers from pinecone biomass: Evaluation and optimization of chemical and mechanical treatment conditions on mechanical properties of nanocellulose films. Ind. Crop. Prod. 2016, 83, 746–754. [Google Scholar] [CrossRef]
- Dos Santos, R.M.; Neto, W.P.F.; Silvério, H.A.; Martins, D.F.; Dantas, N.O.; Pasquini, D. Cellulose nanocrystals from pineapple leaf, a new approach for the reuse of this agro-waste. Ind. Crop. Prod. 2013, 50, 707–714. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crop. Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Siqueira, G.; Tapin-Lingua, S.; Bras, J.; da Silva Perez, D.; Dufresne, A. Morphological investigation of nanoparticles obtained from combined mechanical shearing and enzymatic and acid hydrolysis of sisal fibers. Cellulose 2010, 17, 1147–1158. [Google Scholar] [CrossRef]
- Ohwoavworhua, F.; Adelakun, T. Non-wood fibre production of microcrystalline cellulose from Sorghum caudatum: Characterisation and tableting properties. Indian J. Pharm. Sci. 2010, 72, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortunati, E.; Luzi, F.; Jiménez, A.; Gopakumar, D.; Puglia, D.; Thomas, S.; Kenny, J.; Chiralt, A.; Torre, L. Revalorization of sunflower stalks as novel sources of cellulose nanofibrils and nanocrystals and their effect on wheat gluten bionanocomposite properties. Carbohydr. Polym. 2016, 149, 357–368. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Reshmy, R.; Deepa, T.; Eapen, P.; Sherely, A.P.; Aravind, M.; Raveendran, S.; Parameswaran, B.; Arivalagan, P.; Ranjna, S.; Ayon, T.; et al. Potential of nanocellulose for wastewater treatment. Chemosphere 2021, 281, 130738. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in hydrogels—A review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar] [CrossRef]
- Bharimalla, A.K.; Deshmukh, S.P.; Vigneshwaran, N.; Patil, P.G.; Prasad, V. Nanocellulose-Polymer Composites for Applications in Food Packaging: Current Status, Future Prospects and Challenges. Polym. Plast. Technol. Eng. 2017, 56, 805–823. [Google Scholar] [CrossRef]
- Zielińska, D.; Szentner, K.; Waśkiewicz, A.; Borysiak, S. Production of Nanocellulose by Enzymatic Treatment for Application in Polymer Composites. Materials 2021, 14, 2124. [Google Scholar] [CrossRef]
- Srivastava, K.; Dixit, S.; Pal, D.; Mishra, P.; Srivastava, P.; Srivastava, N.; Hashem, A.; Alqarawi, A.A.; Abd_Allah, E.F. Effect of nanocellulose on mechanical and barrier properties of PVA–banana pseudostem fiber composite films. Environ. Technol. Innov. 2021, 21, 101312. [Google Scholar] [CrossRef]
- Zhu, C.; Monti, S.; Mathew, A.P. Evaluation of nanocellulose interaction with water pollutants using nanocellulose colloidal probes and molecular dynamic simulations. Carbohydr. Polym. 2020, 229, 115510. [Google Scholar] [CrossRef]
- Reshmy, R.; Aravind, M.; Eapen, P.; Sherely, A.P.; Raveendran, S.; Parameswaran, B.; Arivalagan, P.; Ranjna, S.; Ashok, P. Sugarcane bagasse derived nanocellulose reinforced with frankincense (Boswellia serrata): Physicochemical properties, biodegradability and antimicrobial effect for controlling microbial growth for food packaging application. Environ. Technol. Innov. 2021, 21, 101335. [Google Scholar] [CrossRef]
- Zhong, T.; Dhandapani, R.; Liang, D.; Wang, J.; Wolcott, M.P.; Fossen, D.V.; Liu, H. Nanocellulose from recycled indigo-dyed denim fabric and its application in composite films. Carbohydr. Polym. 2020, 240, 116283. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, V.; Grumezescu, A. (Eds.) Materials for Biomedical Engineering: Thermoset and Thermoplastic Polymers; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Kargarzadeh, H.; Huang, J.; Lin, N.; Ahmad, I.; Mariano, M.; Dufresne, A.; Thomas, S.; Gałęski, A. Recent developments in nanocellulose-based biodegradable polymers, thermoplastic polymers, and porous nanocomposites. Prog. Polym. Sci. 2018, 87, 197–227. [Google Scholar] [CrossRef]
- Lu, P.; Yang, Y.; Liu, R.; Liu, X.; Ma, J.; Wu, M.; Wang, S. Preparation of sugarcane bagasse nanocellulose hydrogel as a colourimetric freshness indicator for intelligent food packaging. Carbohydr. Polym. 2020, 249, 116831. [Google Scholar] [CrossRef]
- Ahankari, S.S.; Subhedar, A.R.; Bhadauria, S.S.; Dufresne, A. Nanocellulose in food packaging: A review. Carbohydr. Polym. 2021, 255, 117479. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Q.; Wang, Z.; Pu, J. Facile fabrication of an effective nanocellulose-based aerogel and removal of methylene blue from aqueous system. J. Water Process Eng. 2020, 37, 101511. [Google Scholar] [CrossRef]
- Shahnaz, T.; Padmanaban, V.C.; Narayanasamy, S. Surface modification of nanocellulose using polypyrrole for the adsorptive removal of Congo red dye and chromium in binary mixture. Int. J. Biol. Macromol. 2020, 151, 322–332. [Google Scholar] [CrossRef]
- Mutar, H.R.; Jasim, K.K. Adsorption study of disperse yellow dye on nanocellulose surface. Mater. Today Proc. 2021, in press. [Google Scholar] [CrossRef]
- Tan, H.F.; Ooi, B.; Leo, C. Future perspectives of nanocellulose-based membrane for water treatment. J. Water Process Eng. 2020, 37, 101502. [Google Scholar] [CrossRef]
- Goswami, R.; Mishra, A.; Bhatt, N.; Mishra, A.; Naithani, P. Potential of chitosan/nanocellulose based composite membrane for the removal of heavy metal (chromium ion). Mater. Today Proc. 2021, in press. [Google Scholar] [CrossRef]
- Amiralian, N.; Mustapic, M.; Hossain, M.S.A.; Wang, C.; Konarova, M.; Tang, J.; Na, J.; Khan, A.; Rowan, A. Magnetic nanocellulose: A potential material for removal of dye from water. J. Hazard. Mater. 2020, 394, 122571. [Google Scholar] [CrossRef] [PubMed]
- Tshikovhi, A.; Mishra, S.B.; Mishra, A.K. Nanocellulose-based composites for the removal of contaminants from wastewater. Int. J. Biol. Macromol. 2020, 152, 616–632. [Google Scholar] [CrossRef]
- Putro, J.N.; Kurniawan, A.; Ismadji, S.; Ju, Y.H. Nanocellulose based biosorbents for wastewater treatment: Study of isotherm, kinetic, thermodynamic and reusability. Environ. Nanotechnol. Monit. Manag. 2017, 8, 134–149. [Google Scholar] [CrossRef]
- Marchetti, L.; Andrés, S.C. Use of nanocellulose in meat products. Curr. Opin. Food Sci. 2021, 38, 96–101. [Google Scholar] [CrossRef]
- Alzate-Arbeláez, A.F.; Dorta, E.; López-Alarcón, C.; Cortés, F.B.; Rojano, B.A. Immobilization of Andean berry (Vaccinium Meridionale) Polyphenols Nanocellulose Isol. Banan. Residues: A Nat. Food Addit. Antioxid. Prop. Food Chem. 2019, 294, 503–517. [Google Scholar] [CrossRef]
- Chen, J.; Huang, M.; Kong, L. Flexible Ag/nanocellulose fibers SERS substrate and its applications for in-situ hazardous residues detection on food. Appl. Surf. Sci. 2020, 533, 147454. [Google Scholar] [CrossRef]
- Urbina, L.; Corcuera, M.Á.; Gabilondo, N.; Eceiza, A.; Retegi, A. A review of bacterial cellulose: Sustainable production from agricultural waste and applications in various fields. Cellulose 2021, 28, 8229–8253. [Google Scholar] [CrossRef]
- Xie, Y.; Niu, X.; Yang, J.; Fan, R.; Shi, J.; Ullah, N.; Feng, X.; Chen, L. Active biodegradable films based on the whole potato peel incorporated with bacterial cellulose and curcumin. Int. J. Biol. Macromol. 2020, 150, 480–491. [Google Scholar] [CrossRef]
- Subhedar, A.; Bhadauria, S.; Ahankari, S.; Kargarzadeh, H. Nanocellulose in biomedical and biosensing applications: A review. Int. J. Biol. Macromol. 2021, 166, 587–600. [Google Scholar] [CrossRef]
- Gupta, R.D.; Raghav, N. Differential effect of surfactants tetra-n-butyl ammonium bromide and N-Cetyl-N,N,N-trimethyl ammonium bromide bound to nano-cellulose on binding and sustained release of some non-steroidal anti-inflammatory drugs. Int. J. Biol. Macromol. 2020, 164, 2745–2752. [Google Scholar] [CrossRef] [PubMed]
- Erdagi, S.I.; Ngwabebhoh, F.A.; Yildiz, U. Genipin crosslinked gelatin-diosgenin-nanocellulose hydrogels for potential wound dressing and healing applications. Int. J. Biol. Macromol. 2020, 149, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Petzold-Welcke, K.; Kramer, F.; Richter, T.; Raddatz, V.; Fried, W.; Nietzsche, S.; Bellmann, T.; Fischer, D. Biotech nanocellulose: A review on progress in product design and today’s state of technical and medical applications. Carbohydr. Polym. 2021, 254, 117313. [Google Scholar] [CrossRef]
- Ludwicka, K.; Jedrzejczak-Krzepkowska, M.; Kubiak, K.; Kolodziejczyk, M.; Pankiewicz, T.; Bielecki, S. Medical and Cosmetic Applications of Bacterial NanoCellulose. In Bacterial Nanocellulose; Elsevier: Amsterdam, The Netherlands, 2016; pp. 145–165. [Google Scholar] [CrossRef]
- Xu, H.; Liu, Y.; Xie, Y.; Zhu, E.; Shi, Z.; Yang, Q.; Xiong, C. Doubly cross-linked nanocellulose hydrogels with excellent mechanical properties. Cellulose 2019, 26, 8645–8654. [Google Scholar] [CrossRef]
- Soemphol, W.; Charee, P.; Audtarat, S.; Sompech, S.; Hongsachart, P.; Dasri, T. Characterization of a bacterial cellulose-silica nanocomposite prepared from agricultural waste products. Mater. Res. Express 2020, 7, 015085. [Google Scholar] [CrossRef]
- Vineeth, S.K.; Gadhave, R.V.; Gadekar, P.T. Nanocellulose Applications in Wood Adhesives—Review. Open J. Polym. Chem. 2019, 9, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Kajtna, J.; Šebenik, U. Novel acrylic/nanocellulose microsphere with improved adhesive properties. Int. J. Adhes. Adhes. 2017, 74, 100–106. [Google Scholar] [CrossRef]
- Dastjerdi, Z.; Cranston, E.D.; Dubé, M.A. Pressure sensitive adhesive property modification using cellulose nanocrystals. Int. J. Adhes. Adhes. 2018, 81, 36–42. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose Processing Properties and Potential Applications. Curr. For. Rep. 2019, 5, 76–89. [Google Scholar] [CrossRef]
- Lengowski, E.C.; Júnior, E.A.B.; Kumode, M.M.N.; Carneiro, M.E.; Satyanarayana, K.G. Nanocellulose-Reinforced Adhesives for Wood-Based Panels. In Sustainable Polymer Composites and Nanocomposites; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 1001–1025. [Google Scholar] [CrossRef]
- Hamed, S.A.; Hassan, M.L. A new mixture of hydroxypropyl cellulose and nanocellulose for wood consolidation. J. Cult. Herit. 2019, 35, 140–144. [Google Scholar] [CrossRef]
- Almeida, T.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Bacterial Nanocellulose toward Green Cosmetics: Recent Progresses and Challenges. Int. J. Mol. Sci. 2021, 22, 2836. [Google Scholar] [CrossRef]
- De Amorim, J.D.P.; de Souza, K.C.; Duarte, C.R.; da Silva Duarte, I.; de Assis Sales Ribeiro, F.; Silva, G.S.; de Farias, P.M.A.; Stingl, A.; Costa, A.F.S.; Vinhas, G.M.; et al. Plant and bacterial nanocellulose: Production, properties and applications in medicine, food, cosmetics, electronics and engineering. A review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Stasiak-Różańska, L.; Płoska, J. Study on the Use of Microbial Cellulose as a Biocarrier for 1,3-Dihydroxy-2-Propanone and Its Potential Application in Industry. Polymers 2018, 10, 438. [Google Scholar] [CrossRef] [Green Version]
- Blanco, A.; Monte, M.C.; Campano, C.; Balea, A.; Merayo, N.; Negro, C. Chapter 5. Nanocellulose for Industrial Use: Cellulose Nanofibers (CNF), Cellulose Nanocrystals (CNC), and Bacterial Cellulose (BC). In Handbook of Nanomaterials for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 74–126. [Google Scholar] [CrossRef]
- Reshmy, R.; Philip, E.; Paul, S.A.; Madhavan, A.; Sindhu, R.; Binod, P.; Pandey, A.; Sirohi, R. Nanocellulose-based products for sustainable applications-recent trends and possibilities. Rev. Environ. Sci. Bio/Technol. 2020, 19, 779–806. [Google Scholar] [CrossRef]
- Omran, A.A.B.; Mohammed, A.A.B.A.; Sapuan, S.M.; Ilyas, R.A.; Asyraf, M.R.M.; Koloor, S.S.R.; Petrů, M. Micro- and Nanocellulose in Polymer Composite Materials: A Review. Polymers 2021, 13, 231. [Google Scholar] [CrossRef] [PubMed]
- Feczkó, T.; Kovács, M.; Voncina, B. Improvement of fatigue resistance of spirooxazine in ethyl cellulose and poly(methyl methacrylate) nanoparticles using a hindered amine light stabilizer. J. Photochem. Photobiol. A Chem. 2012, 247, 1–7. [Google Scholar] [CrossRef]
- Ho, N.A.D.; Leo, C. A review on the emerging applications of cellulose, cellulose derivatives and nanocellulose in carbon capture. Environ. Res. 2021, 197, 111100. [Google Scholar] [CrossRef]
- Li, Y.; Jia, P.; Xu, J.; Wu, Y.; Jiang, H.; Li, Z. The aminosilane functionalization of cellulose nanofibrils and the mechanical and CO2 adsorption characteristics of their aerogel. Ind. Eng. Chem. Res. 2020, 59, 2874–2882. [Google Scholar] [CrossRef]
- Wei, J.; Geng, S.; Hedlund, J.; Oksman, K. Lightweight, flexible, and multifunctional anisotropic nanocellulose-based aerogels for CO2 adsorption. Cellulose 2020, 27, 2695–2707. [Google Scholar] [CrossRef] [Green Version]

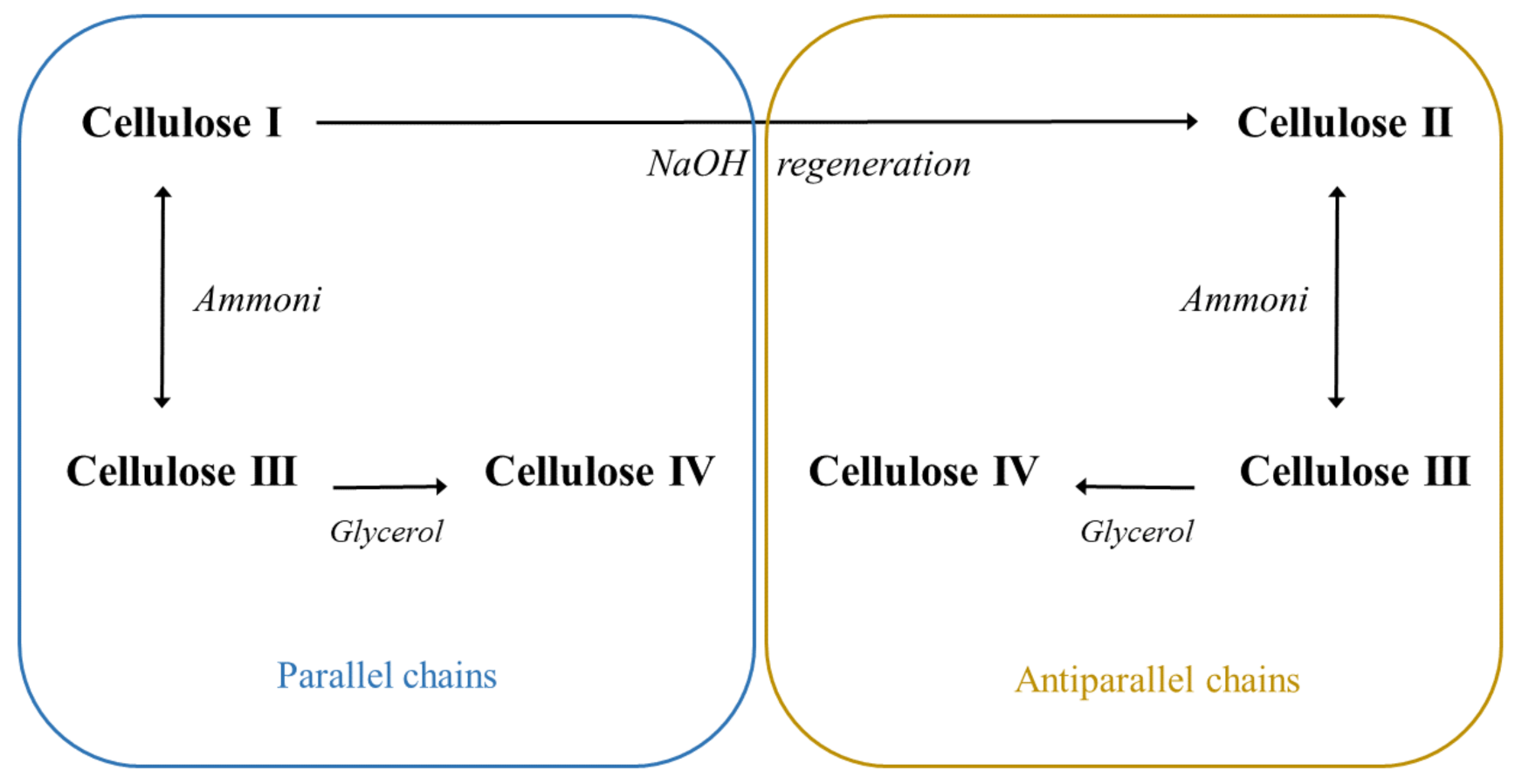
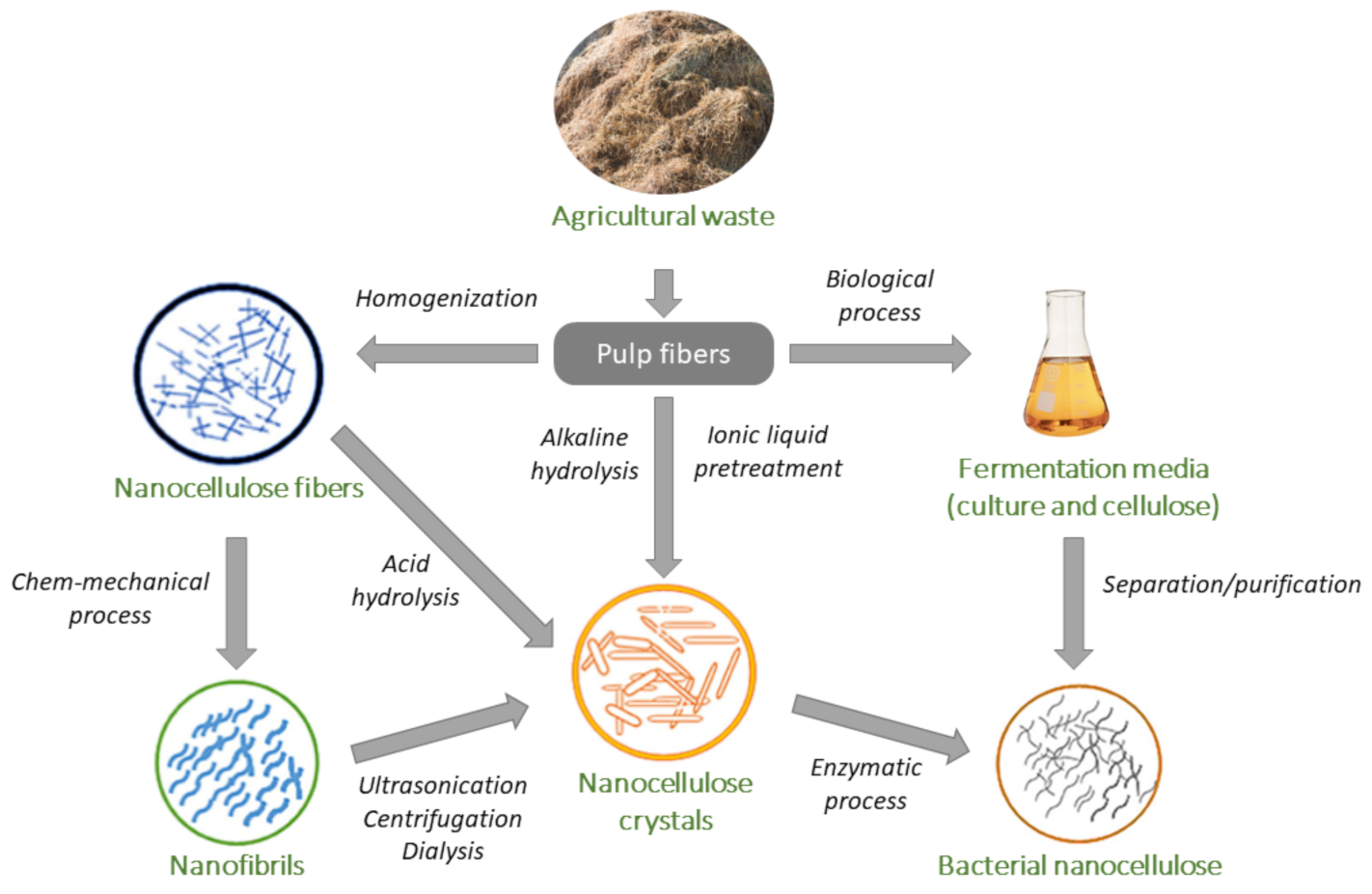
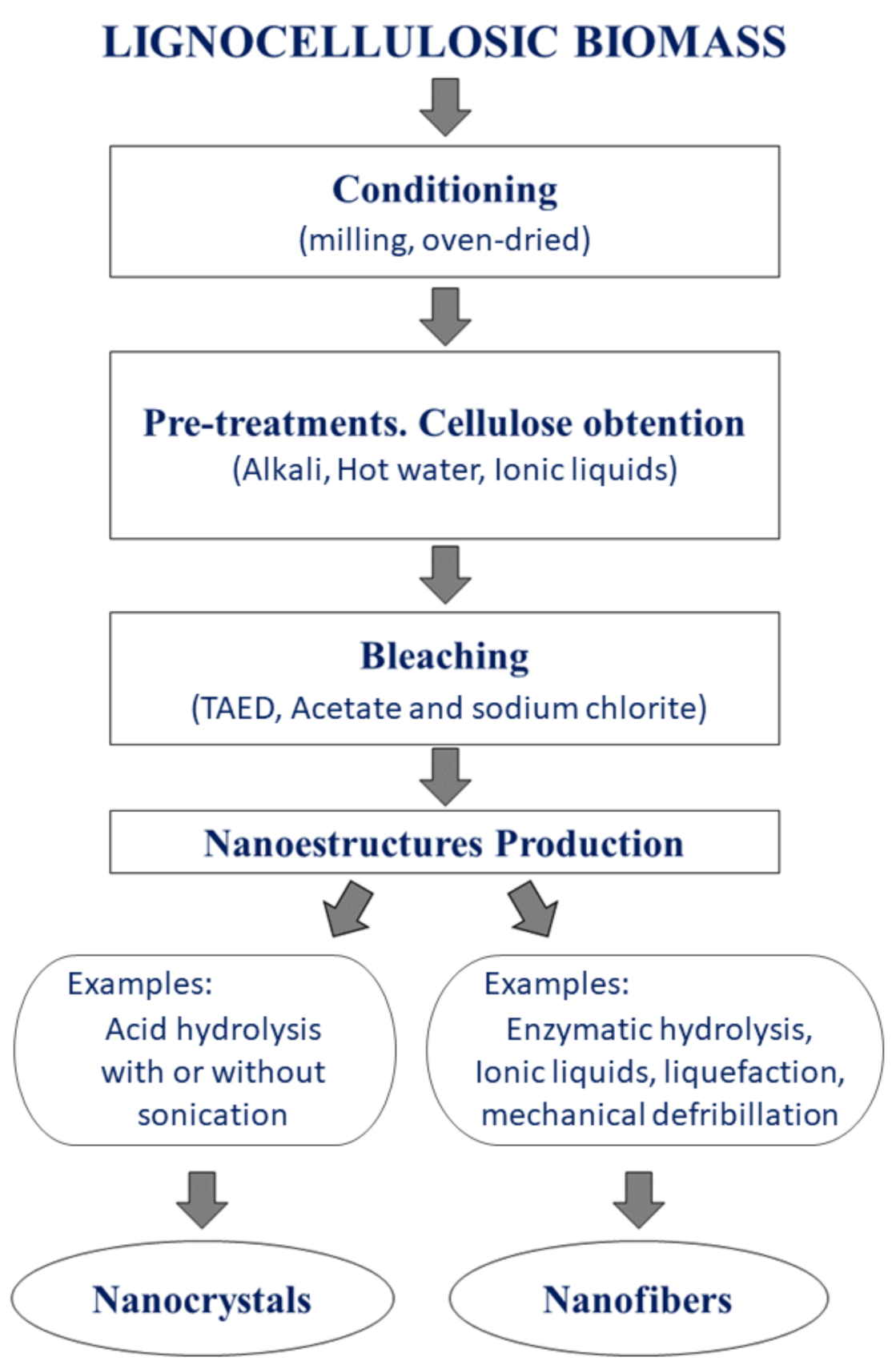

| Nanocellulose Product | Diameter, nm | Length, nm |
|---|---|---|
| Cellulose nanofibers (CNF) Microfibril | 2–10 | >10,000 |
| Cellulose nanocrystals (CNC) | 2–20 | 100–600 |
| Bacterial nanocellulose (BNC) | 4–10 | 100–2000 |
| Cellulose nanofibers (CNF) Microfibrillated | 10–40 | >1000 |
| Microcrytalline cellulose (MCC) | >1000 | >1000 |
| % Cellulose | % Hemicellulose | % Lignin | |
|---|---|---|---|
| Aspen | 53 | 22 | 20 |
| Bamboo | 40 | 20 | 21 |
| Barley straw | 38 | 35 | 16 |
| Bean hulls | 52 | 26 | 10 |
| Coconut husk fiber | 25 | 12 | 40 |
| Corncob | 45 | 35 | 15 |
| Corn husks | 29 | 40 | 11 |
| Corn stover | 38–40 | 24–26 | 7–19 |
| Cotton | 40 | 23 | 23 |
| Jute | 61–71 | 14–20 | 12–13 |
| Mango seeds | 55 | 21 | 24 |
| Olive tree pruning | 30–40 | 24–27 | 18–23 |
| Pineapple leaf fibre | 75 | 13 | 10 |
| Pine | 47 | 20 | 27 |
| Pistachio shells | 54 | 20 | 25 |
| Poplar | 49 | 24 | 20 |
| Raw banana fibre | 70 | 20 | 6 |
| Rice straw | 30–35 | 25–35 | 12–23 |
| Sorghum bagasse | 34–45 | 18–27 | 14–21 |
| Soy hulls | 48 | 24 | 6 |
| Spruce | 43 | 30 | 28 |
| Sugarcane bagasse | 40–50 | 20–24 | 25–30 |
| Switchgrass | 5–20 | 30–50 | 10–40 |
| Sunflower | 37 | 21 | 17 |
| Wheat straw | 30–38 | 21–50 | 15–23 |
| Biomass | Process | Product | Ref. | |
|---|---|---|---|---|
| Pretreatment | Treatment | |||
| Apple | NaOH 10%, 160 min, | Acid hydrolysis 45% HSO, | Cellulose | [19] |
| pomace | 70 °C | 50 °C, 45 min + ultrasonication | nanocrystals | |
| Argan press | NaOH 12% + NaSO 8%, | Purified cellulose | [18] | |
| cake | 90 min, 80 °C | powder | ||
| Bamboo | Microwave liquefaction + NaClO | Cellulose | [68] | |
| 0.1%, 75 °C, 1 h + ultrasonication | nanofibers | |||
| Banana | KOH 5%, 14 h + | Acid hydrolysis HSO 1%, | Cellulose | [69] |
| peels | NaClO 1%, 70 °C, 1 h | 80 °C, 2 h 3 times | nanofibers | |
| Citrulluss | NaOH 1M, 24 h, 70 °C + | Acid hydrolysis HSO 40%, | Cellulose | [70] |
| colocynthis | bleaching with NaClO | 30 min + sonication | nanofibers | |
| Corncob | LHW 190 °C, 30 min, | Ionic liquid 4% BmimCl | Cellulose | [1] |
| + Alkali 2% NaOH, | nanofibers | |||
| 90 min, 90 °C | ||||
| Corn | NaOH 5%, 121 °C + | Acid hydrolysis HSO 64%, | Cellulose | [71] |
| straw | HO 2% + TAED 0.2% | 25 °C, 90 min | whiskers | |
| Dunchi | HO 2% + TAED 0.2% | Acid hydrolysis HSO 64%, | Cellulose | [72] |
| fibers | 25 °C, 60 min | nanofibers | ||
| Oil palm | NaOH 6%, 24 h, 20 °C | Acid hydrolysis HSO 64%, | Cellulose | [73] |
| trunk | 45 °C, 60 min | nanocrystals | ||
| Olive tree | TEMPO-ox + defibrillation | Cellulose | [74] | |
| pruning | high-pressure microfluidizer | nanofibers | ||
| Pine | NaOH 5%, 14 h | Acidified sodium chlorite 6%, | Cellulose | [75] |
| cones | 70 °C, 1 h | nanofibers | ||
| Pineapple | NaOH 2%, 4 h, 100 °C + | Acid hydrolysis HSO 64%, | Cellulose | [76] |
| leaf | bleaching 80 °C, 4 h | 45 °C, 30 min | nanocrystals | |
| Rice | NaOH 4%, 2 h + | Acid hydrolysis HSO 10 M, | Cellulose | [77] |
| husks | bleaching 130 °C, 4 h | 50 °C, 40 min + sonication | nanocrystals | |
| Sisal | NaOH 4%, 80 °C, 2 h + | Enzymatic hydrolysis | Microfibrillated | [78] |
| fibers | bleaching 80 °C, 4 h | (cellulases) | cellulose | |
| Sorghum | NaOH + bleaching | Acid hydrolysis HCl 2.5 N, | Microcrystaline | [79] |
| stalks | with NaClO | 105 °C, 15 min | cellulose | |
| Sunflower | NaOH 5%, 2 h, 98 °C + | Acid hydrolysis HSO 64%, | Cellulose | [80] |
| stalks | sodium chlorite 5% | 45 °C, 30 min | nanofibers | |
| Vine | NaOH 4%, 120 min, 80 °C | Acid hydrolysis HSO 64%, | Cellulose | [65] |
| shoots | + bleaching with acetate | 50 °C, 30 min | nanocrystals | |
| and sodium chlorite | ||||
| Yute | NaOH 5%, 60 min, | Mechanical | Cellulose | [50] |
| fiber | 80 °C + TAED | defibrillation | nanofibers | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateo, S.; Peinado, S.; Morillas-Gutiérrez, F.; La Rubia, M.D.; Moya, A.J. Nanocellulose from Agricultural Wastes: Products and Applications—A Review. Processes 2021, 9, 1594. https://doi.org/10.3390/pr9091594
Mateo S, Peinado S, Morillas-Gutiérrez F, La Rubia MD, Moya AJ. Nanocellulose from Agricultural Wastes: Products and Applications—A Review. Processes. 2021; 9(9):1594. https://doi.org/10.3390/pr9091594
Chicago/Turabian StyleMateo, Soledad, Silvia Peinado, Francisca Morillas-Gutiérrez, M. Dolores La Rubia, and Alberto J. Moya. 2021. "Nanocellulose from Agricultural Wastes: Products and Applications—A Review" Processes 9, no. 9: 1594. https://doi.org/10.3390/pr9091594
APA StyleMateo, S., Peinado, S., Morillas-Gutiérrez, F., La Rubia, M. D., & Moya, A. J. (2021). Nanocellulose from Agricultural Wastes: Products and Applications—A Review. Processes, 9(9), 1594. https://doi.org/10.3390/pr9091594








