Conjugated Mass Transfer of CO2 Absorption through Concentric Circular Gas–Liquid Membrane Contactors
Abstract
:1. Introduction
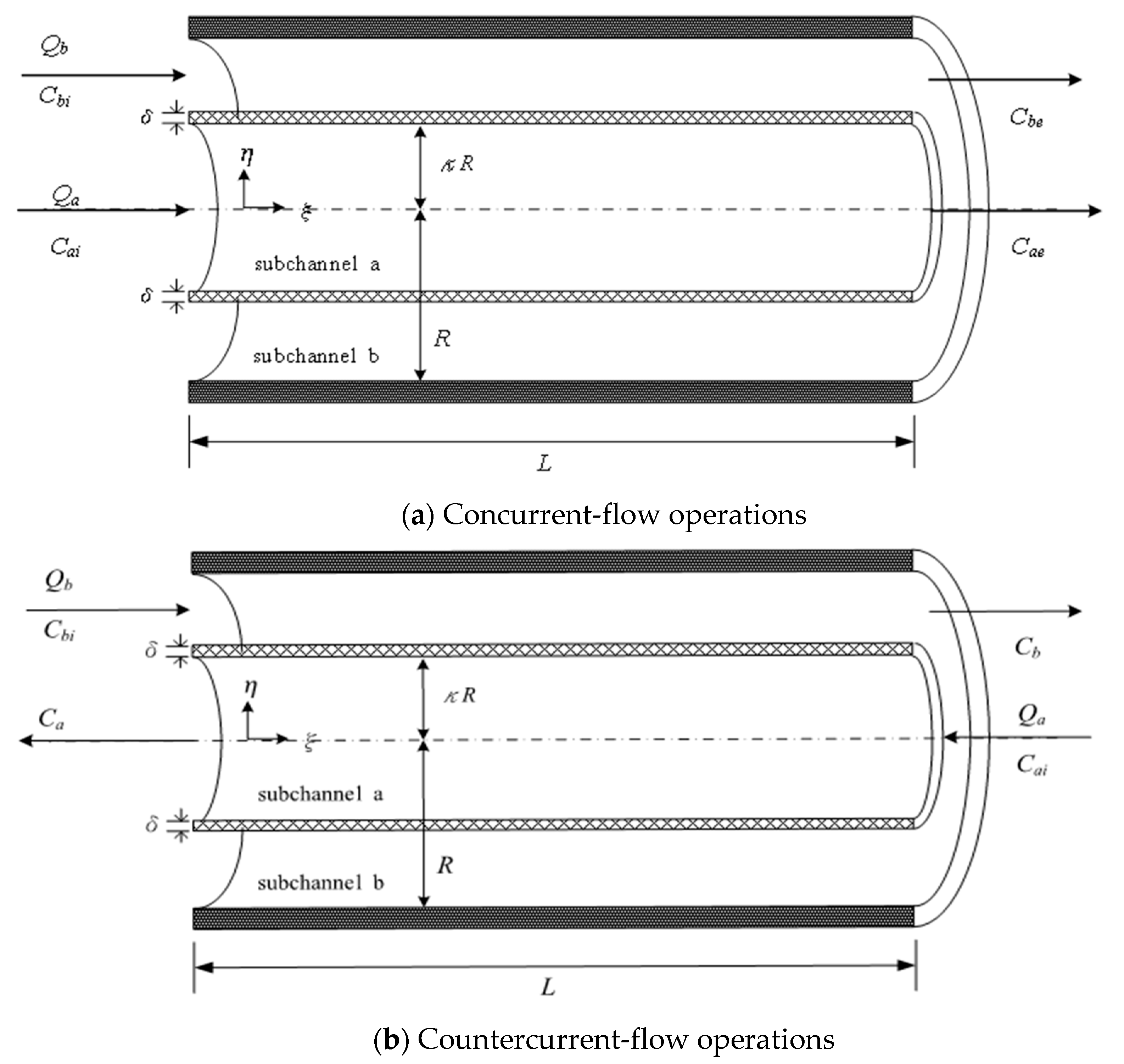
2. Mathematical Formulations
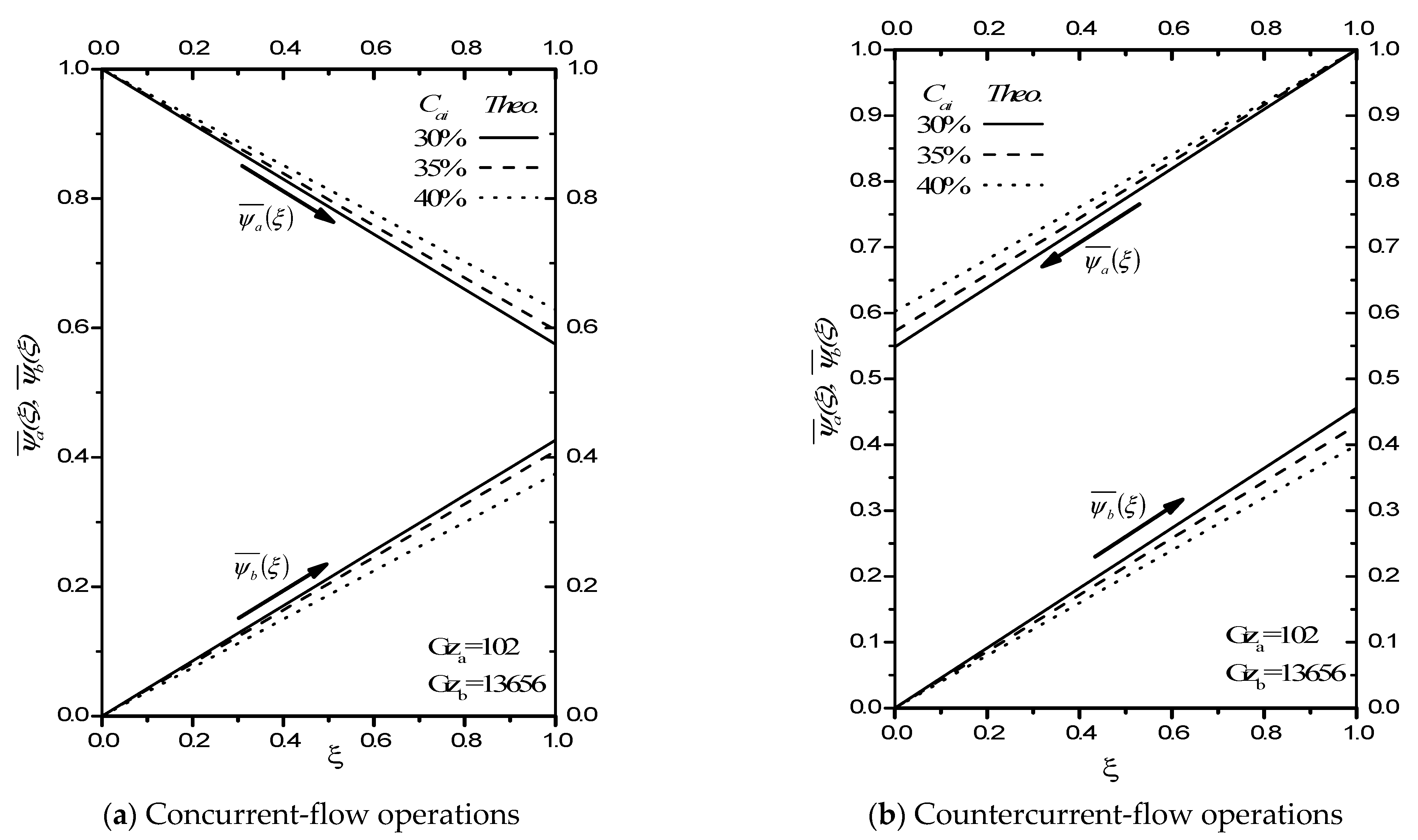
2.1. Concurrent-Flow Operations
2.2. Countercurrent-Flow Operations
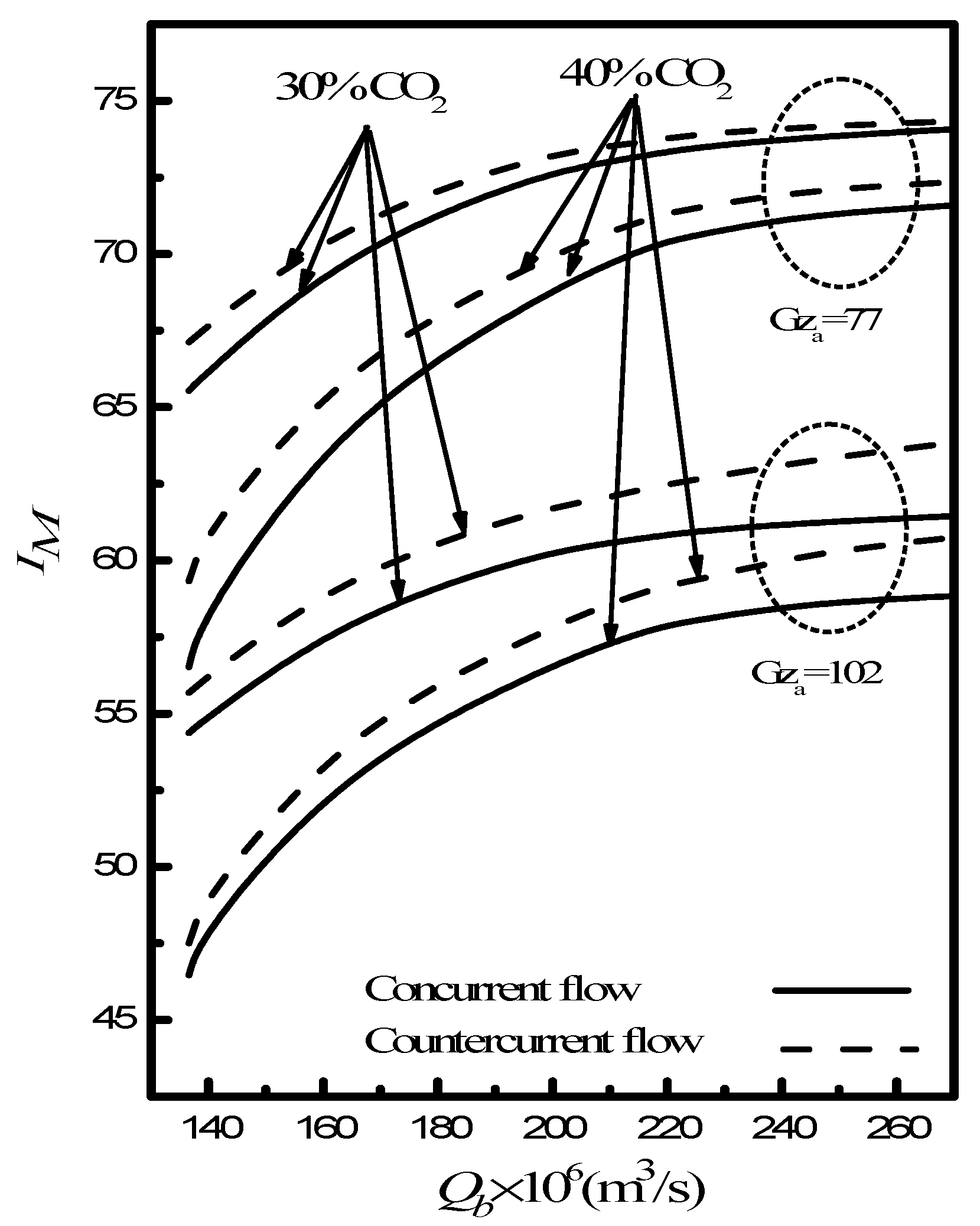
2.3. Absorption Efficiency
3. Experimental Apparatus
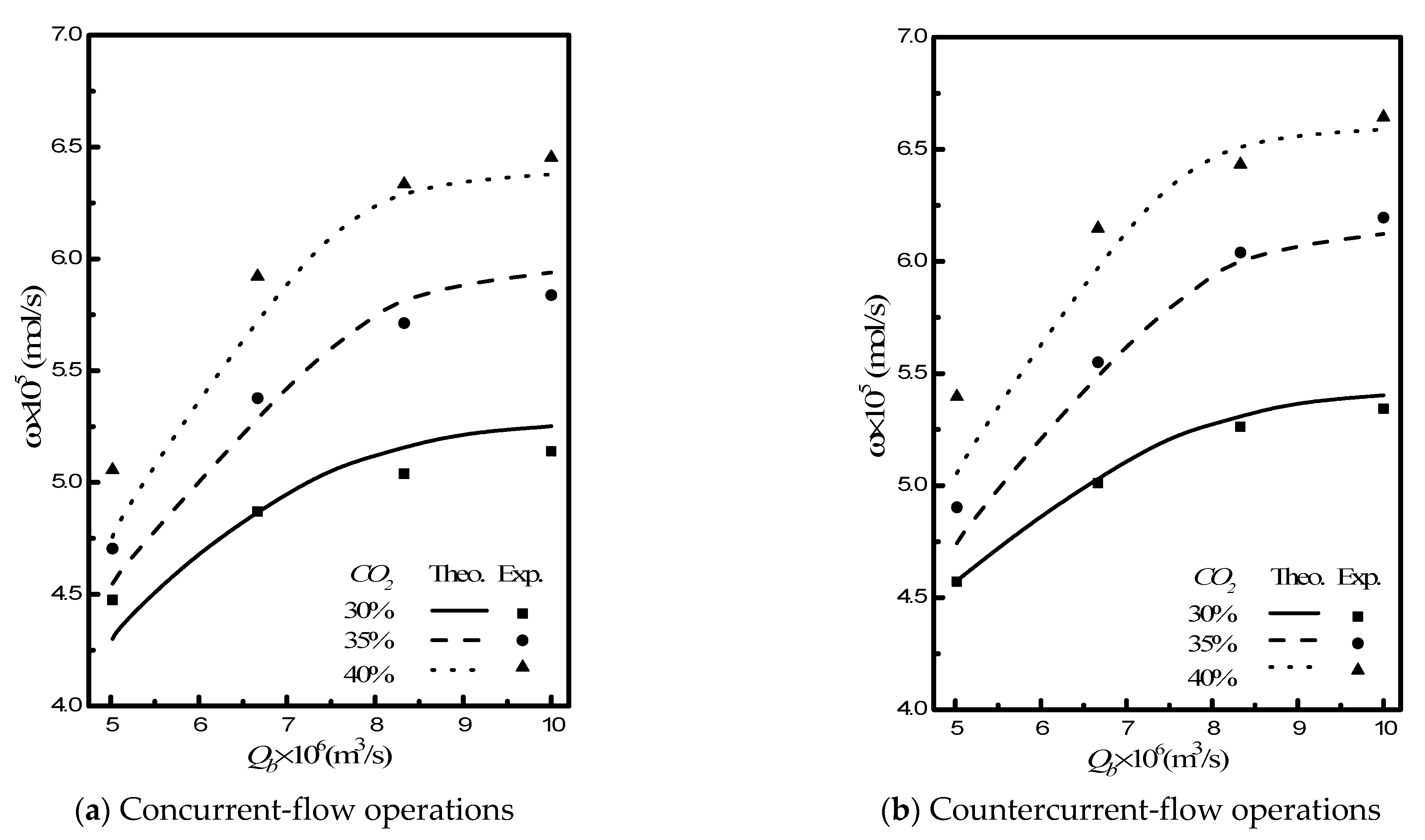
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| B | conduit width, m |
| C | concentration in the stream, mole/m3 |
| Da | ordinary diffusion coefficient of CO2 in N2, m2/s |
| Db | ordinary diffusion coefficient of CO2 in MEA solution, m2/s |
| dmn | coefficient in the eigen-function Fa,m |
| E | the accuracy derivation between theoretical predictions and experimental results |
| emn | coefficient in the eigen-function Fb,m |
| Fm | eigen-function associated with eigenvalue λm |
| Gz | mass-transfer Graetz number |
| H | dimensionless Henry’s law constant |
| Kex | equilibrium constant |
| K′ex | reduced equilibrium constant |
| kξ | local mass transfer coefficient of CO2, m/s |
| IM | absorption efficiency |
| L | conduit length, m |
| Nexp | the number of experimental measurements |
| Q | volumetric flow rate of conduit, m3/s |
| Sm | coefficient in the eigenfunction |
| Shξ | local Sherwood number |
| averaged Sherwood number | |
| R | inside radius of the shell, m |
| r | axial coordinate, m |
| v | velocity distribution of fluid, m/s |
| average velocity of fluid, m/s | |
| z | longitudinal coordinate, m |
| Greek letters | |
| κ | ratio of channel thickness |
| δ | thickness of the membrane, m |
| ε | porosity of the membrane |
| η | dimensionless transversal coordinate, x/W |
| λm | eigenvalue |
| ξ | longitudinal coordinate |
| ω | absorption rate, mol/s |
| Ψ | dimensionless concentration |
| Subscripts | |
| a | in the gas feed flow channel |
| b | in the liquid absorbent flow channel |
| i | at the inlet |
| e | at the outlet |
References
- Gabelman, A.; Hwang, S.T. Hollow fiber membrane contactors. J. Membr. Sci. 1999, 159, 61–106. [Google Scholar] [CrossRef]
- Li, K.; Teo, W.K. Use of permeation and absorption methods for CO2 removal in hollow fiber membrane modules. Sep. Purif. Technol. 1998, 13, 79–88. [Google Scholar] [CrossRef]
- Karoor, S.; Sirkar, K.K. Gas absorption studies in microporous hollow fiber membrane modules. Ind. Eng. Chem. Res. 1993, 32, 674–684. [Google Scholar] [CrossRef]
- Kim, Y.S.; Yang, S.M. Absorption of carbon dioxide through hollow fiber membranes using various aqueous absorbents. Sep. Purif. Technol. 2000, 21, 101–109. [Google Scholar] [CrossRef]
- Oyenekan, B.A.; Rochelle, G.T. Alternative stripper configurations for CO2 capture by aqueous amines. AIChE J. 2007, 53, 3144–3154. [Google Scholar] [CrossRef]
- Lee, Y.; Noble, R.D.; Yeom, B.Y.; Park, Y.I.; Lee, K.H. Analysis of CO2 removal by hollow fiber membrane contactors. J. Membr. Sci. 2001, 194, 57–67. [Google Scholar] [CrossRef]
- Dindore, V.Y.; Brilman, D.W.F.; Feron, P.H.M.; Versteeg, G.F. CO2 absorption at elevated pressures using a hollow fiber membrane contactor. J. Membr. Sci. 2004, 235, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Feron, P.H.M.; Jansen, A.E. CO2 separation with polyolefin membrane contactors and dedicated absorption liquids: Performance and prospects. Sep. Purif. Technol. 2002, 27, 231–242. [Google Scholar] [CrossRef]
- Li, J.L.; Chen, B.H. Review of CO2 absorption using chemical solvents in hollow fiber membrane contactors. Sep. Purif. Technol. 2005, 41, 109–122. [Google Scholar] [CrossRef]
- Rangwala, H.A. Absorption of carbon dioxide into aqueous solutions using hollow fiber membrane contactors. J. Membr. Sci. 1996, 112, 229–240. [Google Scholar] [CrossRef]
- Koo, S.; Sangani, A.S. Mass transfer coefficients for laminar longitudinal flow in hollow-fibre contactors. J. Fluid Mech. 2003, 484, 255–282. [Google Scholar] [CrossRef] [Green Version]
- Dindore, V.Y.; Brilman, D.W.F.; Versteeg, G.F. Modeling of cross-flow membrane contactors: Physical mass transfer processes. J. Membr. Sci. 2005, 251, 209–222. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.P.; Lin, H.T.; Ho, C.D. An analytical study of laminar co-current flow gas absorption through a parallel-plate gas-liquid membrane contactor. J. Membr. Sci. 2006, 278, 181–185. [Google Scholar] [CrossRef]
- Nunge, R.J.; Gill, W.N. Analysis of heat transfer in some countercurrent flows. Int. J. Heat Mass Transfer 1965, 8, 873–886. [Google Scholar] [CrossRef]
- Ho, C.D.; Yeh, H.M.; Chiang, S.C. A study of mass transfer efficiency in a parallel-plate channel with external refluxes. Chem. Eng. J. 2002, 85, 253–262. [Google Scholar] [CrossRef]
- Zheng, Q.; Dong, L.; Chen, J.; Gao, G.; Fei, W. Absorption solubility calculation and process simulation for CO2 capture. CIESC J. 2010, 61, 1740–1744. [Google Scholar]
- Li, W.B.; Chen, J. Kinetics of absorption of CO2 into aqueous MEA solutions. Sciencepaper 2009, 4, 0849–0856. [Google Scholar]
- Ho, C.D.; Chen, L.; Chen, L.; Liou, J.W.; Jen, L.Y. Theoretical and experimental studies of CO2 absorption by the amine solvent system in parallel-plate membrane contactors. Sep. Purif. Technol. 2018, 198, 128–136. [Google Scholar] [CrossRef]
- Nunge, R.J.; Gill, W.N. An analytical study of laminar counterflow double-pipe heat exchangers. AIChE J. 1996, 12, 279–289. [Google Scholar] [CrossRef]
- Ho, C.D. Improvement in performance of double-flow laminar countercurrent mass exchangers. J. Chem. Eng. Jpn. 2000, 33, 545–551. [Google Scholar] [CrossRef]
- Moffat, R.J. Describing the uncertainties in experimental results. Exp. Thermal Fluid Sci. 1988, 1, 3–17. [Google Scholar] [CrossRef] [Green Version]






| m | λ0 | λ1 | λ2 | λ3 | λ4 | λ5 | S a,0 | S a,1× 104 | S a,2× 104 | S a,3× 105 | S a,4× 105 | S a,5× 106 | |
| Concurrent-flow operations | |||||||||||||
| 3 | 0.0 | −0.027 | −1.316 | −3.158 | - | - | 0.087 | 7.99 | −3.86 | 8.82 | - | - | 0.5624 |
| 4 | 0.0 | −0.027 | −1.316 | −3.158 | −6.687 | - | 0.083 | 7.57 | −3.41 | 4.42 | −3.62 | - | 0.5791 |
| 5 | 0.0 | −0.027 | −1.316 | −3.158 | −6.687 | −7.609 | 0.084 | 7.86 | −3.19 | 4.63 | −3.76 | 4.30 | 0.5791 |
| Countercurrent-flow operations | |||||||||||||
| 3 | 0.0 | −0.011 | −1.487 | −3.158 | - | - | 0.077 | 7.53 | −1.37 | 8.63 | - | - | 0.5284 |
| 4 | 0.0 | −0.011 | −1.487 | −3.158 | 0.022 | - | 0.074 | 6.69 | −1.21 | 4.52 | −3.53 | - | 0.5532 |
| 5 | 0.0 | −0.011 | −1.487 | −3.158 | 0.022 | 3.621 | 0.074 | 6.41 | −1.71 | 4.51 | −3.61 | 4.43 | 0.5532 |
| Fixed Parameters | Valuable Parameters | ||
|---|---|---|---|
| Outer diameter of shell (mm) | 28 | MEA absorbent flow rates (cm3/s) | 5.0~10.0 |
| Inner diameter of shell (mm) | 17 | CO2/N2 gas flow rate (cm3/s) | 5.0 cm |
| Effective length of tube (cm) | 20 | Inlet CO2 concentrations (%) | 30, 35, 40 |
| Outer diameter of tube (mm) | 15 | MEA solution (wt %) | 30 |
| Membrane pore size (μm) | 0.2 | Henry’s law constant H | 0.73 |
| Membrane porosity | 0.72 | Diffusion coefficient Da (cm2/s) | 1.67 × 10−3 |
| Membrane thickness (μm) | 98 (PTFE) | Diffusion coefficient Db (cm2/s) | 5.0 × 10−5 |
| 32 (PP) | Equilibrium constant Kex(298 K) | 1.25 × 10−5 | |
| Cai (%) | Concurrent Flow | Countercurrent Flow |
|---|---|---|
| E (%) | E (%) | |
| 30 | 6.63 | 7.74 |
| 40 | 6.89 | 7.71 |
| 45 | 6.92 | 6.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, C.-D.; Chang, H.; Chen, Y.-H.; Lim, J.-W.; Liou, J.-W. Conjugated Mass Transfer of CO2 Absorption through Concentric Circular Gas–Liquid Membrane Contactors. Processes 2021, 9, 1580. https://doi.org/10.3390/pr9091580
Ho C-D, Chang H, Chen Y-H, Lim J-W, Liou J-W. Conjugated Mass Transfer of CO2 Absorption through Concentric Circular Gas–Liquid Membrane Contactors. Processes. 2021; 9(9):1580. https://doi.org/10.3390/pr9091580
Chicago/Turabian StyleHo, Chii-Dong, Hsuan Chang, Yih-Hang Chen, Jun-Wei Lim, and Jing-Wei Liou. 2021. "Conjugated Mass Transfer of CO2 Absorption through Concentric Circular Gas–Liquid Membrane Contactors" Processes 9, no. 9: 1580. https://doi.org/10.3390/pr9091580
APA StyleHo, C.-D., Chang, H., Chen, Y.-H., Lim, J.-W., & Liou, J.-W. (2021). Conjugated Mass Transfer of CO2 Absorption through Concentric Circular Gas–Liquid Membrane Contactors. Processes, 9(9), 1580. https://doi.org/10.3390/pr9091580









