Continuous Cooling Crystallization in a Coiled Flow Inverter Crystallizer Technology—Design, Characterization, and Hurdles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substance Systems
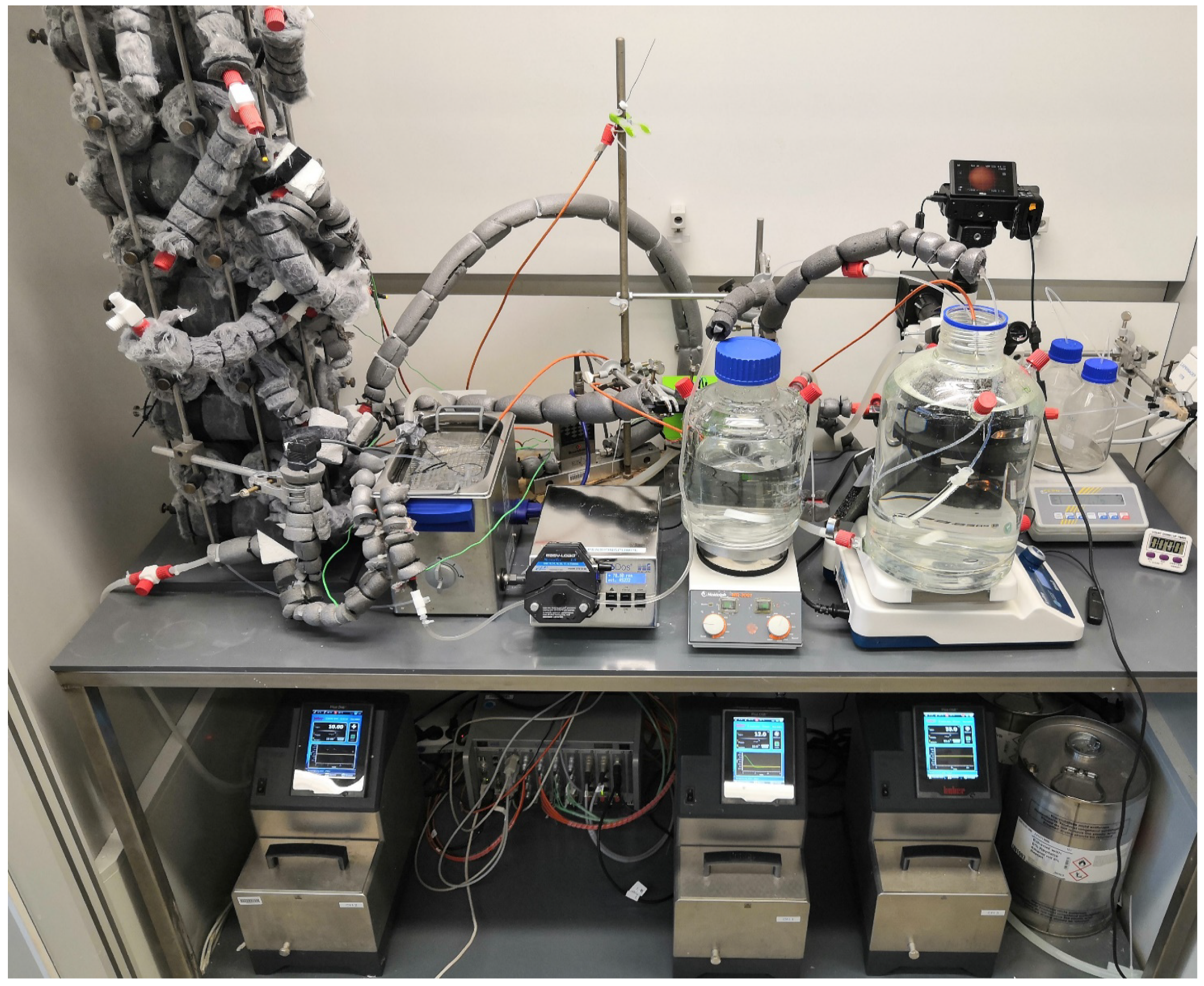
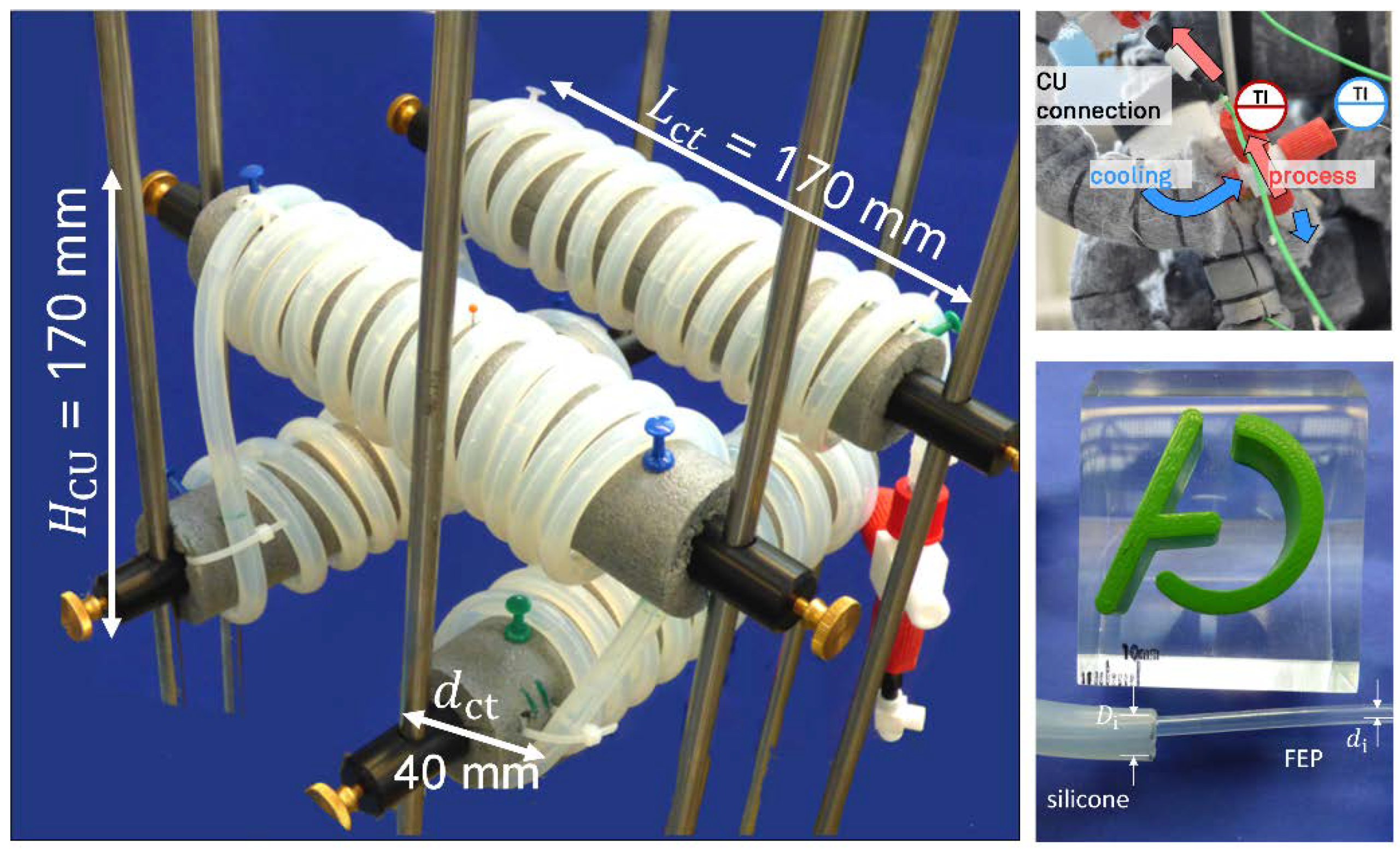
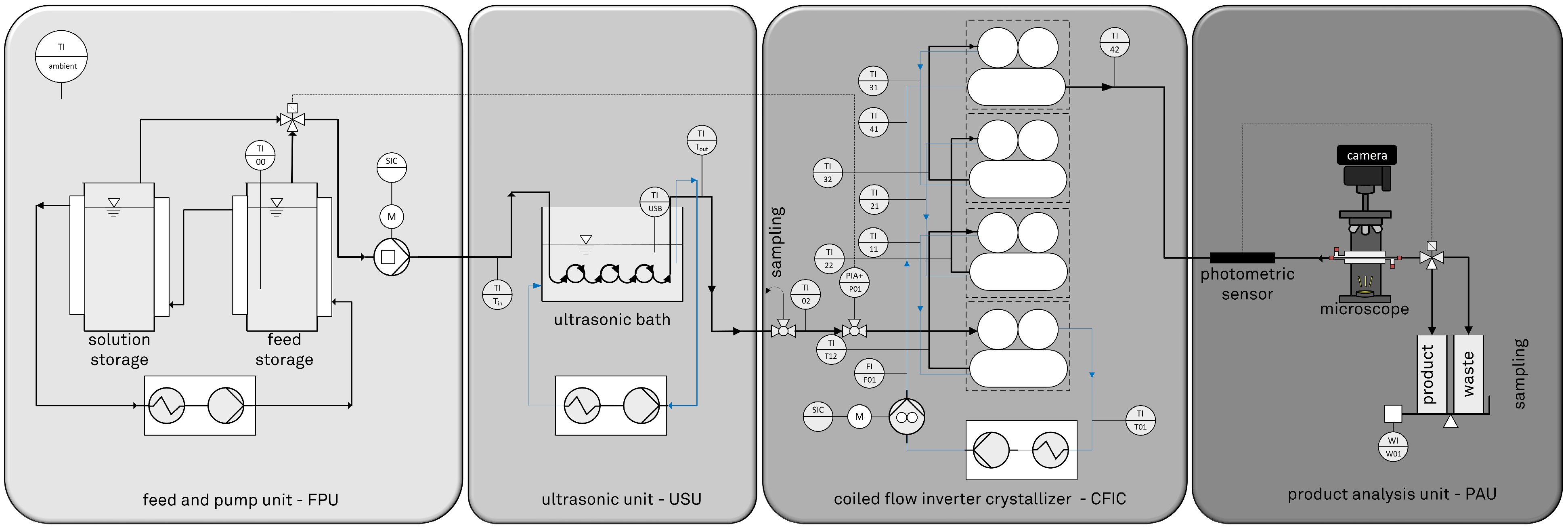
2.2. Design, Setup, and Process Parameters
2.3. Analytics
2.4. Experimental Procedure
2.4.1. Residence Time Distribution for the CFIC
2.4.2. Crystal Growth Experiments in the CFIC
2.4.3. Anti-Plugging Strategies
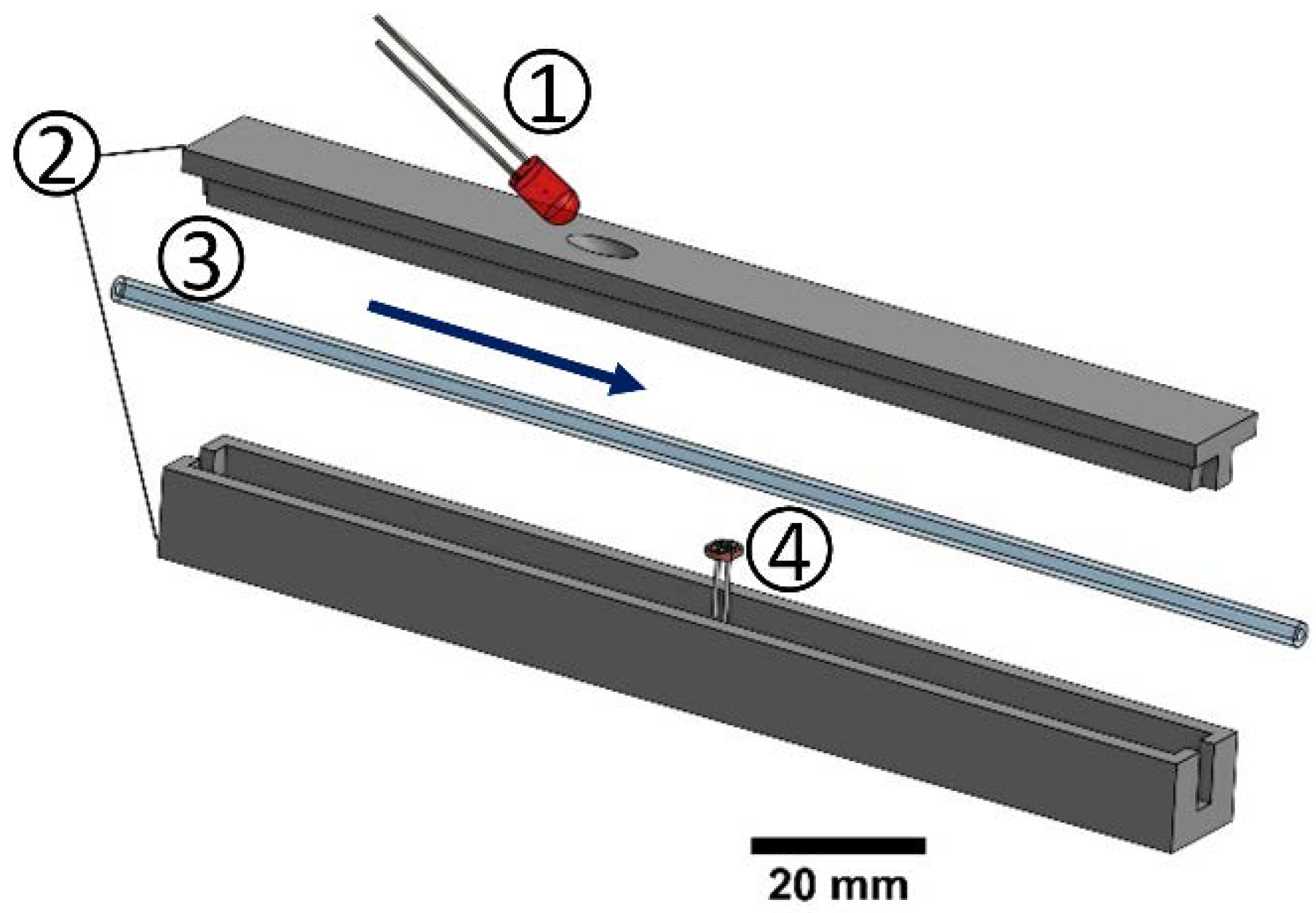
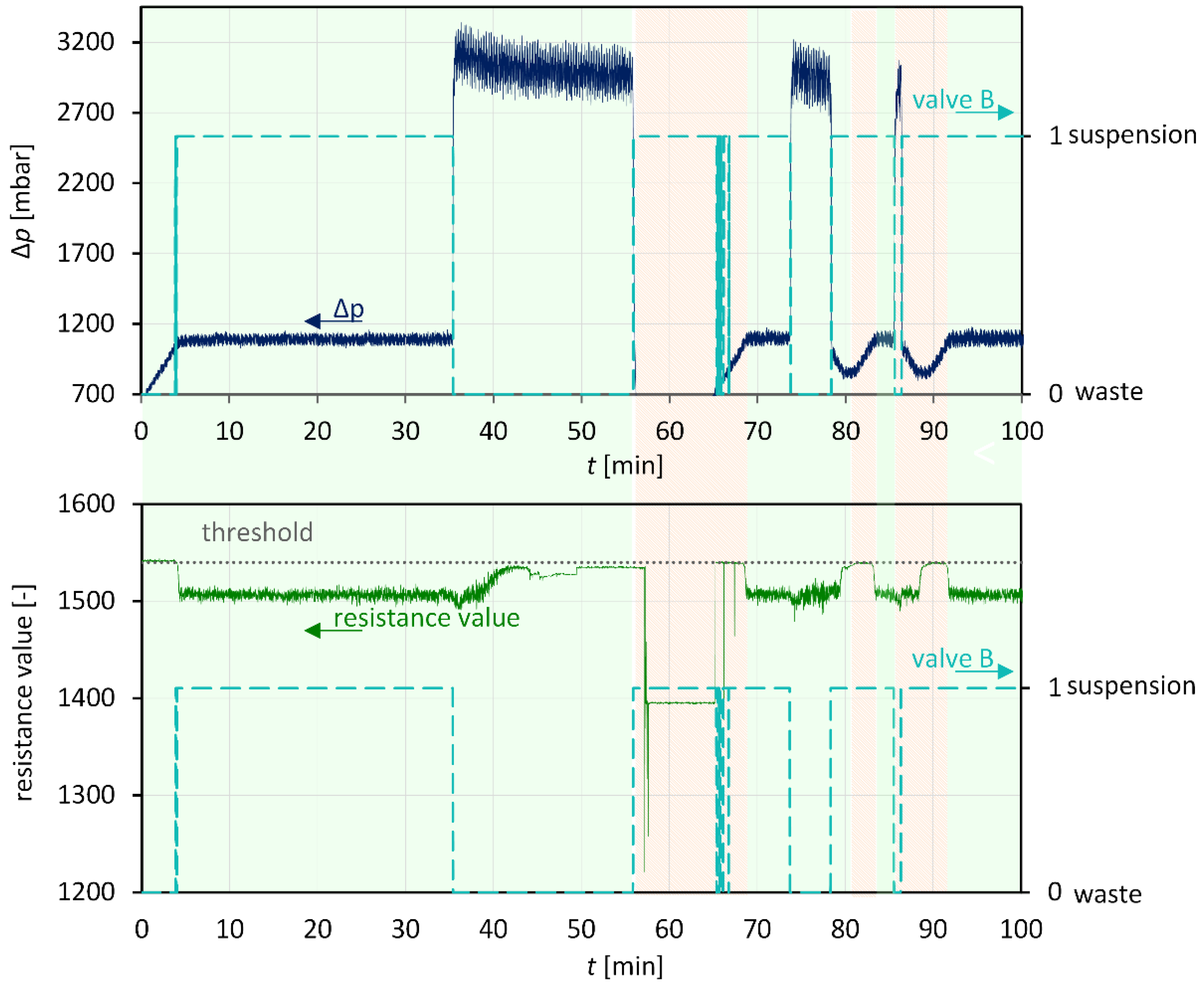
2.4.4. Suspension Detection with Inline Sensor
2.4.5. Combination: Nucleation and Crystal Growth
3. Results
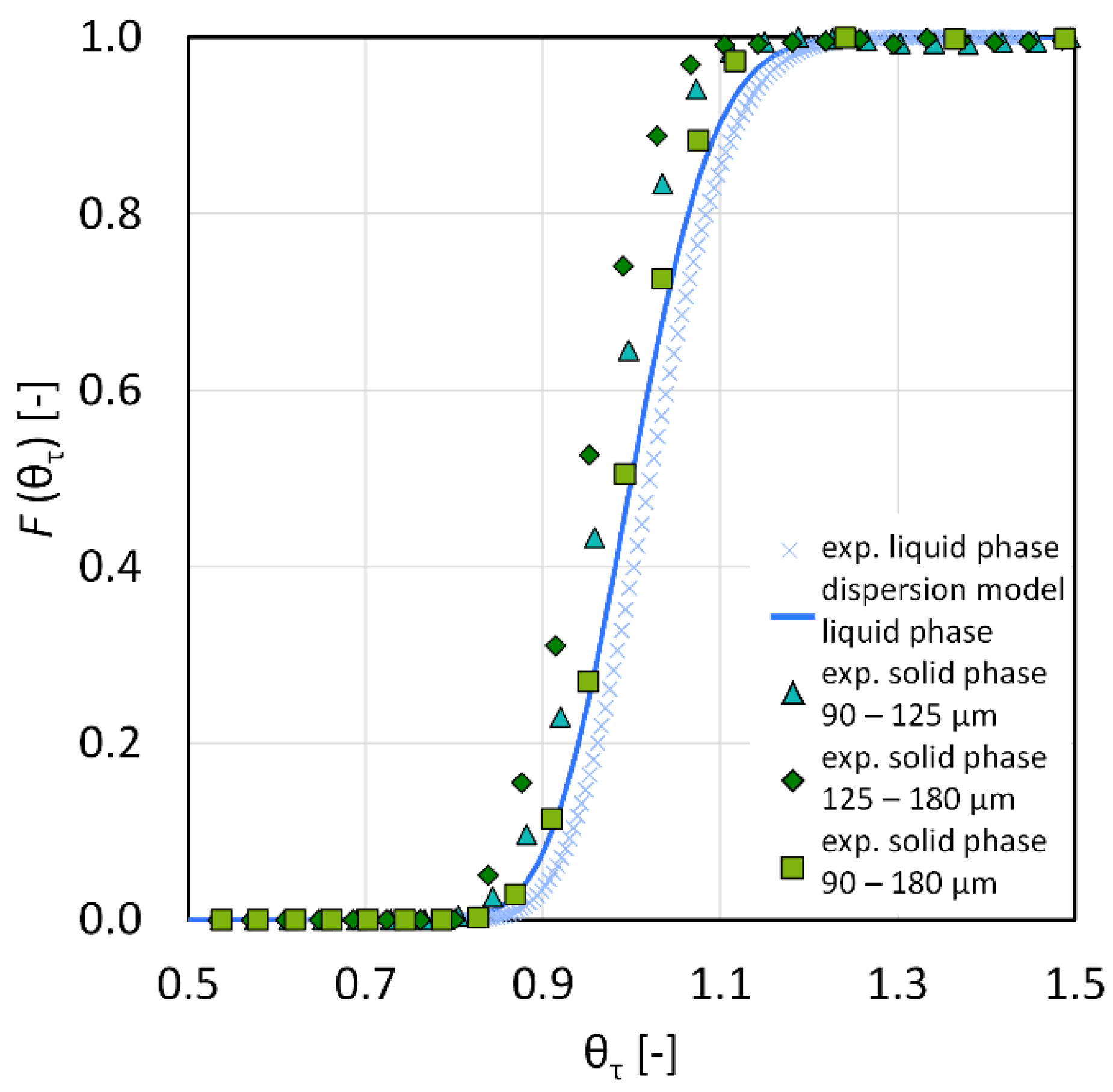
3.1. Residence Time Distribution
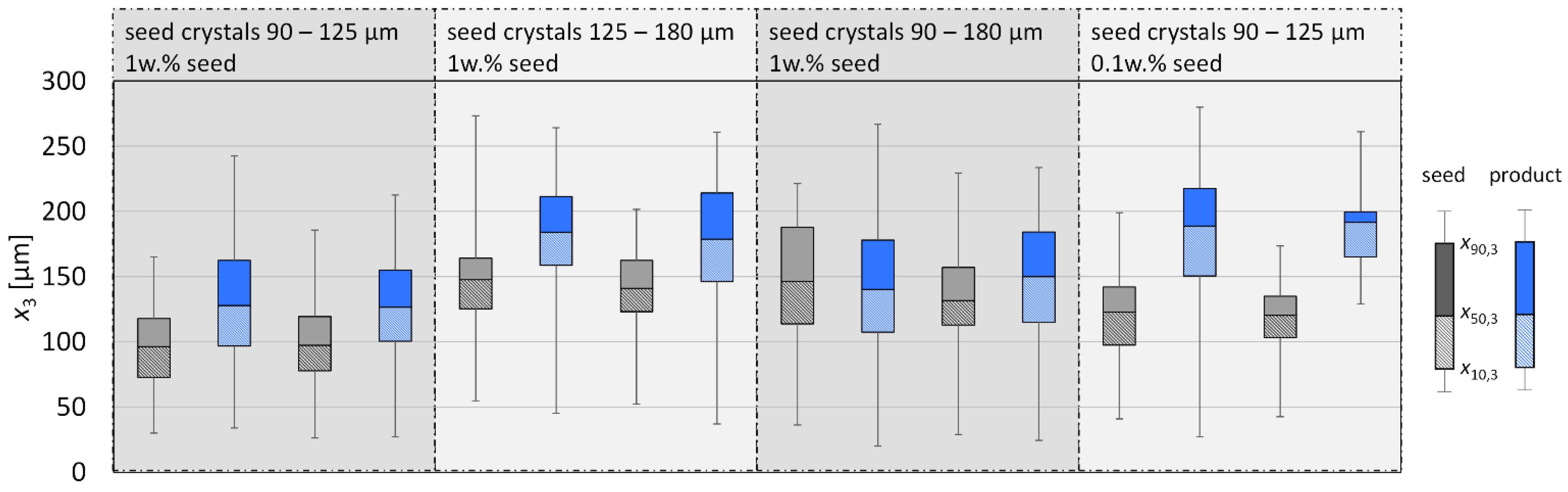
3.2. Crystal Growth Experiments
3.2.1. l-Alanine
3.2.2. Glycine
- Feasibility of another substance system shall be shown;
- A higher solid amount should be reached due to the higher dependency of solubility of glycine in water than l-alanine in water;
- Its structure is similar to l-alanine.
3.3. Anti-Plugging Strategies and Suspension Detection
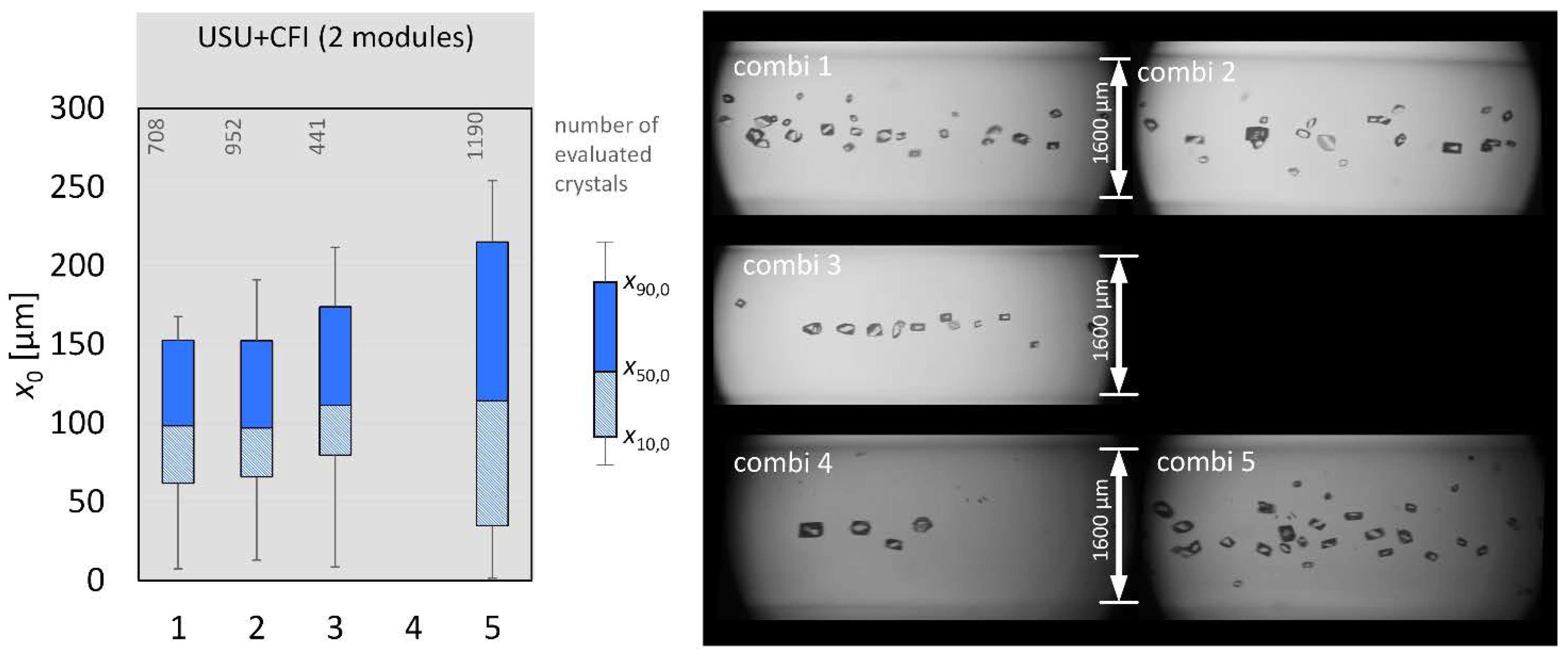
3.4. Combination: Nucleation and Crystal Growth
4. Discussion
5. Conclusions
- Residence time distribution (RTD) for different phases and particle sizes;
- Crystal growth for different sieved seed particle sizes, seed amount, and two different amino acids;
- For combined nucleation in an ultrasonic bath and crystal growth units in continuous flow.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Symbols | Dimensionless Numbers | ||
| coefficients | Bodenstein number | ||
| heat capacity | Frd,crit | critical densimetric Froude number according to a empirical correlation | |
| inner tube diameter | densimetric Froude number | ||
| friction factor | Reynolds number | ||
| distribution sum curve | Indices | ||
| gravitational constant | * | saturated (equilibrated) | |
| mean growth rate | ambient | ||
| heat transfer coefficient | 0,tracer | tracer concentration | |
| length | l-alanine | ||
| mass flow rate | cooling agent | ||
| pressure loss | (time) until crystals are visible | ||
| crystal size distribution sum curve | experimental (time) | ||
| gravimetrically determined saturation | glycine | ||
| time | liquid phase | ||
| actual residence time | logartithmic normal size distribution | ||
| temperature | process | ||
| mean temperature | product crystal | ||
| mean flow velocity | relative | ||
| volume | suspension | ||
| volume flow rate | solution | ||
| mass fraction | solvent | ||
| mean mass fraction | Abbreviations | ||
| particle mass fraction | CFI | coiled flow inverter | |
| particle size | CFIC | coiled flow inverter crystallizer | |
| mass loading | CG | crystal growth | |
| yield | combi | combined experiments with nucleation and crystal growth | |
| mean yield | CSD | crystal size distribution | |
| Greek Symbols | CU | crystallization unit | |
| difference | DTB | Draft Tube Baffle | |
| dynamic viscosity | erf | error function | |
| dimensionless time | FEA | Functional Equipment Assemblies | |
| mean dimensionless time | FEP | fluorinated ethylene propylene | |
| density | MSMPR | Multistage Continuous Mixed-Suspension, Mixed-Product Removal | |
| density liquid | MZW | meta stable zone width | |
| density difference between solid and liquid phase | RTD | residence time distribution | |
| standard deviation | SFC | slug flow crystallizer | |
| hydraulic residence time | |||
Appendix A
| l-alanine [42] | −1.4024∙10−2 | 3.2451∙100 | −6.0342∙10−4 | 3.2874∙10−1 |
| glycine | 7.8260∙10−3 | 1.6994∙100 | −8.6319∙10−4 | 4.3729∙10−1 |

| Equipment | Model | Manufacturer | Country | Name (in the Flowchart) |
|---|---|---|---|---|
| peristaltic pump | LabDos® Easy-Load | HiTec Zang GmbH | Germany | - |
| peristaltic pump head | Masterflex L7s Easy-Load® | Cole-Parmer GmbH | US | - |
| gear pump | ISMATEX REGLO-Z Digital | Cole-Parmer GmbH | US | - |
| gear pump head | ISMATEC GA-X21.CFS.C | Cole-Parmer GmbH | US | - |
| flow control | mini CORI-FLOW™ | Bronkhorst High-Tech B.V. | Netherlands | FI_F01 |
| scale | Kern 572 | KERN & SOHN GmbH | Germany | WI_W01 |
| process control system | LabManager® | HiTec Zang GmbH | Germany | - |
| resistance thermometers | RM-Typ WL-1,5-1Pt-B-100-2 | RÖSSEL-Messtechnik GmbH | Germany | TI_ambient, TI_00, TI_USB, TI_01-41 |
| thermocouple | type-K | OMEGA Engineering | US | TI_TIin, TI_TIout, TI_02-42 |
| cryostat (1–3) | Pilot ONE ministat 125 | Peter Huber Kältemaschinenbau AG | Germany | - |
| magnetic valve | Typ 6626–TwinPower | Bürkert GmbH & Co. KG | Germany | magnetic valve A and B |
| pressure sensor | A-10 | WIKA Alexander Wiegand SE & Co. KG | Germany | PIA+_P01 |
| stirrer plate | MR 3001 | Heidolph Instruments GmbH & Co. KG | Germany | - |
| microscope | Bresser Science ADL 601P | Bresser GmbH | Germany | - |
| camera | Z6 | Nikon GmbH | Japan | - |
| separation analyzer | LUMiReader® PSA 453 | LUM GmbH | Germany | - |
| scale | MS1003S/01 | Mettler-Toledo GmbH | US | - |
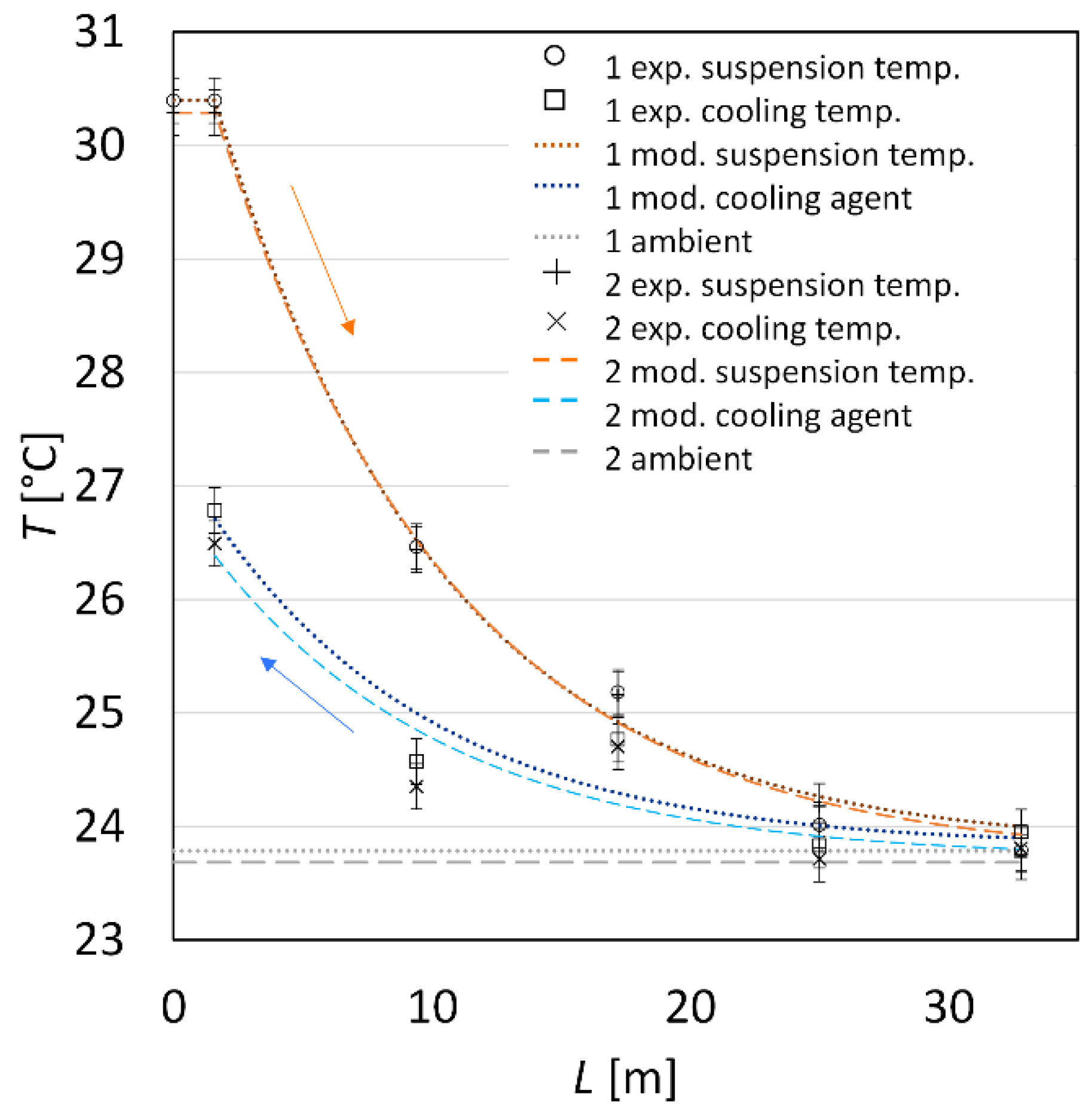
| Sample (-) | (g·min−1) | (g·min−1) | (°C) | (°C) | (°C) |
|---|---|---|---|---|---|
| seed90–125 1 | 18.6 ± 0.4 | 19.5 ± 0.2 | 30.9 | 23.3 | 24.0 |
| seed90–125 2 | 19.8 ± 0.7 | 20.2 ± 0.2 | 30.4 | 23.9 | 22.9 |
| seed125–180 1 | 17.6 ± 0.6 | 20.4 ± 0.1 | 30.4 | 23.8 | 23.8 |
| seed125–180 2 | 17.9 ± 0.5 | 21.5 ± 0.1 | 30.3 | 23.7 | 23.7 |
| seed90–180 1 | 15.6 ± 0.5 | 19.9 ± 0.1 | 30.0 | 23.2 | 25.9 |
| seed90–180 2 | 18.8 ± 0.6 | 21.3 ± 0.1 | 30.5 | 23.9 | 23.6 |
| seed90–125 3 | 15.4 ± 0.3 | 16.3 ± 0.1 | 30.1 | 23.4 | 23.3 |
| seed90–125 4 | 16.3 ± 0.4 | 17.7 ± 0.1 | 30.0 | 23.4 | 23.3 |
| glycine90–125 | 26.0 ± 0.9 | 20.1 ± 0.2 | 30.4 | 25.0 | 23.1 |
| 01 | wait until !PERISTALTICPUMP.ON = 1 {pump needs to be turned on} | |
| 02 | repeat | |
| 03 | T1 | !MAGNETIC_VALVE_A=0 {rinse with solvent(water)} |
| 04 | wait 10 seconds | |
| 05 | s=current_time | |
| 06 | repeat | |
| 07 | if (!PI_00<1250) and (!PI_00>20) and (!PERISTALTICPUMP.ON=1) then | |
| 08 | !MAGNETIC_VALVE_A=1 {pump suspension} | |
| 09 | wait 1 second | |
| 10 | suspension=current_time - s | |
| 11 | end if | |
| 12 | until (suspension>00:13:00) or (!PI_00>1250) or (!PI_00<20) | |
| 13 | !MAGNETIC_VALVE_A=0 | |
| 14 | if (!PI_00>1250) or (!PI_00<20) then | |
| 15 | wait until (!PI_00<900) and (!PI_00>500) | |
| 16 | else | |
| 17 | continue with T1 | |
| 18 | end if | |
| 19 | until !PERISTALTICPUMP.ON=0 | |
| 20 | !MAGNETIC_VALVE_A=0 |
| 01 | wait until !PERISTALTICPUMP.ON=1 |
| 02 | !MAGNETIC_VALVE_A=0 {rinse with solvent(water)} |
| 03 | wait 30seconds |
| 04 | if (!PI_00<1250) and (!PI_00>20) and (!PERISTALTICPUMP.ON=1) then |
| 05 | !MAGNETIC_VALVE_A=1 {pump suspension} |
| 06 | t_start = current_time |
| 07 | wait until (!PI_00>1250) or (!PI_00<20) |
| 08 | !MAGNETIC_VALVE_A=0 |
| 09 | rinse_time = current_time – t_start {time till clogging} |
| 10 | wait until !PI_00<800 {stabillization of the pressure} |
| 11 | else |
| 12 | rinse_time=00:13:00 {error condtion} |
| 13 | end if |
| 14 | repeat |
| 15 | ! MAGNETIC_VALVE_A=0 {rinse with solvent (water)} |
| 16 | wait 60 seconds |
| 17 | t=current_time |
| 18 | repeat |
| 19 | if (!PI_00<1250) and (!PI_00>20) and (!PERISTALTICPUMP.ON=1) then |
| 20 | ! MAGNETIC_VALVE_A=1 {pump suspension} |
| 21 | wait 1 second |
| 22 | duration=current_time-t |
| 23 | end if |
| 24 | until (duration=rinse_time) or (!PI_00>1250) or (!MASSFLOWRATE.A<0) then |
| 25 | ! MAGNETIC_VALVE_A=0 |
| 26 | if (!MASSFLOWRATE.A>1.5) or (!MASSFLOWRATE.A<0) then |
| 27 | wait until (!PI_00<900) and (!PI_00>500) |
| 28 | end if |
| 29 | until (!PERISTALTICPUMP.ON=0) |
| 30 | ! MAGNETIC_VALVE_A=0 |
| 01 | wait until (!MAGNETIC_VALVE_A=1) and (!PERISTALTICPUMP.ON=1) |
| 02 | repeat |
| 03 | if (!ARDUINO.RESISTOR<1567) and (!PI_00<1250) then |
| 04 | !MAGNETIC_VALVE_B=1 {product tank} |
| 05 | wait until (!ARDUINO.RESISTOR>1567) or (!PERISTALTICPUMP.ON=0) or (!PI_00>1250) |
| 06 | !MAGNETIC_VALVE_B=0 {waste tank} |
| 07 | wait until (!ARDUINO.RESISTOR<1567) or (!PERISTALTICPUMP.ON=0) |
| 08 | end if |
| 09 | until !PERISTALTICPUMP.ON=0 |
| 10 | !MAGNETIC_VALVE_B=0 |
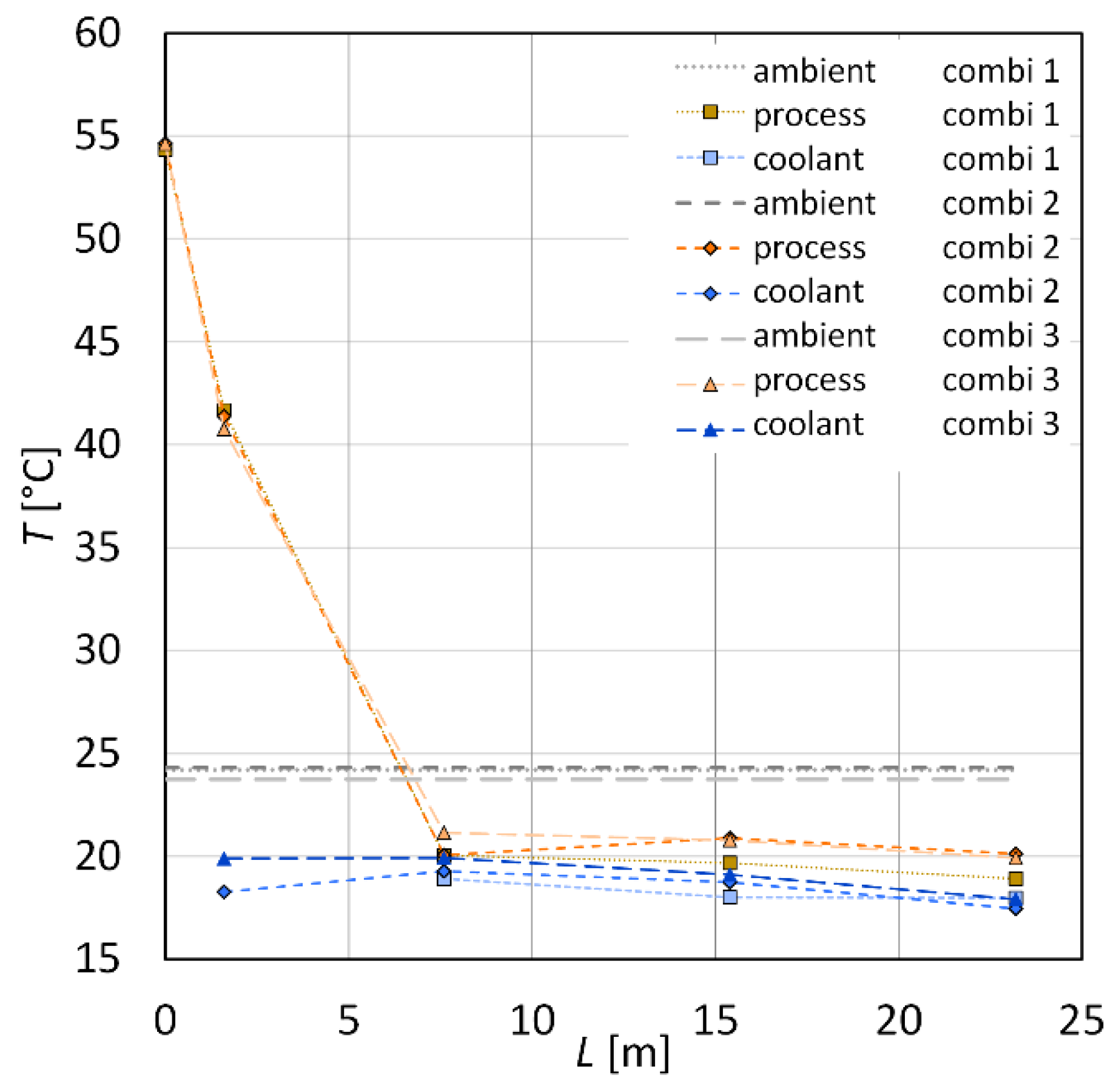
| Sample | (g·min−1) | (g·min−1) | (°C) | (°C) | (°C) | (°C) | (°C) |
|---|---|---|---|---|---|---|---|
| combi 1 | 17.9 ± 0.5 | 19.5 ± 0.1 | 54.3 | 18.3 | 20.0 | 18.9 | 24.2 ± 0.2 |
| combi 2 | 17.4 ± 0.5 | 19.9 ± 0.1 | 54.6 | 18.3 | 20.1 | 20.1 | 24.3 ± 0.2 |
| combi 3 | 16.4 ± 0.5 | 19.7 ± 0.1 | 54.6 | 19.9 | 21.2 | 19.9 | 23.8 ± 0.2 |
| combi 4 | 17.3 ± 1.1 | 18.1 ± 0.1 | 53.9 | 12.6 | 19.9 | 21.8 | 26.1 ± 0.4 |
| combi 5 | 17.2 ± 0.8 | 18.4 ± 0.1 | 53.6 | 12.5 | 19.7 | 23.0 | 26.3 ± 0.4 |
References
- Myerson, A.S. Handbook of Industrial Crystallization, 2nd ed.; Butterworth-Heinemann: Boston, MA, USA, 2002; ISBN 9780080533513. [Google Scholar]
- Hofmann, G. (Ed.) Kristallisation in der Industriellen Praxis; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Domokos, A.; Nagy, B.; Szilágyi, B.; Marosi, G.; Nagy, Z.K. Integrated Continuous Pharmaceutical Technologies—A Review. Org. Process. Res. Dev. 2021, 25, 721–739. [Google Scholar] [CrossRef]
- Hou, G.; Power, G.; Barrett, M.; Glennon, B.; Morris, G.; Zhao, Y. Development and Characterization of a Single Stage Mixed-Suspension, Mixed-Product-Removal Crystallization Process with a Novel Transfer Unit. Cryst. Growth Des. 2014, 14, 1782–1793. [Google Scholar] [CrossRef]
- Chianese, A.; Kramer, H.J.M. (Eds.) Industrial Crystallization Process Monitoring and Control; Wiley-VCH: Weinheim, Germany, 2012; ISBN 9783527331734. [Google Scholar]
- Fernandez Rivas, D.; Kuhn, S. Synergy of Microfluidics and Ultrasound: Process Intensification Challenges and Opportunities. Top. Curr. Chem. 2016, 374, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deora, N.S.; Misra, N.N.; Deswal, A.; Mishra, H.N.; Cullen, P.J.; Tiwari, B.K. Ultrasound for Improved Crystallisation in Food Processing. Food Eng. Rev. 2013, 5, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Castillo-Peinado, L.d.l.S.; Luque de Castro, M.D. The role of ultrasound in pharmaceutical production: Sonocrystallization. J. Pharm. Pharmacol. 2016, 68, 1249–1267. [Google Scholar] [CrossRef]
- Kim, H.; Suslick, K. The Effects of Ultrasound on Crystals: Sonocrystallization and Sonofragmentation. Crystals 2018, 8, 280. [Google Scholar] [CrossRef] [Green Version]
- Jordens, J.; Canini, E.; Gielen, B.; van Gerven, T.; Braeken, L. Ultrasound Assisted Particle Size Control by Continuous Seed Generation and Batch Growth. Crystals 2017, 7, 195. [Google Scholar] [CrossRef]
- Narducci, O.; Jones, A.G.; Kougoulos, E. Continuous crystallization of adipic acid with ultrasound. Chem. Eng. Sci. 2011, 66, 1069–1076. [Google Scholar] [CrossRef]
- Nalajala, V.S.; Moholkar, V.S. Investigations in the physical mechanism of sonocrystallization. Ultrason. Sonochem. 2011, 18, 345–355. [Google Scholar] [CrossRef]
- Nguyen, T.; Khan, A.; Bruce, L.; Forbes, C.; O’Leary, R.; Price, C. The Effect of Ultrasound on the Crystallisation of Paracetamol in the Presence of Structurally Similar Impurities. Crystals 2017, 7, 294. [Google Scholar] [CrossRef] [Green Version]
- Kudo, S.; Takiyama, H. Production of Fine Organic Crystalline Particles by Using Milli Segmented Flow Crystallizer. J. Chem. Eng. Jpn. JCEJ 2012, 45, 305–309. [Google Scholar] [CrossRef]
- Sharma, D.; Murthy, Z.V.P.; Patel, S.R. Recovery of lactose from aqueous solution by application of ultrasound through millichannel. Int. J. Food Eng. 2021, 17, 571–581. [Google Scholar] [CrossRef]
- Gracin, S.; Uusi-Penttilä, M.; Rasmuson, Å.C. Influence of Ultrasound on the Nucleation of Polymorphs of p -Aminobenzoic Acid. Cryst. Growth Des. 2005, 5, 1787–1794. [Google Scholar] [CrossRef]
- Jordens, J.; Gielen, B.; Braeken, L.; van Gerven, T. Determination of the effect of the ultrasonic frequency on the cooling crystallization of paracetamol. Chem. Eng. Process. Process. Intensif. 2014, 84, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Kordylla, A.; Koch, S.; Tumakaka, F.; Schembecker, G. Towards an optimized crystallization with ultrasound: Effect of solvent properties and ultrasonic process parameters. J. Cryst. Growth 2008, 310, 4177–4184. [Google Scholar] [CrossRef]
- Han, B.; Ezeanowi, N.C.; Koiranen, T.O.; Häkkinen, A.T.; Louhi-Kultanen, M. Insights into Design Criteria for a Continuous, Sonicated Modular Tubular Cooling Crystallizer. Cryst. Growth Des. 2018, 18, 7286–7295. [Google Scholar] [CrossRef]
- Ezeanowi, N.; Pajari, H.; Laitinen, A.; Koiranen, T. Monitoring the Dynamics of a Continuous Sonicated Tubular Cooling Crystallizer. Cryst. Growth Des. 2020, 20, 1458–1466. [Google Scholar] [CrossRef]
- Furuta, M.; Mukai, K.; Cork, D.; Mae, K. Continuous crystallization using a sonicated tubular system for controlling particle size in an API manufacturing process. Chem. Eng. Process. Process. Intensif. 2016, 102, 210–218. [Google Scholar] [CrossRef]
- Pohl, B. Charakterisierung der Mischung und Fällung bei Kontinuierlichen Sonochemischen Reaktoren unter Besonderer Berücksichtigung der Reaktorform. Doctoral Dissertation, Technischen Universität Bergakademie, Freiberg, Germany, 20 December 2012. [Google Scholar]
- Eder, R.J.P.; Schrank, S.; Besenhard, M.O.; Roblegg, E.; Gruber-Woelfler, H.; Khinast, J.G. Continuous Sonocrystallization of Acetylsalicylic Acid (ASA): Control of Crystal Size. Cryst. Growth Des. 2012, 12, 4733–4738. [Google Scholar] [CrossRef]
- Besenhard, M.O.; Neugebauer, P.; Ho, C.-D.; Khinast, J.G. Crystal Size Control in a Continuous Tubular Crystallizer. Cryst. Growth Des. 2015, 15, 1683–1691. [Google Scholar] [CrossRef]
- Schmalenberg, M.; Weick, L.K.; Kockmann, N. Nucleation in continuous flow cooling sonocrystallization for coiled capillary crystallizers. J. Flow Chem. 2021. [Google Scholar] [CrossRef]
- Ruecroft, G.; Hipkiss, D.; Ly, T.; Maxted, N.; Cains, P.W. Sonocrystallization: The Use of Ultrasound for Improved Industrial Crystallization. Org. Process. Res. Dev. 2005, 9, 923–932. [Google Scholar] [CrossRef]
- Sander, J.R.G.; Zeiger, B.W.; Suslick, K.S. Sonocrystallization and sonofragmentation. Ultrason. Sonochem. 2014, 21, 1908–1915. [Google Scholar] [CrossRef]
- Lührmann, M.-C.; Timmermann, J.; Schembecker, G.; Wohlgemuth, K. Enhanced Product Quality Control through Separation of Crystallization Phenomena in a Four-Stage MSMPR Cascade. Cryst. Growth Des. 2018, 18, 7323–7334. [Google Scholar] [CrossRef]
- Capellades, G.; Joshi, P.U.; Dam-Johansen, K.; Mealy, M.J.; Christensen, T.V.; Kiil, S. Characterization of a Multistage Continuous MSMPR Crystallization Process Assisted by Image Analysis of Elongated Crystals. Cryst. Growth Des. 2018, 18, 6455–6469. [Google Scholar] [CrossRef]
- Takiyama, H.; Matsuoka, M. Design of seed crystal specifications for start-up operation of a continuous MSMPR crystallizer. Powder Technol. 2001, 121, 99–105. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, S.; Du, S.; Wang, J.; Gong, J. Progress of Pharmaceutical Continuous Crystallization. Engineering 2017, 3, 354–364. [Google Scholar] [CrossRef]
- Wang, T.; Lu, H.; Wang, J.; Xiao, Y.; Zhou, Y.; Bao, Y.; Hao, H. Recent progress of continuous crystallization. J. Ind. Eng. Chem. 2017, 54, 14–29. [Google Scholar] [CrossRef]
- Onyemelukwe, I.I.; Parsons, A.R.; Wheatcroft, H.P.; Robertson, A.; Nagy, Z.K.; Rielly, C.D. The Role of Residence Time Distribution in the Continuous Steady-State Mixed Suspension Mixed Product Removal Crystallization of Glycine. Cryst. Growth Des. 2019, 19, 66–80. [Google Scholar] [CrossRef]
- Nguyen, A.-T.; Kim, J.-M.; Chang, S.-M.; Kim, W.-S. Taylor Vortex Effect on Phase Transformation of Guanosine 5-Monophosphate in Drowning-Out Crystallization. Ind. Eng. Chem. Res. 2010, 49, 4865–4872. [Google Scholar] [CrossRef]
- Kim, J.-E.; Kim, W.-S. Synthesis of Core–Shell Particles of Nickel–Manganese–Cobalt Hydroxides in a Continuous Couette-Taylor Crystallizer. Cryst. Growth Des. 2017, 17, 3677–3686. [Google Scholar] [CrossRef]
- Xu, K.; Wang, C.; Wang, X.; Qian, Y. Laboratory experiments on simultaneous removal of K and P from synthetic and real urine for nutrient recycle by crystallization of magnesium-potassium-phosphate-hexahydrate in a draft tube and baffle reactor. Chemosphere 2012, 88, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Schmalenberg, M.; Mensing, L.; Lindemann, S.; Krell, T.; Kockmann, N. Miniaturized Draft Tube Baffle Crystallizer for Continuous Cooling Crystallization. Chem. Eng. Res. Des. under review.
- Briggs, N.E.B.; Schacht, U.; Raval, V.; McGlone, T.; Sefcik, J.; Florence, A.J. Seeded Crystallization of β- l -Glutamic Acid in a Continuous Oscillatory Baffled Crystallizer. Org. Process. Res. Dev. 2015, 19, 1903–1911. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Ni, X.-W. Reactive Crystallization of Paracetamol in a Continuous Oscillatory Baffled Reactor. Org. Process. Res. Dev. 2019, 23, 882–890. [Google Scholar] [CrossRef]
- Orehek, J.; Teslić, D.; Likozar, B. Continuous Crystallization Processes in Pharmaceutical Manufacturing: A Review. Org. Process. Res. Dev. 2021, 25, 16–42. [Google Scholar] [CrossRef]
- Eder, R.J.P.; Radl, S.; Schmitt, E.; Innerhofer, S.; Maier, M.; Gruber-Woelfler, H.; Khinast, J.G. Continuously Seeded, Continuously Operated Tubular Crystallizer for the Production of Active Pharmaceutical Ingredients. Cryst. Growth Des. 2010, 10, 2247–2257. [Google Scholar] [CrossRef]
- Hohmann, L.; Greinert, T.; Mierka, O.; Turek, S.; Schembecker, G.; Bayraktar, E.; Wohlgemuth, K.; Kockmann, N. Analysis of Crystal Size Dispersion Effects in a Continuous Coiled Tubular Crystallizer: Experiments and Modeling. Cryst. Growth Des. 2018, 18, 1459–1473. [Google Scholar] [CrossRef]
- Wiedmeyer, V.; Anker, F.; Bartsch, C.; Voigt, A.; John, V.; Sundmacher, K. Continuous Crystallization in a Helically Coiled Flow Tube: Analysis of Flow Field, Residence Time Behavior, and Crystal Growth. Ind. Eng. Chem. Res. 2017, 56, 3699–3712. [Google Scholar] [CrossRef] [Green Version]
- Termühlen, M.; Etmanski, M.M.; Kryschewski, I.; Kufner, A.C.; Schembecker, G.; Wohlgemuth, K. Continuous slug flow crystallization: Impact of design and operating parameters on product quality. Chem. Eng. Res. Des. 2021, 170, 290–303. [Google Scholar] [CrossRef]
- Besenhard, M.O.; Neugebauer, P.; Scheibelhofer, O.; Khinast, J.G. Crystal Engineering in Continuous Plug-Flow Crystallizers. Cryst. Growth Des. 2017, 17, 6432–6444. [Google Scholar] [CrossRef] [PubMed]
- Kurt, S.K.; Akhtar, M.; Nigam, K.D.P.; Kockmann, N. Continuous Reactive Precipitation in a Coiled Flow Inverter: Inert Particle Tracking, Modular Design, and Production of Uniform CaCO 3 Particles. Ind. Eng. Chem. Res. 2017, 56, 11320–11335. [Google Scholar] [CrossRef]
- Hussain, M.N.; Jordens, J.; John, J.J.; Braeken, L.; van Gerven, T. Enhancing pharmaceutical crystallization in a flow crystallizer with ultrasound: Anti-solvent crystallization. Ultrason. Sonochem. 2019, 59, 104743. [Google Scholar] [CrossRef]
- Degli Esposti, L.; Dotti, A.; Adamiano, A.; Fabbi, C.; Quarta, E.; Colombo, P.; Catalucci, D.; Luca, C.D.; Iafisco, M. Calcium Phosphate Nanoparticle Precipitation by a Continuous Flow Process: A Design of Experiment Approach. Crystals 2020, 10, 953. [Google Scholar] [CrossRef]
- Benitez-Chapa, A.G.; Nigam, K.D.P.; Alvarez, A.J. Process Intensification of Continuous Antisolvent Crystallization Using a Coiled Flow Inverter. Ind. Eng. Chem. Res. 2020, 59, 3934–3942. [Google Scholar] [CrossRef]
- Del Méndez Río, J.R.; Rousseau, R.W. Batch and Tubular-Batch Crystallization of Paracetamol: Crystal Size Distribution and Polymorph Formation. Cryst. Growth Des. 2006, 6, 1407–1414. [Google Scholar] [CrossRef]
- Eder, R.J.P.; Schmitt, E.K.; Grill, J.; Radl, S.; Gruber-Woelfler, H.; Khinast, J.G. Seed loading effects on the mean crystal size of acetylsalicylic acid in a continuous-flow crystallization device. Cryst. Res. Technol. 2011, 46, 227–237. [Google Scholar] [CrossRef]
- Hohmann, L.; Gorny, R.; Klaas, O.; Ahlert, J.; Wohlgemuth, K.; Kockmann, N. Design of a Continuous Tubular Cooling Crystallizer for Process Development on Lab-Scale. Chem. Eng. Technol. 2016, 39, 1268–1280. [Google Scholar] [CrossRef]
- Kurt, S.K.; Warnebold, F.; Nigam, K.D.; Kockmann, N. Gas-liquid reaction and mass transfer in microstructured coiled flow inverter. Chem. Eng. Sci. 2017, 169, 164–178. [Google Scholar] [CrossRef]
- Hohmann, L.; Schmalenberg, M.; Prasanna, M.; Matuschek, M.; Kockmann, N. Suspension flow behavior and particle residence time distribution in helical tube devices. Chem. Eng. J. 2019, 360, 1371–1389. [Google Scholar] [CrossRef]
- Hohmann, L.; Löbnitz, L.; Menke, C.; Santhirakumaran, B.; Stier, P.; Stenger, F.; Dufour, F.; Wiese, G.; zur Horst-Meyer, S.; Kusserow, B.; et al. Continuous Downstream Processing of Amino Acids in a Modular Miniplant. Chem. Eng. Technol. 2018, 41, 1152–1164. [Google Scholar] [CrossRef]
- Schmalenberg, M.; Hohmann, L.; Kockmann, N. Miniaturized Tubular Cooling Crystallizer with Solid-Liquid Flow for Process Development. Proc. ASME ICNMM 2018, 51197, V001T02A006. [Google Scholar] [CrossRef]
- Wiedmeyer, V.; Voigt, A.; Sundmacher, K. Crystal Population Growth in a Continuous Helically Coiled Flow Tube Crystallizer. Chem. Eng. Technol. 2017, 40, 1584–1590. [Google Scholar] [CrossRef] [Green Version]
- Wiedmeyer, V. Continuous Crystallization in a Helically Coiled Flow Tube Crystallizer. Doctoral Dissertation, Otto-von-Guericke-Universität Magdeburg, Magdeburg, Germany, 30 June 2020. [Google Scholar]
- Neugebauer, P.; Khinast, J.G. Continuous Crystallization of Proteins in a Tubular Plug-Flow Crystallizer. Cryst. Growth Des. 2015, 15, 1089–1095. [Google Scholar] [CrossRef]
- Ferreira, J.; Castro, F.; Rocha, F.; Kuhn, S. Protein crystallization in a droplet-based microfluidic device: Hydrodynamic analysis and study of the phase behaviour. Chem. Eng. Sci. 2018, 191, 232–244. [Google Scholar] [CrossRef]
- Neugebauer, P.; Cardona, J.; Besenhard, M.O.; Peter, A.; Gruber-Woelfler, H.; Tachtatzis, C.; Cleary, A.; Andonovic, I.; Sefcik, J.; Khinast, J.G. Crystal Shape Modification via Cycles of Growth and Dissolution in a Tubular Crystallizer. Cryst. Growth Des. 2018, 18, 4403–4415. [Google Scholar] [CrossRef]
- Su, M.; Gao, Y. Air–Liquid Segmented Continuous Crystallization Process Optimization of the Flow Field, Growth Rate, and Size Distribution of Crystals. Ind. Eng. Chem. Res. 2018, 57, 3781–3791. [Google Scholar] [CrossRef]
- Mou, M.; Jiang, M. Fast Continuous Non-Seeded Cooling Crystallization of Glycine in Slug Flow: Pure α-Form Crystals with Narrow Size Distribution. J. Pharm. Innov. 2020, 15, 281–294. [Google Scholar] [CrossRef]
- Mozdzierz, N.J.; Lee, Y.; Hong, M.S.; Benisch, M.H.; Rasche, M.L.; Tropp, U.E.; Jiang, M.; Myerson, A.S.; Braatz, R.D. Mathematical modeling and experimental validation of continuous slug-flow tubular crystallization with ultrasonication-induced nucleation and spatially varying temperature. Chem. Eng. Res. Des. 2021, 169, 275–287. [Google Scholar] [CrossRef]
- Nalesso, S.; Bussemaker, M.J.; Sear, R.P.; Hodnett, M.; Lee, J. A review on possible mechanisms of sonocrystallisation in solution. Ultrason. Sonochem. 2019, 57, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, E.; Ebihara, S.; Hirasawa, I. Investigation of primary nucleation phenomena of acetylsalicylic acid crystals induced by ultrasonic irradiation—Ultrasonic energy needed to activate primary nucleation. J. Cryst. Growth 2006, 295, 97–101. [Google Scholar] [CrossRef]
- Louhi-Kultanen, M.; Karjalainen, M.; Rantanen, J.; Huhtanen, M.; Kallas, J. Crystallization of glycine with ultrasound. Int. J. Pharm. 2006, 320, 23–29. [Google Scholar] [CrossRef]
- Kurotani, M.; Hirasawa, I. Polymorph control of sulfamerazine by ultrasonic irradiation. J. Cryst. Growth 2008, 310, 4576–4580. [Google Scholar] [CrossRef]
- Hatakka, H.; Alatalo, H.; Louhi-Kultanen, M.; Lassila, I.; Haeggström, E. Closed-Loop Control of Reactive Crystallization PART II: Polymorphism Control of L-Glutamic Acid by Sonocrystallization and Seeding. Chem. Eng. Technol. 2010, 33, 751–756. [Google Scholar] [CrossRef]
- Schmalenberg, M.; Sallamon, F.; Haas, C.; Kockmann, N. Temperature-Controlled Minichannel Flow-Cell for Non-Invasive Particle Measurements in Solid-Liquid Flow. Proc. ASME ICNMM 2020, 83693, V001T16A005. [Google Scholar] [CrossRef]
- Schindel, A.-L.; Schmalenberg, M.; Polyakova, M.; Grünewald, M.; Kockmann, N. Matching Matrix for the Selection of Continuous Crystallization Technologies Exemplified by the CFI-Crystallizer. Available online: https://fluidvt.ruhr-uni-bochum.de/mitarbeiter/frau-anna-lena-schindel-m-sc/ (accessed on 9 November 2020).
- Verein Deutscher Ingenieure; VDI-Gesellschaft Verfahrenstechnik und Chemieingenieurwesen. VDI-Wärmeatlas: Mit 320 Tabellen, 11., Bearb. und erw. Aufl.; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 9783642199813. [Google Scholar]
- Ogawa, T.; Mizutani, K.; Yasuda, M. The Volume, Adiabatic Compressibility, and Viscosity of Amino Acids in Aqueous Alkali-chloride Solutions. BCSJ 1984, 57, 2064–2068. [Google Scholar] [CrossRef]
- Ogawa, T.; Yasuda, M.; Mizutani, K. Volume and Adiabatic Compressibility of Amino Acids in Urea–Water Mixtures. BCSJ 1984, 57, 662–666. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.; Wang, J.; Liu, W.; Lu, J. Apparent molar volumes and viscosity B-coefficients of some α-amino acids in aqueous solutions from 278.15 to 308.15 K. Thermochim. Acta 1999, 334, 17–27. [Google Scholar] [CrossRef]
- Wohlgemuth, K.; Schembecker, G. Modeling induced nucleation processes during batch cooling crystallization: A sequential parameter determination procedure. Comput. Chem. Eng. 2013, 52, 216–229. [Google Scholar] [CrossRef]
- Sullivan, R.; Pyda, M.; Pak, J.; Wunderlich, B.; Thompson, J.R.; Pagni, R.; Pan, H.; Barnes, C.; Schwerdtfeger, P.; Compton, R. Search for Electroweak Interactions in Amino Acid Crystals. II. The Salam Hypothesis. J. Phys. Chem. A 2003, 107, 6674–6680. [Google Scholar] [CrossRef]
- VWR. Glycin (H-Gly-OH) ≥98%, GPR RECTAPUR®|VWR. Available online: https://de.vwr.com/store/product/2355458/glycin-h-gly-oh-98-gpr-rectapur (accessed on 26 May 2021).
- Saxena, A.K.; Nigam, K.D.P. Coiled configuration for flow inversion and its effect on residence time distribution. AIChE J. 1984, 30, 363–368. [Google Scholar] [CrossRef]
- Gelhausen, M.G.; Kurt, S.K.; Kockmann, N. Mixing and Heat Transfer in Helical Capillary Flow Reactors with Alternating Bends. Proc. ASME ICNMM 2014, 46278, V001T13A001. [Google Scholar] [CrossRef]
- Klutz, S.; Kurt, S.K.; Lobedann, M.; Kockmann, N. Narrow residence time distribution in tubular reactor concept for Reynolds number range of 10–100. Chem. Eng. Res. Des. 2015, 95, 22–33. [Google Scholar] [CrossRef]
- Schmalenberg, M.; Krieger, W.; Kockmann, N. Modular Coiled Flow Inverter with Narrow Residence Time Distribution for Process Development and Production. Chem. Ing. Tech. 2019, 91, 567–575. [Google Scholar] [CrossRef]
- Bode, K.; Hooper, R.J.; Paterson, W.R.; Wilson, D.I.; Augustin, W.; Scholl, S. Reinigung von Molkeproteinablagerungen mit pulsierender Strömung. Chem. Ing. Tech. 2006, 78, 613–620. [Google Scholar] [CrossRef]
- Wang, Y.; Su, M.; Bai, Y. Mechanism of Glycine Crystal Adhesion and Clogging in a Continuous Tubular Crystallizer. Ind. Eng. Chem. Res. 2020, 59, 25–33. [Google Scholar] [CrossRef]
- Klose, A.; Merkelbach, S.; Menschner, A.; Hensel, S.; Heinze, S.; Bittorf, L.; Kockmann, N.; Schäfer, C.; Szmais, S.; Eckert, M.; et al. Orchestration Requirements for Modular Process Plants in Chemical and Pharmaceutical Industries. Chem. Eng. Technol. 2019, 42, 2282–2291. [Google Scholar] [CrossRef] [Green Version]
- Höving, S.; Bobers, J.; Kockmann, N. Low-cost multi-purpose sensor for measurements in continuous two-phase capillary flow. To be submitted.
- Borchert, C.; Sundmacher, K. Crystal Aggregation in a Flow Tube: Image-Based Observation. Chem. Eng. Technol. 2011, 34, 545–556. [Google Scholar] [CrossRef]
- Huo, Y.; Liu, T.; Liu, H.; Ma, C.Y.; Wang, X.Z. In-situ crystal morphology identification using imaging analysis with application to the L-glutamic acid crystallization. Chem. Eng. Sci. 2016, 148, 126–139. [Google Scholar] [CrossRef]
- Emig, G.; Klemm, E. Chemische Reaktionstechnik; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Kurt, S.K.; Gelhausen, M.G.; Kockmann, N. Axial Dispersion and Heat Transfer in a Milli/Microstructured Coiled Flow Inverter for Narrow Residence Time Distribution at Laminar Flow. Chem. Eng. Technol. 2015, 38, 1122–1130. [Google Scholar] [CrossRef]
- Steenweg, C.; Seifert, A.I.; Schembecker, G.; Wohlgemuth, K. Characterization of a Modular Continuous Vacuum Screw Filter for Small-Scale Solid–Liquid Separation of Suspensions. Org. Process. Res. Dev. 2021, 25, 926–940. [Google Scholar] [CrossRef]
- Hohmann, L. Equipment and Process Design for Small-Scale Continuous Crystallization. Doctoral Dissertation, TU Dortmund University, Dortmund, Germany, 9 April 2019. [Google Scholar]
- Palazoglu, T.; Sandeep, K.P. Effect of tube curvature ratio on the residence time distribution of multiple particles in helical tubes. LWT Food Sci. Technol. 2004, 37, 387–393. [Google Scholar] [CrossRef]
- Fu, T.; Ma, Y.; Funfschilling, D.; Zhu, C.; Li, H.Z. Squeezing-to-dripping transition for bubble formation in a microfluidic T-junction. Chem. Eng. Sci. 2010, 65, 3739–3748. [Google Scholar] [CrossRef]
- Termühlen, M.; Strakeljahn, B.; Schembecker, G.; Wohlgemuth, K. Characterization of slug formation towards the performance of air-liquid segmented flow. Chem. Eng. Sci. 2019, 207, 1288–1298. [Google Scholar] [CrossRef]
- Termühlen, M.; Strakeljahn, B.; Schembecker, G.; Wohlgemuth, K. Quantification and evaluation of operating parameters’ effect on suspension behavior for slug flow crystallization. Chem. Eng. Sci. 2021, 243, 116771. [Google Scholar] [CrossRef]
- Hohmann, L.; Kurt, S.K.; Pouya Far, N.; Vieth, D.; Kockmann, N. Micro-/Milli-Fluidic Heat-Exchanger Characterization by Non-Invasive Temperature Sensors. Proc. ASME ICNMM 2016, 50343, V001T12A002. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Ching, C.B. Solubility of Form α and Form γ of Glycine in Aqueous Solutions. J. Chem. Eng. Data 2008, 53, 1133–1137. [Google Scholar] [CrossRef]
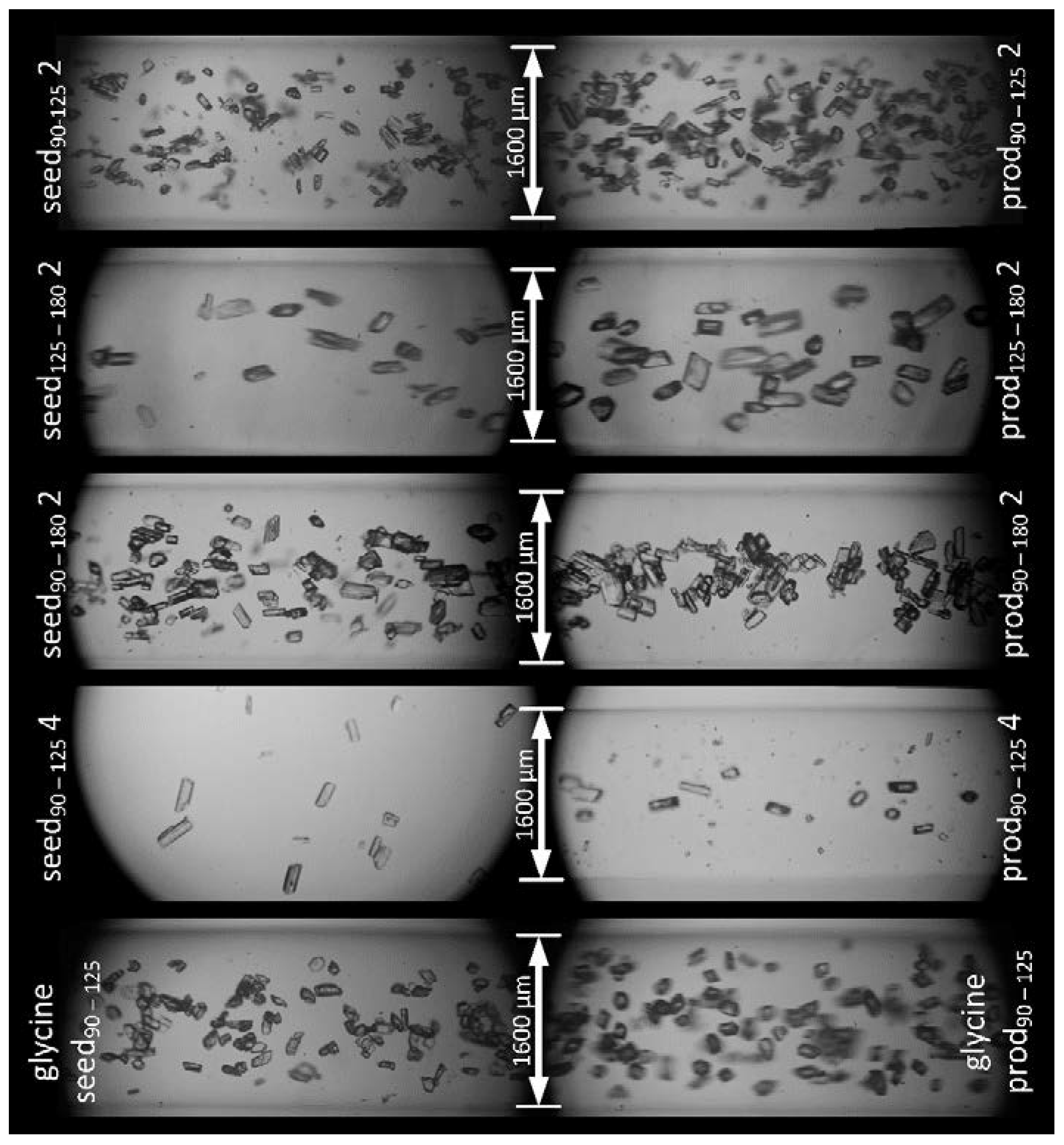
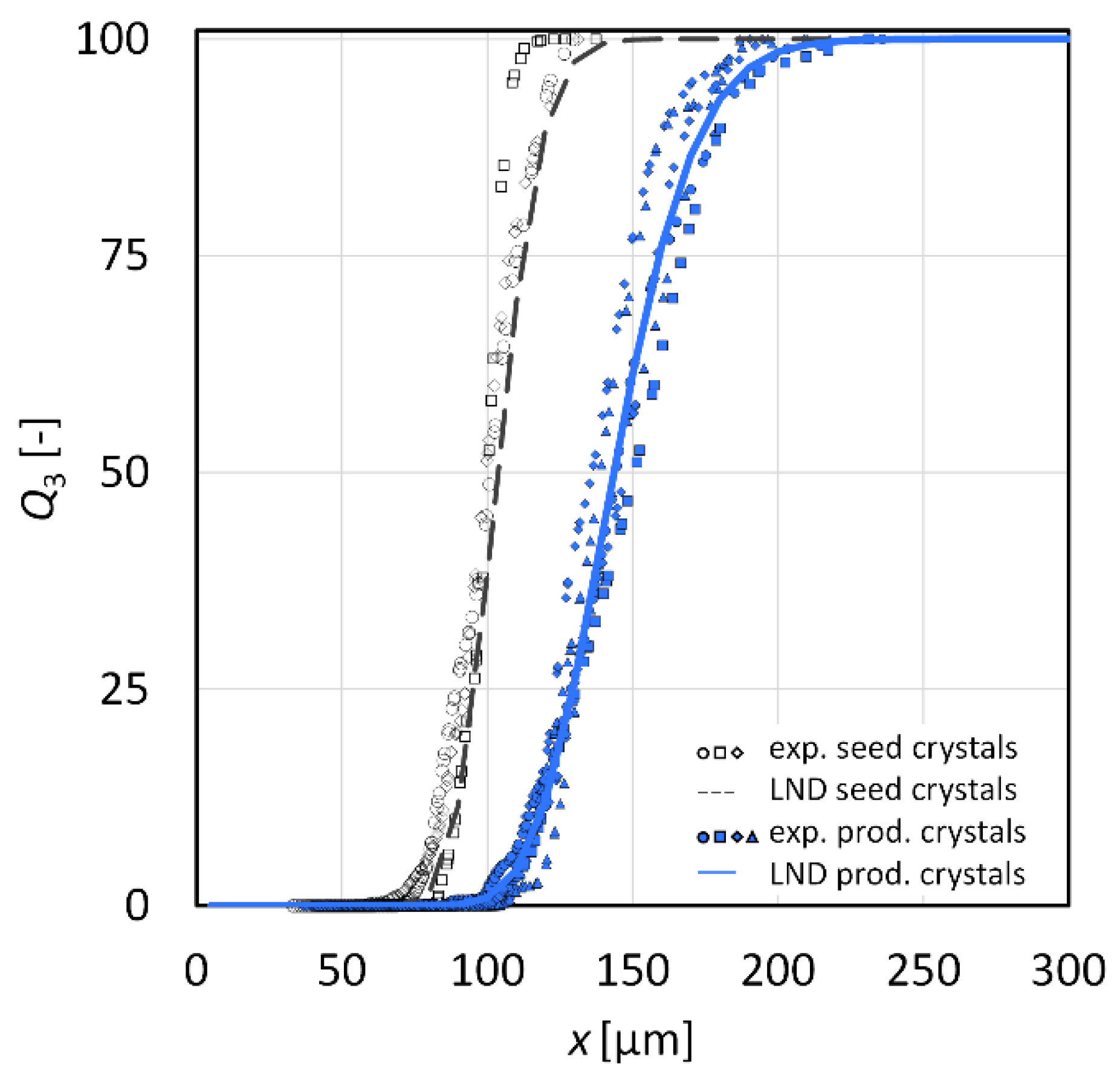
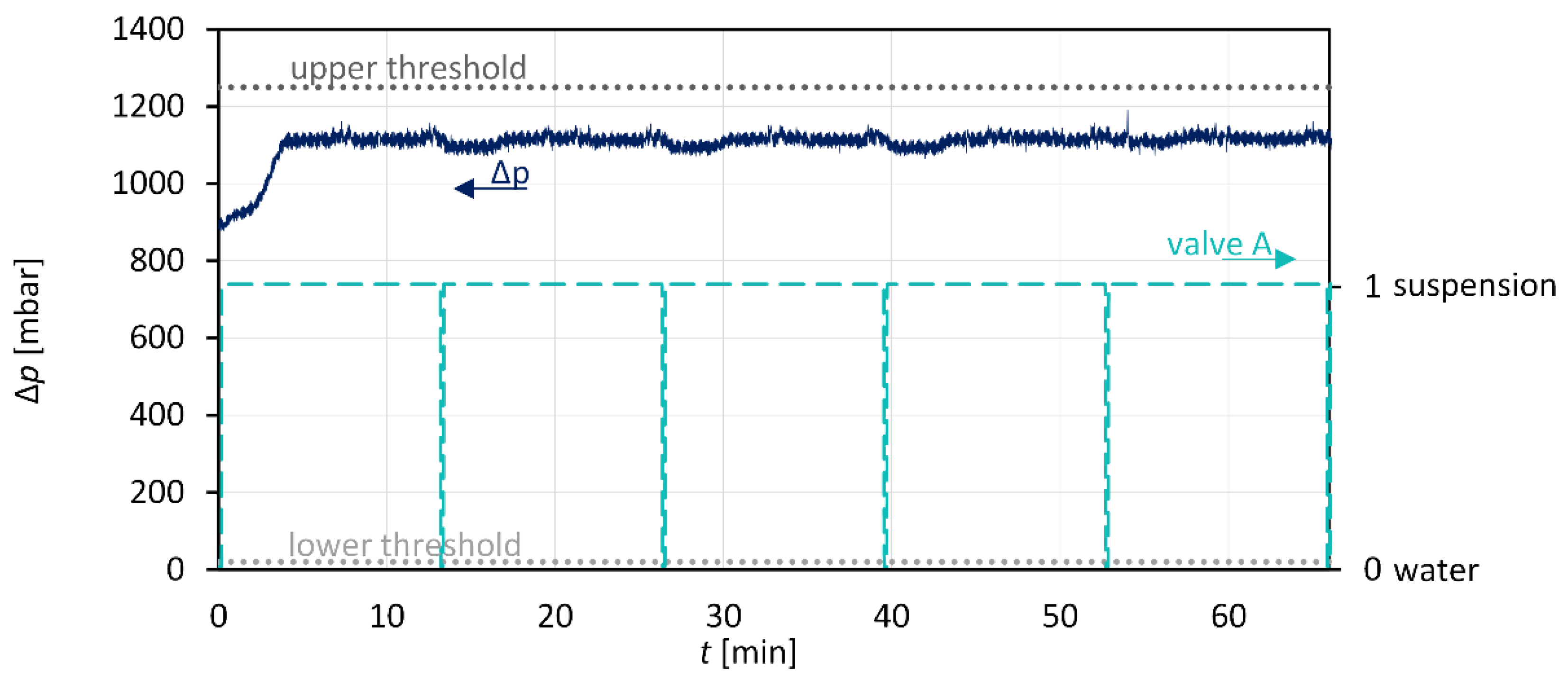
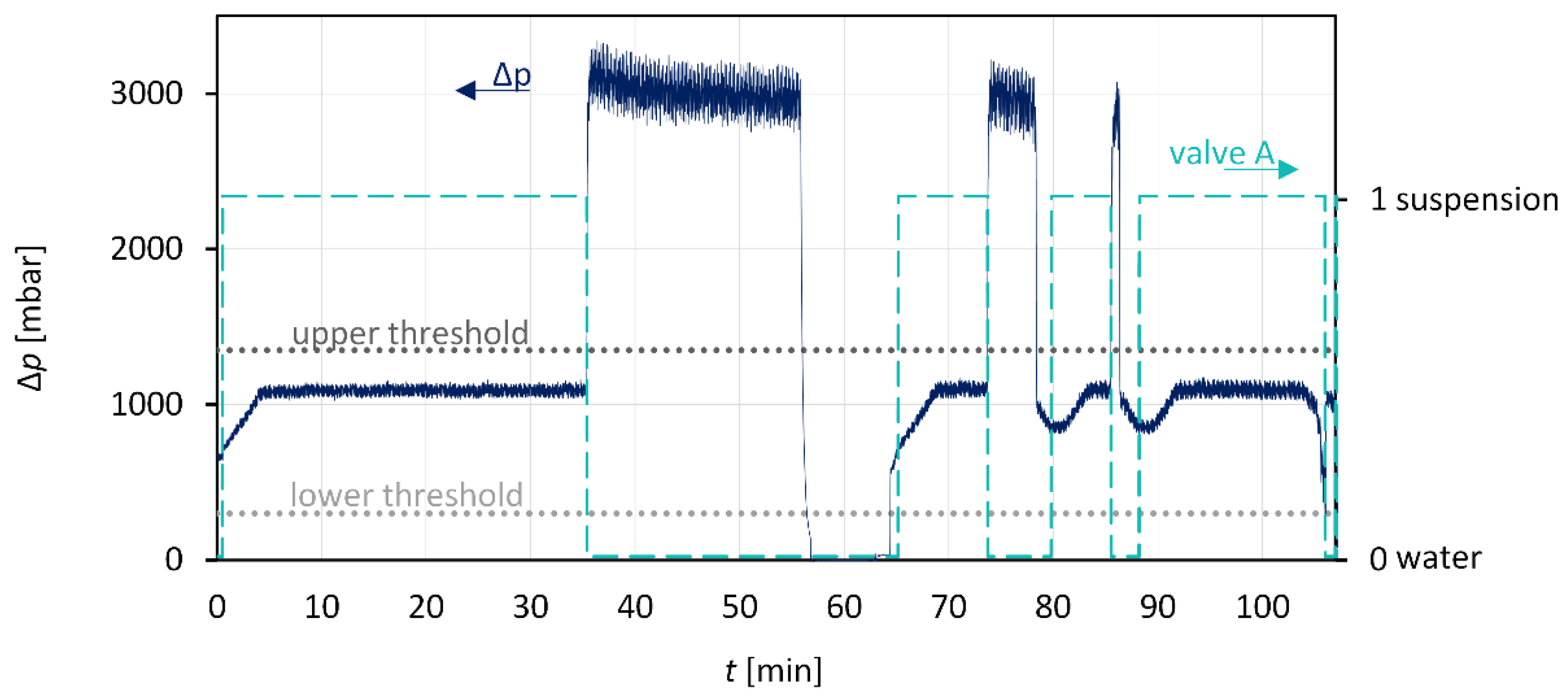
| Sample Name | Investigation | Seed/Tracer Particles | |
|---|---|---|---|
| Sieve Size (µm) | Solid Weight Fraction (Set Point) (w.%) | ||
| RTDS,90–125 | RTD | 90–125 | 1 |
| RTDS,125–180 | RTD | 125–180 | 1 |
| RTDS,90–180 | RTD | 90–180 | 1 |
| RTDL | RTD | - | 0 |
| seed90–125 1 | CG | 90–125 | 1 |
| seed90–125 2 | CG | 90–125 | 1 |
| seed125–180 1 | CG | 125–180 | 1 |
| seed125–180 2 | CG | 125–180 | 1 |
| seed90–180 1 | CG | 90–180 | 1 |
| seed90–180 2 | CG | 90–180 | 1 |
| seed90–125 3 | CG | 90–125 | 0.1 |
| seed90–125 4 | CG | 90–125 | 0.1 |
| glycine90–125 | CG | 90–125 | 1 |
| combi 1 | nucleation + CG | - | 0 |
| combi 2 | nucleation + CG | - | 0 |
| combi 3 | nucleation + CG | - | 0 |
| combi 4 | nucleation + CG | - | 0 |
| combi 5 | nucleation + CG | - | 0 |
| Sample Name | (g∙min−1) | (s) | τ (s) | (-) | (-) |
|---|---|---|---|---|---|
| RTDS, 90–125 | 16.2 ± 0.3 | 256 ± 1 | 266 ± 5 | 0.960 | 308 ± 73 |
| RTDS, 125–180 | 16.5 ± 0.1 | 248 ± 2 | 260 ± 2 | 0.951 | 286 ± 20 |
| RTDS, 90–180 | 17.3 ± 0.5 | 240 ± 1 | 249 ± 6 | 0.962 | 353 ± 65 |
| RTDL | 16.6 ± 0.5 | 227 ± 8 | 226 ± 7 | 1.006 | 383 ± 5 |
| Sample | (μm∙s−1) | Clogging | ||||||
|---|---|---|---|---|---|---|---|---|
| seed90–125 1 | 0.00802 | 0.00872 | 66 ± 3 | 31.5 | 0.148 | 3.55 | 115 | no |
| seed90–125 2 * | 0.00628 | 0.00868 | 60 ± 8 | 29.4 | 0.147 | 3.34 | 66 | no |
| seed125–180 1 | 0.00984 | 0.00725 | 65 ± 4 | 36.4 | 0.161 | 3.76 | 57 + 20 | yes |
| seed125–180 2 | 0.00759 | 0.00570 | 55 ± 9 | 37.8 | 0.170 | 3.69 | 10 + 37 | yes |
| seed90–180 1 | 0.00828 | 0.00659 | 51 ± 6 | -6 | -0.024 | 4.24 | 23 | yes |
| seed90–180 2 ** | 0.01158 | 0.00394 | 58 ± 11 | 18.5 | 0.088 | 3.52 | 35 + 8 + 6 + 16 | yes |
| seed90–125 3 | 0.00144 | 0.00127 | 18 ± 5 | 66.0 | 0.256 | 4.29 | 39 | yes |
| seed90–125 4 | 0.00112 | 0.00042 | 7 ± 2 | 71.5 | 0.294 | 4.05 | 45 | yes |
| glycine90–125 ** | 0.00615 | 0.01457 | 71 ± 4 | 39.9 | 0.248 | 2.69 | 32 | yes |
| Sample | l-Alanine Batch Number | |||
|---|---|---|---|---|
| 1 | 1941180707 | 1.371 | 3.6 | 18 |
| 2 | 1941180707 | 1.356 | 3.4 | 12 |
| 3 | 1941180707 | 1.314 | 3.5 | 20 |
| 4 | 1941200315 | 1.352 | 3.4 | 9 |
| 5 | 1941200315 | 1.328 | 3.3 | 7 |
| Criterion | CFIC (This Work and Reference [56]) | CFI Hohmann et al. [42] | SFC Termühlen et al. [44] |
|---|---|---|---|
| Inner Diameter (mm) | 1.6 | 4 | 3.18 |
| Tube Length (m) | 7.8 ∙ number.CU | 6.54 | 7, 13.25 and 26.5 |
| Flow Rate | ≈16 g min−1 | 30, 40 g min−1 | 20, 40 mL min−1 |
| Residence Time (min) | ≈1 ∙ number.CU | 2.75, 2.15 | 10.5, 5.25 |
| Cooling Strategy | tube-in-tube counter-current (with water) | housed tube counter-current (with gas) | tube-in-tube co-current (water as active insulation) |
| Crystal Growth Rate (µm s−1) | 0.088–0.170 (seed90–180 1 excluded) | 0.055 to 0.079 | 0.127 to 0.411 |
| Operation Time | 2.6τ to 30τ 10–115 min | ≥3τ | 3.8τ to 7.6τ 40 min |
| Tendency to Clogging | relatively high | stable | seems stable |
| Other Factors |
|
| |
| |||
| Criterion | USU+CFIC (This Work) | Eder et al., 2012 [23] | Besenhard et al., 2015 [24] | Han et al., 2018 [19] Ultrasonicated Conditions Only |
|---|---|---|---|---|
| Substance System | l-alanine in water | acetylsalicylic acid in ethanol | phthalic acid in water | |
| Inner Diameter (mm) | 1.6 | 2 | 4 | |
| Tube Length (m) | 6 + 15.6 | 3 + 27 | 15 | 12/18 |
| Tube Material | FEP | polysiloxane | stainless steel | |
| Flow Rate | ≈16 g min−1 | 15 mL min−1 | 22 (feed) + 4–11 (seed) mL min−1 | 50–150 mL min−1 |
| Residence Time (s) | ≈45 + 120 | ≈215, 265 | ≈100 | 60–271 |
| Cooling Strategy | cooled USU and tube-in-tube counter-current in the CFIC (with water) | step-wise (7 levels) (with cryostats) | step-wise (6 levels) (with cryostats) | step-wise (2 levels) (with cryostats) |
| Operation Mode |
|
|
|
|
| Ultrasound Device | ultrasonic bath, 37 kHz, | ultrasonic bath, 35 kHz | ultrasonic baths, 35 kHz | |
| Operation Time | 7–20 min | ≈25 min | 5 ∙ (10 min + 2.5 min rinsing) = 60 min | three to four residence times |
| Location of Crystal Samples | non-invasive inline | crystallizers outlet (filtrated, washed, dried) | online in an external chamber | after filtering and drying |
| Particle Size (µm) | 69–215 | 50–190 | 90–130 | seed ≈ 11 product ≈ 10–400 |
| Tendency to Clogging | relatively high and variable | n/a | no clogging occurred | operation time for three or four residence times was defined as feasible |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmalenberg, M.; Kreis, S.; Weick, L.K.; Haas, C.; Sallamon, F.; Kockmann, N. Continuous Cooling Crystallization in a Coiled Flow Inverter Crystallizer Technology—Design, Characterization, and Hurdles. Processes 2021, 9, 1537. https://doi.org/10.3390/pr9091537
Schmalenberg M, Kreis S, Weick LK, Haas C, Sallamon F, Kockmann N. Continuous Cooling Crystallization in a Coiled Flow Inverter Crystallizer Technology—Design, Characterization, and Hurdles. Processes. 2021; 9(9):1537. https://doi.org/10.3390/pr9091537
Chicago/Turabian StyleSchmalenberg, Mira, Stephanie Kreis, Lena K. Weick, Christian Haas, Fabian Sallamon, and Norbert Kockmann. 2021. "Continuous Cooling Crystallization in a Coiled Flow Inverter Crystallizer Technology—Design, Characterization, and Hurdles" Processes 9, no. 9: 1537. https://doi.org/10.3390/pr9091537
APA StyleSchmalenberg, M., Kreis, S., Weick, L. K., Haas, C., Sallamon, F., & Kockmann, N. (2021). Continuous Cooling Crystallization in a Coiled Flow Inverter Crystallizer Technology—Design, Characterization, and Hurdles. Processes, 9(9), 1537. https://doi.org/10.3390/pr9091537





