Cell Length Growth in the Fission Yeast Cell Cycle: Is It (Bi)linear or (Bi)exponential?
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Media, and Film Techniques
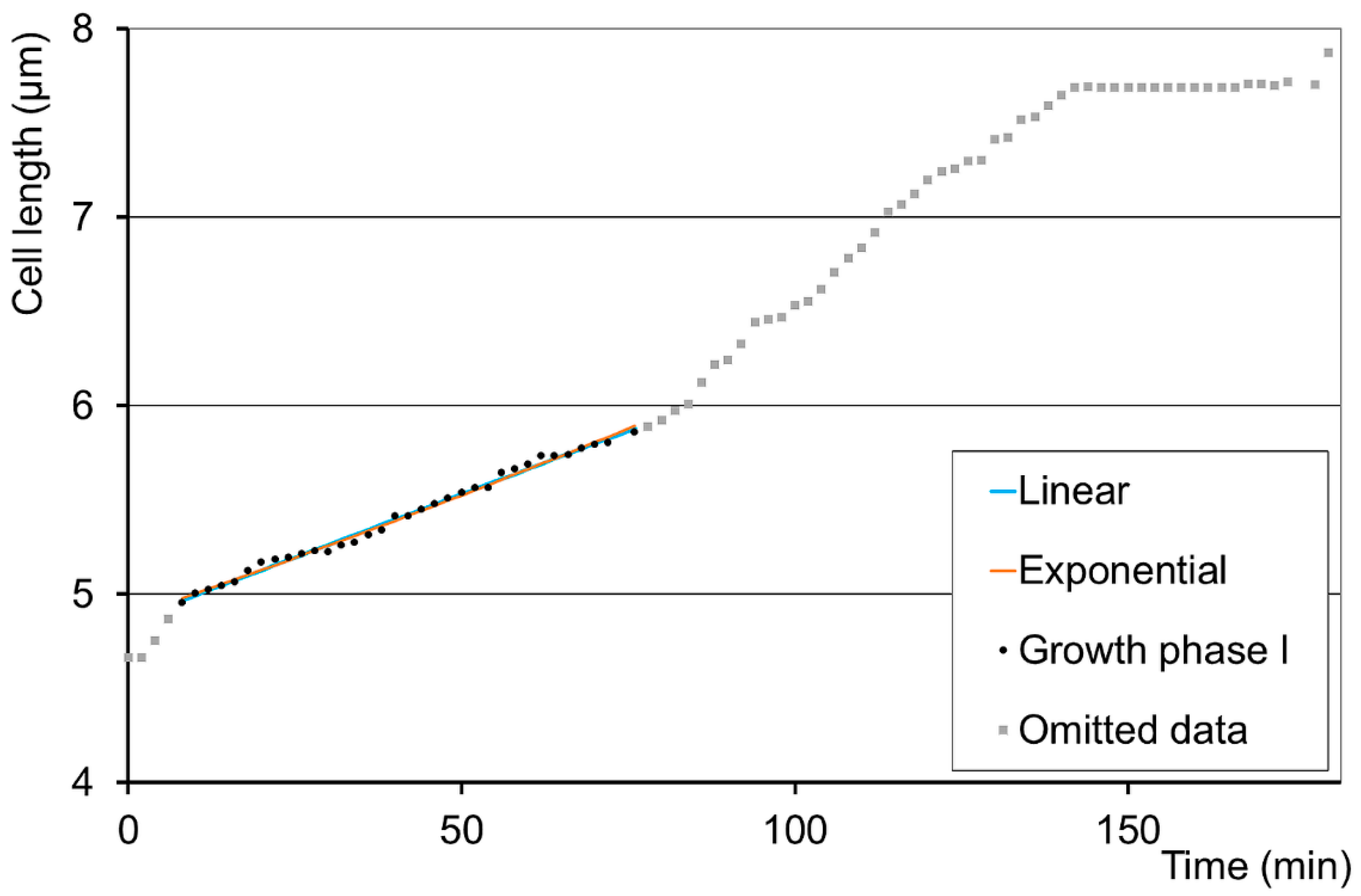
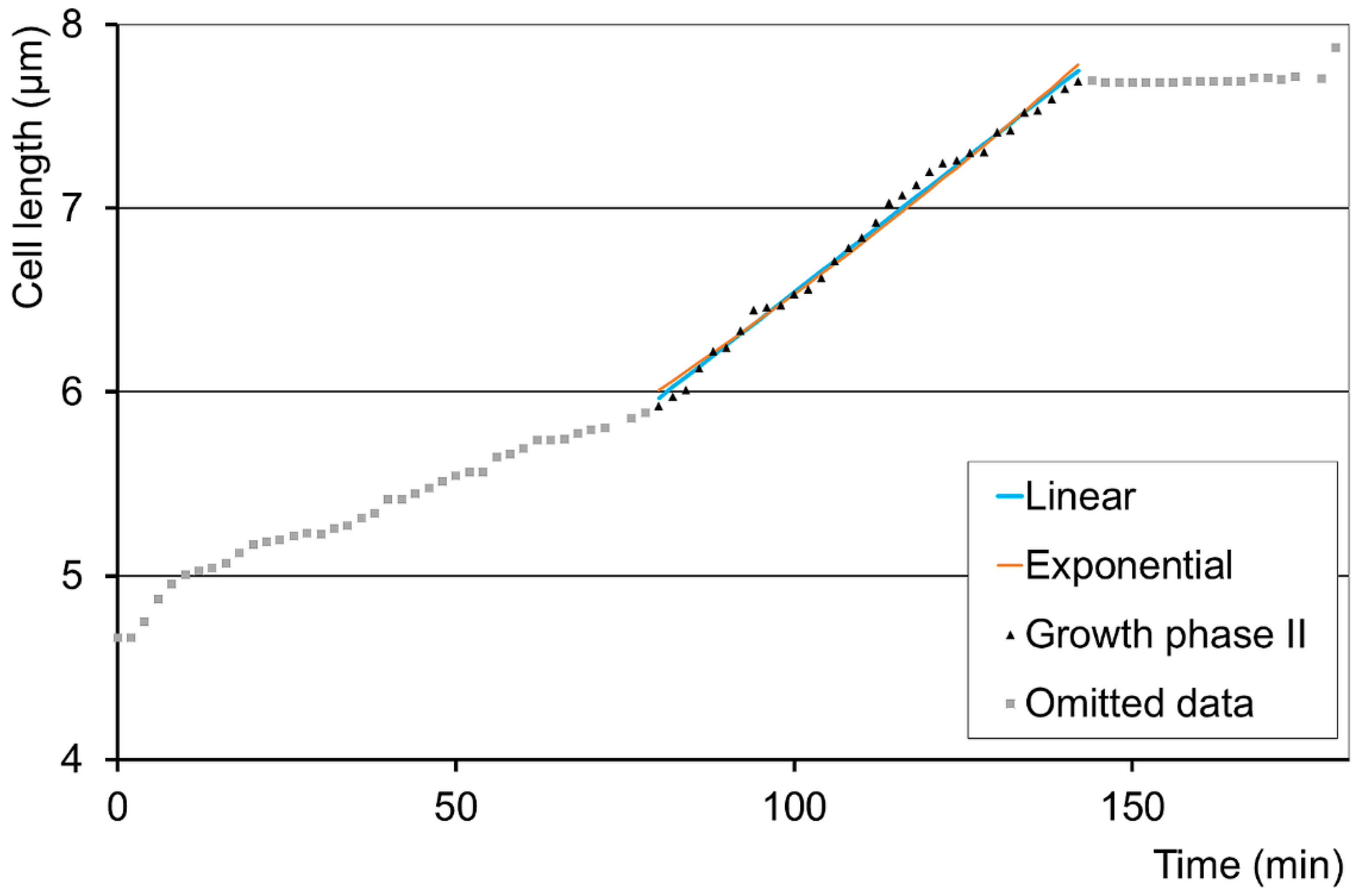
2.2. Cell Length Measurements and Model Fittings
2.3. Further Analyses of Bilinear Patterns
3. Results
3.1. Growth Patterns in Different Cell Cultures
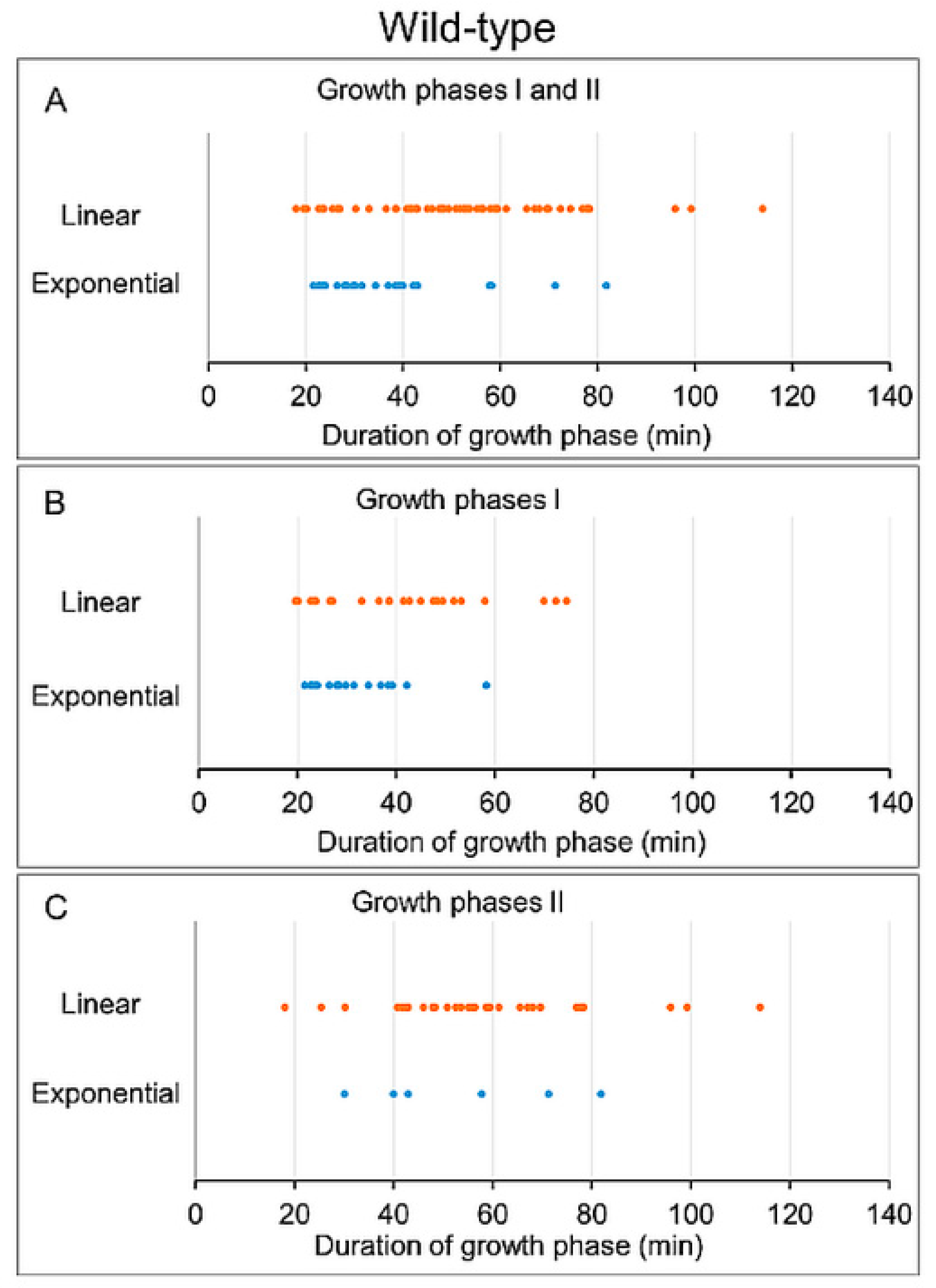
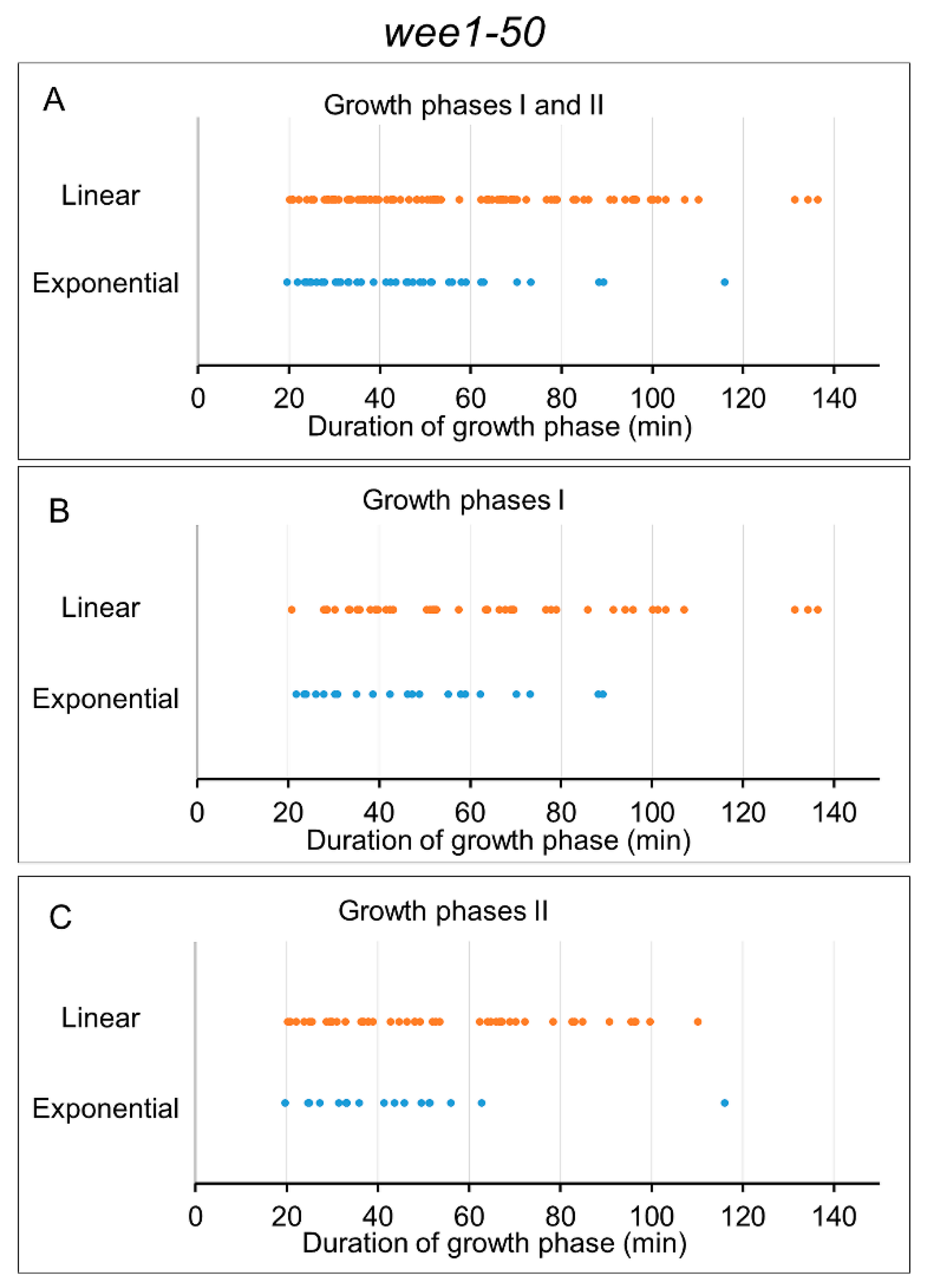
3.2. Further Growth Pattern Analyses of the Adequate Bilinear Cases
3.3. Analysis of Growth Phases in Wild-Type Cells
3.4. Analysis of Growth Phases in Wee1-50 Mutant Cells
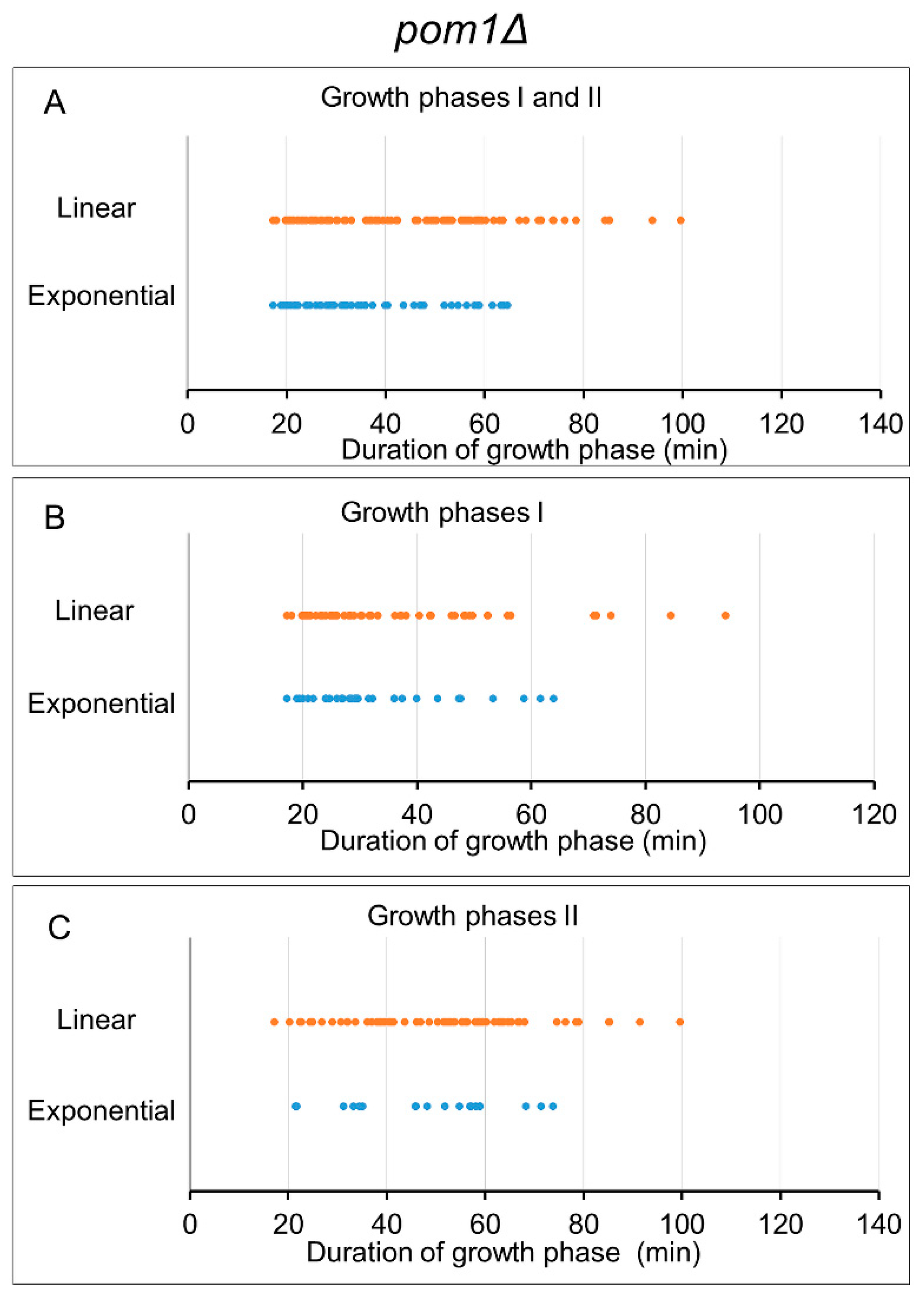
3.5. Analysis of Growth Phases in Pom1δ Mutant Cells
3.6. Analysis of Both Growth Segments in the Same Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitchison, J.M.; Nurse, P. Growth in cell length in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 1985, 75, 357–376. [Google Scholar] [CrossRef] [PubMed]
- May, J.W.; Mitchison, J.M. Pattern of polar extension of the cell wall in the fission yeast Schizosaccharomyces pombe. Can. J. Microbiol. 1995, 41, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, S.; Tolic-Norrelykke, I.M. Growth pattern of single fission yeast cells is bilinear and depends on temperature and DNA synthesis. Biophys. J. 2009, 96, 4336–4347. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S. Schizosaccharomyces pombe grows exponentially during the division cycle with no rate change points. FEMS Yeast Res. 2013, 13, 650–658. [Google Scholar] [CrossRef]
- Horváth, A.; Rácz-Mónus, A.; Buchwald, P.; Sveiczer, A. Cell length growth in fission yeast: An analysis of its bilinear character and the nature of its rate change transition. FEMS Yeast Res. 2013, 13, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Pickering, M.; Hollis, L.N.; D’Souza, E.; Rhind, N. Fission yeast cells grow approximately exponentially. Cell Cycle 2019, 18, 869–879. [Google Scholar] [CrossRef]
- Knapp, B.D.; Odermatt, P.; Rojas, E.R.; Cheng, W.; He, X.; Huang, K.C.; Chang, F. Decoupling of Rates of Protein Synthesis from Cell Expansion Leads to Supergrowth. Cell Syst. 2019, 9, 434–445. [Google Scholar] [CrossRef]
- Neumann, F.R.; Nurse, P. Nuclear size control in fission yeast. J. Cell Biol. 2007, 179, 593–600. [Google Scholar] [CrossRef]
- Moreno, S.; Nurse, P. Regulation of progression through the G1 phase of the cell cycle by the rum1+ gene. Nature 1994, 367, 236–242. [Google Scholar] [CrossRef]
- Allard, C.A.H.; Opalko, H.E.; Liu, K.W.; Medoh, U.; Moseley, J.B. Cell size-dependent regulation of Wee1 localization by Cdr2 cortical nodes. J. Cell Biol. 2018, 217, 1589–1599. [Google Scholar] [CrossRef]
- Gerganova, V.; Bhatia, P.; Vincenzetti, V.; Martin, S.G. Direct and indirect regulation of Pom1 cell size pathway by the protein phosphatase 2C Ptc1. Mol. Biol. Cell 2021, 32, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.G.; Berthelot-Grosjean, M. Polar gradients of the DYRK-family kinase Pom1 couple cell length with the cell cycle. Nature 2009, 459, 852–856. [Google Scholar] [CrossRef]
- Moseley, J.B.; Mayeux, A.; Paoletti, A.; Nurse, P. A spatial gradient coordinates cell size and mitotic entry in fission yeast. Nature 2009, 459, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Opalko, H.E.; Nasa, I.; Kettenbach, A.N.; Moseley, J.B. A mechanism for how Cdr1/Nim1 kinase promotes mitotic entry by inhibiting Wee1. Mol. Biol. Cell 2019, 30, 3015–3023. [Google Scholar] [CrossRef]
- Rhind, N.; Ohira, M.J.; D’Souza, E.; Magner, M.; Mayhew, M.B.; Keifenheim, D.; Sun, X.-M.; Marguerat, S. Size-Dependent Expression of the Mitotic Activator Cdc25 Suggests a Mechanism of Size Control in Fission Yeast. Curr. Biol. 2017, 27, 1491–1497. [Google Scholar] [CrossRef]
- Navarro, F.J.; Weston, L.; Nurse, P. Global control of cell growth in fission yeast and its coordination with the cell cycle. Curr. Opin. Cell Biol. 2012, 24, 833–837. [Google Scholar] [CrossRef]
- Patterson, J.O.; Rees, P.; Nurse, P. Noisy Cell-Size-Correlated Expression of Cyclin B Drives Probabilistic Cell-Size Homeostasis in Fission Yeast. Curr. Biol. 2019, 29, 1379–1386.e4. [Google Scholar] [CrossRef]
- Nurse, P.; Fantes, P.A. Cell cycle controls in fission yeast: A genetic analysis. In The Cell Cycle; John, P.C.L., Ed.; Cambridge University Press: Cambridge, UK, 1981; pp. 85–98. [Google Scholar]
- Sveiczer, A.; Novak, B.; Mitchison, J.M. Mitotic control in the absence of cdc25 mitotic inducer in fission yeast. J. Cell Sci. 1999, 112, 1085–1092. [Google Scholar] [CrossRef]
- Horváth, A.; Rácz-Mónus, A.; Buchwald, P.; Sveiczer, Á. Cell length growth patterns in fission yeast reveal a novel size control mechanism operating in late G2 phase. Biol. Cell 2016, 108, 259–277. [Google Scholar] [CrossRef]
- Sveiczer, A.; Novak, B.; Mitchison, J.M. The size control of fission yeast revisited. J. Cell Sci. 1996, 109, 2947–2957. [Google Scholar] [CrossRef]
- Nagy, Z.; Medgyes-Horváth, A.; Vörös, E.; Sveiczer, Á. Strongly oversized fission yeast cells lack any size control and tend to grow linearly rather than bilinearly. Yeast 2021, 38, 206–221. [Google Scholar] [CrossRef]
- Coudreuse, D.; Nurse, P. Driving the cell cycle with a minimal CDK control network. Nature 2010, 468, 1074–1079. [Google Scholar] [CrossRef]
- Schmoller KM The phenomenology of cell size control. Curr. Opin. Cell Biol. 2017, 49, 53–58. [CrossRef] [PubMed]
- Scotchman, E.; Kume, K.; Navarro, F.J.; Nurse, P. Identification of mutants with increased variation in cell size at onset of mitosis in fission yeast. J. Cell Sci. 2021, 134. [Google Scholar] [CrossRef] [PubMed]
- Odermatt, P.D.; Miettinen, T.P.; Lemière, J.; Kang, J.H.; Bostan, E.; Manalis, S.R.; Huang, K.C.; Chang, F. Variations of intracellular density during the cell cycle arise from tip-growth regulation in fission yeast. Elife 2021, 10, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Sveiczer, Á.; Horváth, A.; Buchwald, P. Is there a universal rule for cellular growth?—Problems in studying and interpreting this phenomenon. FEMS Yeast Res. 2014, 14, 679–682. [Google Scholar] [CrossRef][Green Version]
- Mitchison, J.M. Growth during the cell cycle. Int. Rev. Cytol. 2003, 226, 165–258. [Google Scholar]
- Sveiczer, A.; Tyson, J.J.; Novak, B. Modelling the fission yeast cell cycle. Brief. Funct. Genom. Proteom. 2004, 2, 298–307. [Google Scholar] [CrossRef]
- Chica, N.; Rozalén, A.E.; Pérez-Hidalgo, L.; Rubio, A.; Novak, B.; Moreno, S. Nutritional Control of Cell Size by the Greatwall-Endosulfine-PP2A·B55 Pathway. Curr. Biol. 2016, 26, 319–330. [Google Scholar] [CrossRef]
- Atilgan, E.; Magidson, V.; Khodjakov, A.; Chang, F. Morphogenesis of the fission yeast cell through cell wall expansion. Curr. Biol. 2015, 25, 2150–2157. [Google Scholar] [CrossRef]
- Hayles, J.; Nurse, P. A journey into space. Nat. Rev. Mol. Cell. Biol. 2001, 2, 647–656. [Google Scholar] [CrossRef]
- Verde, F. On growth and form: Control of cell morphogenesis in fission yeast. Curr. Opin. Microbiol. 1998, 1, 712–718. [Google Scholar] [CrossRef]
- Facchetti, G.; Knapp, B.; Flor-Parra, I.; Chang, F.; Howard, M. Reprogramming Cdr2-Dependent Geometry-Based Cell Size Control in Fission Yeast. Curr. Biol. 2019, 29, 350–358. [Google Scholar] [CrossRef]
- Creanor, J.; Mitchison, J.M. Patterns of protein synthesis during the cell cycle of the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 1982, 58, 263–285. [Google Scholar] [CrossRef]
- Buchwald, P.; Sveiczer, A. The time-profile of cell growth in fission yeast: Model selection criteria favoring bilinear models over exponential ones. Theor. Biol. Med. Model. 2006, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Drake, T.; Wiley, D.J.; Buchwald, P.; Vavylonis, D.; Verde, F. Oscillatory dynamics of Cdc42 GTPase in the control of polarized growth. Science 2012, 337, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Sveiczer, Á.; Horváth, A. How do fission yeast cells grow and connect growth to the mitotic cycle? Curr. Genet. 2017, 63, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Reshes, G.; Vanounou, S.; Fishov, I.; Feingold, M. Cell shape dynamics in Escherichia coli. Biophys. J. 2008, 94, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Bryan, A.K.; Goranov, A.; Amon, A.; Manalis, S.R. Measurement of mass, density, and volume during the cell cycle of yeast. Proc. Natl. Acad. Sci. USA 2010, 107, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Hannebelle, M.T.M.; Ven, J.X.Y.; Toniolo, C.; Eskandarian, H.A.; Vuaridel-Thurre, G.; McKinney, J.D.; Fantner, G.E. A biphasic growth model for cell pole elongation in mycobacteria. Nat. Commun. 2020, 11, 452. [Google Scholar] [CrossRef]
- Nurse, P.; Thuriaux, P.; Nasmyth, K. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol. Gen. Genet. 1976, 146, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, P. A general bilinear model to describe growth or decline time profiles. Math. Biosci. 2007, 205, 108–136. [Google Scholar] [CrossRef]
- Fantes, P.A.; Nurse, P. Control of the timing of cell division in fission yeast: Cell size mutants reveal a second control pathway. Exp. Cell Res. 1978, 115, 317–329. [Google Scholar] [CrossRef]
- Mitchison, J.M. Cell cycle growth and periodicities. In Molecular Biology of the Fission Yeast; Nasim, A., Young, P., Johnson, B.F., Eds.; Academic Press: New York, NY, USA, 1989; pp. 205–242. [Google Scholar]
- Sorrell, D.A.; Marchbank, A.; McMahon, K.; Dickinson, R.J.; Rogers, H.J.; Francis, D. A WEE1 homologue from Arabidopsis thaliana. Planta 2002, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Z.; Medgyes-Horváth, A.; Szalay, C.; Sipiczki, M.; Sveiczer, Á. Phylogenetic Analyses of Proteins Coordinating G2 Size Control in Fission Yeast. Period. Polytech. Chem. Eng. 2019, 63, 555–568. [Google Scholar] [CrossRef]
- Wood, E.; Nurse, P. Pom1 and cell size homeostasis in fission yeast. Cell Cycle 2013, 12, 3228–3236. [Google Scholar] [CrossRef]
- Bähler, J.; Pringle, J.R. Pom1p, a fission yeast protein kinase that provides positional information for both polarized growth and cytokinesis. Genes Dev. 1998, 12, 1356–1370. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.G.; Chang, F. New end take off: Regulating cell polarity during the fission yeast cell cycle. Cell Cycle 2005, 4, 1046–1049. [Google Scholar] [CrossRef][Green Version]

| Wild-Type | wee1-50 | pom1Δ | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Bilinear | 40 | 65.6 | 65 | 69.9 | 85 | 59.0 |
| Linear | 15 | 24.6 | 22 | 23.7 | 47 | 32.6 |
| Exponential | 6 | 9.8 | 6 | 6.4 | 12 | 8.3 |
| Total | 61 | 100.0 | 93 | 100.0 | 144 | 100.0 |
| WT | ||||||
|---|---|---|---|---|---|---|
| N | Mean ± SD (min) | |||||
| Total | Exp | Lin | Exp | Lin | p | |
| All growth phases | 23 | 57 | 37.4 ± 16.0 | 52.6 ± 20.7 | * 0.001 | |
| Growth phase I | 17 | 23 | 31.6 ± 9.4 | 42.0 ± 16.5 | * 0.026 | |
| Growth phase II | 6 | 34 | 54.0 ± 19.9 | 59.8 ± 20.3 | 0.520 | |
| <40 | All growth phases | 16 | 14 | 29.2 ± 5.7 | 27.5 ± 7.0 | 0.468 |
| Growth phase I | 15 | 11 | 29.1 ± 5.9 | 28.2 ± 7.2 | 0.740 | |
| Growth phase II | 1 | 3 | 30.1 | 24.6 ± 6.1 | − | |
| ≥40 | All growth phases | 7 | 43 | 56.3 ± 15.9 | 60.8 ± 16.6 | 0.513 |
| Growth phase I | 2 | 12 | 50.2 ± 11.4 | 54.5 ± 11.6 | 0.708 | |
| Growth phase II | 5 | 31 | 58.8 ± 18.0 | 63.2 ± 17.8 | 0.628 | |
| wee1-50 | ||||||
|---|---|---|---|---|---|---|
| N | Mean ± SD (min) | |||||
| Total | Exp | Lin | Exp | Lin | p | |
| All growth phases | 39 | 91 | 45.7 ± 21.0 | 59.1 ± 28.0 | * 0.003 | |
| Growth phase I | 21 | 44 | 47.6 ± 20.7 | 63.9 ± 30.6 | * 0.014 | |
| Growth phase II | 18 | 47 | 43.4 ± 21.7 | 54.7 ± 24.7 | 0.080 | |
| <40 | All growth phases | 18 | 31 | 29.0 ± 5.3 | 31.1 ± 6.0 | 0.200 |
| Growth phase I | 9 | 13 | 28.7 ± 5.6 | 32.3 ± 5.5 | 0.149 | |
| Growth phase II | 9 | 18 | 29.3 ± 5.3 | 30.3 ± 6.3 | 0.673 | |
| ≥40 | All growth phases | 21 | 60 | 59.9 ± 18.7 | 73.6 ± 23.4 | * 0.010 |
| Growth phase I | 12 | 31 | 61.7 ± 15.7 | 77.1 ± 26.8 | * 0.026 | |
| Growth phase II | 9 | 29 | 57.6 ± 22.9 | 69.9 ± 18.9 | 0.170 | |
| pom1Δ | ||||||
|---|---|---|---|---|---|---|
| N | Mean ± SD (min) | |||||
| Total | Exp | Lin | Exp | Lin | p | |
| All growth phases | 50 | 120 | 36.6 ± 14.2 | 45.8 ± 19.5 | * 0.001 | |
| Growth phase I | 31 | 54 | 33.4 ± 12.9 | 36.8 ± 17.6 | 0.312 | |
| Growth phase II | 19 | 66 | 41.7 ± 15.1 | 53.1 ± 17.9 | * 0.009 | |
| <40 | All growth phases | 33 | 52 | 27.6 ± 6.1 | 27.6 ± 6.5 | 0.997 |
| Growth phase I | 24 | 35 | 27.5 ± 6.1 | 26.2 ± 5.9 | 0.451 | |
| Growth phase II | 9 | 17 | 28.0 ± 6.3 | 30.4 ± 7.0 | 0.382 | |
| ≥40 | All growth phases | 17 | 68 | 54.0 ± 7.6 | 59.7 ± 13.8 | * 0.027 |
| Growth phase I | 7 | 19 | 53.7 ± 7.9 | 56.2 ± 15.4 | 0.602 | |
| Growth phase II | 10 | 49 | 54.1 ± 7.8 | 61.0 ± 13.0 | * 0.038 | |
| WT | wee1-50 | pom1Δ | Σ = WT + wee1-50 + pom1Δ | |
|---|---|---|---|---|
| Lin-Lin | 9 | 15 | 8 | 32 (71%) |
| Exp-Lin | 3 | 1 | 2 | 6 (13%) |
| Lin-Exp | 0 | 3 | 1 | 4 (9%) |
| Exp-Exp | 0 | 3 | 0 | 3 (7%) |
| All | 12 | 22 | 11 | 45 (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pesti, B.; Nagy, Z.; Papp, L.A.; Sipiczki, M.; Sveiczer, Á. Cell Length Growth in the Fission Yeast Cell Cycle: Is It (Bi)linear or (Bi)exponential? Processes 2021, 9, 1533. https://doi.org/10.3390/pr9091533
Pesti B, Nagy Z, Papp LA, Sipiczki M, Sveiczer Á. Cell Length Growth in the Fission Yeast Cell Cycle: Is It (Bi)linear or (Bi)exponential? Processes. 2021; 9(9):1533. https://doi.org/10.3390/pr9091533
Chicago/Turabian StylePesti, Benedek, Zsófia Nagy, László Attila Papp, Matthias Sipiczki, and Ákos Sveiczer. 2021. "Cell Length Growth in the Fission Yeast Cell Cycle: Is It (Bi)linear or (Bi)exponential?" Processes 9, no. 9: 1533. https://doi.org/10.3390/pr9091533
APA StylePesti, B., Nagy, Z., Papp, L. A., Sipiczki, M., & Sveiczer, Á. (2021). Cell Length Growth in the Fission Yeast Cell Cycle: Is It (Bi)linear or (Bi)exponential? Processes, 9(9), 1533. https://doi.org/10.3390/pr9091533







