Anaerobic Acidogenic Fermentation of Cellobiose by Immobilized Cells: Prediction of Organic Acids Production by Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture and Growth Media
2.2. Cell Immobilization and Anaerobic Acidogenic Fermentation
2.3. Scanning Electron Microscope Imaging
2.4. Organic Acids Analysis
2.5. Experimental Design and Statistical Analysis
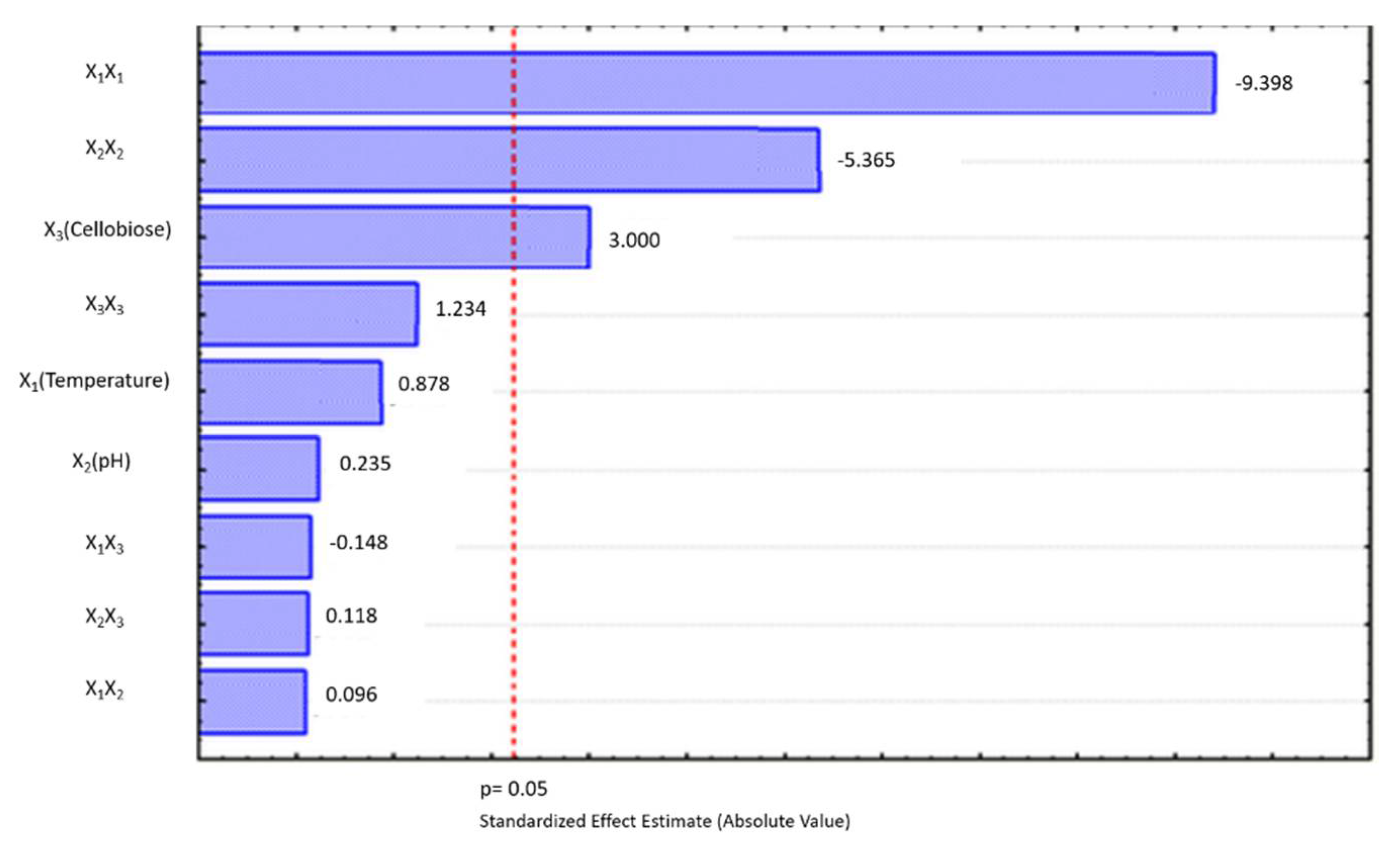
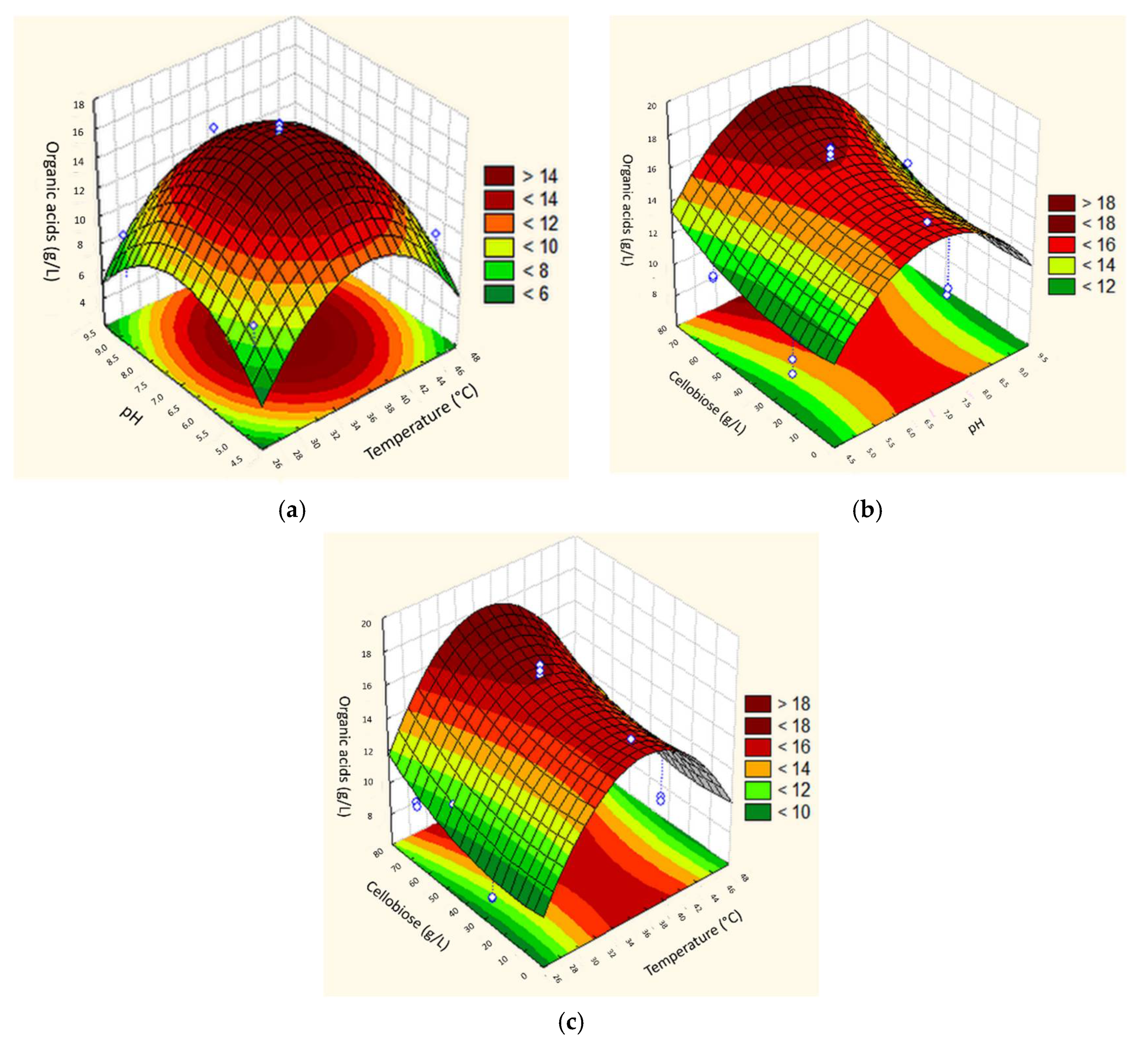
3. Results and Discussion
3.1. Cell Immobilization
3.2. Anaerobic Acidogenic Fermentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Menon, V.; Rao, M. Trends in bioconversion of lignocellulose: Biofuels, platform chemicals & biorefinery concept. Prog. Energy Combust. Sci. 2012, 38, 522–550. [Google Scholar]
- Deng, W.; Zhang, Q.; Wang, Y. Catalytic transformations of cellulose and cellulose-derived carbohydrates into organic acids. Catal. Today 2014, 234, 31–41. [Google Scholar] [CrossRef]
- Sanna, A. Advanced biofuels from thermochemical processing of sustainable biomass in Europe. BioEnergy Res. 2014, 7, 36–47. [Google Scholar] [CrossRef]
- Fontanille, P.; Kumar, V.; Christophe, G.; Nouaille, R.; Larroche, C. Bioconversion of volatile fatty acids into lipids by the oleaginous yeast Yarrowia lipolytica. Bioresour. Technol. 2012, 114, 443–449. [Google Scholar] [CrossRef] [PubMed]
- De La Rubia, M.A.; Raposo, F.; Rincón, B.; Borja, R. Evaluation of the hydrolytic–acidogenic step of a two-stage mesophilic anaerobic digestion process of sunflower oil cake. Bioresour. Technol. 2009, 100, 4133–4138. [Google Scholar] [CrossRef] [PubMed]
- Syngiridis, K.; Bekatorou, A.; Kallis, M.; Kandylis, P.; Kanellaki, M.; Koutinas, A.A. γ-Alumina as a process advancing tool for a new generation biofuel. Bioresour. Technol. 2013, 132, 45–48. [Google Scholar] [CrossRef]
- Syngiridis, K.; Bekatorou, A.; Kandylis, P.; Larroche, C.; Kanellaki, M.; Koutinas, A.A. Favouring butyrate production for a new generation biofuel by acidogenic glucose fermentation using cells immobilised on γ-alumina. Bioresour. Technol. 2014, 161, 118–123. [Google Scholar] [CrossRef]
- Lappa, K.; Kandylis, P.; Bekatorou, A.; Bastas, N.; Klaoudatos, S.; Athanasopoulos, N.; Kanellaki, M.; Koutinas, A.A. Continuous acidogenesis of sucrose, raffinose and vinasse using mineral kissiris as promoter. Bioresour. Technol. 2015, 188, 43–48. [Google Scholar] [CrossRef]
- Koutinas, A.; Kanellaki, M.; Bekatorou, A.; Kandylis, P.; Pissaridi, K.; Dima, A.; Boura, K.; Lappa, K.; Tsafrakidou, P.; Stergiou, P.-Y.; et al. Economic evaluation of technology for a new generation biofuel production using wastes. Bioresour. Technol. 2016, 200. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Wu, J. Enhancement of methane production in anaerobic digestion process: A review. Appl. Energy 2019, 240, 120–137. [Google Scholar] [CrossRef]
- Tomei, M.C.; Braguglia, C.M.; Cento, G.; Mininni, G. Modeling of anaerobic digestion of sludge. Crit. Rev. Environ. Sci. Technol. 2009, 39, 1003–1051. [Google Scholar] [CrossRef]
- Tsafrakidou, P.; Bekatorou, A.; Koutinas, A.A.; Kordulis, C.; Banat, I.M.; Petsi, T.; Sotiriou, M. Αcidogenic fermentation of wheat straw after chemical and microbial pretreatment for biofuel applications. Energy Convers. Manag. 2018, 160, 509–517. [Google Scholar] [CrossRef]
- Kandylis, P.; Bekatorou, A.; Pissaridi, K.; Lappa, K.; Dima, A.; Kanellaki, M.; Koutinas, A.A. Acidogenesis of cellulosic hydrolysates for new generation biofuels. Biomass Bioenergy 2016, 91, 210–216. [Google Scholar] [CrossRef]
- Liang, J.; Nabi, M.; Zhang, P.; Zhang, G.; Cai, Y.; Wang, Q.; Zhou, Z.; Ding, Y. Promising biological conversion of lignocellulosic biomass to renewable energy with rumen microorganisms: A comprehensive review. Renew. Sustain. Energy Rev. 2020, 134, 110335. [Google Scholar] [CrossRef]
- Harris, D.; DeBolt, S. Synthesis, regulation and utilization of lignocellulosic biomass. Plant Biotechnol. J. 2010, 8, 244–262. [Google Scholar] [CrossRef]
- Bajpai, P. Structure of lignocellulosic biomass. In Pretreatment of Lignocellulosic Biomass for Biofuel Production; Springer: Berlin/Heidelberg, Germany, 2016; pp. 7–12. [Google Scholar]
- Bansal, P.; Hall, M.; Realff, M.J.; Lee, J.H.; Bommarius, A.S. Modeling cellulase kinetics on lignocellulosic substrates. Biotechnol. Adv. 2009, 27, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Juturu, V.; Wu, J.C. Microbial cellulases: Engineering, production and applications. Renew. Sustain. Energy Rev. 2014, 33, 188–203. [Google Scholar] [CrossRef]
- Fischer, C.R.; Klein-Marcuschamer, D.; Stephanopoulos, G. Selection and optimization of microbial hosts for biofuels production. Metab. Eng. 2008, 10, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Anandharaj, S.J.; Gunasekaran, J.; Udayakumar, G.P.; Meganathan, Y.; Sivarajasekar, N. Biobutanol: Insight, Production and Challenges. Sustain. Dev. Energy Environ. 2020, 25–37. [Google Scholar] [CrossRef]
- Kumar, M.; Rathour, R.; Gupta, J.; Pandey, A.; Gnansounou, E.; Thakur, I.S. 2—Bacterial production of fatty acid and biodiesel: Opportunity and challenges. In Refining Biomass Residues for Sustainable Energy and Bioproducts; Academic Press: Cambridge, MA, USA, 2020; pp. 21–49. [Google Scholar] [CrossRef]
- Francis, F.; Sabu, A.; Nampoothiri, K.M.; Ramachandran, S.; Ghosh, S.; Szakacs, G.; Pandey, A. Use of response surface methodology for optimizing process parameters for the production of α-amylase by Aspergillus oryzae. Biochem. Eng. J. 2003, 15, 107–115. [Google Scholar] [CrossRef]
- Neter, J.; Kutner, M.H.; Nachtsheim, C.J.; Wasserman, W. Applied Linear Statistical Models; McGraw-Hill: New York, NY, USA, 1996. [Google Scholar]
- Koutinas, A.A.; Toutoutzidakis, G.; Kana, K.; Kouinis, I. Methane fermentation promoted by γ-alumina pellets. J. Ferment. Bioeng. 1991, 72, 64–67. [Google Scholar] [CrossRef]
- Kanellaki, M.; Koutinas, A.A.; Kana, K.; Nicolopoulou, M.; Papadimitriou, A.; Lycourghiotis, A. Ethanol production by Saccharomyces cerevisiae promoted by γ-alumina. Biotechnol. Bioeng. 1989, 34, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Boura, K.; Kandylis, P.; Bekatorou, A.; Kolliopoulos, D.; Vasileiou, D.; Panas, P.; Kanellaki, M.; Koutinas, A.A. New generation biofuel from whey: Successive acidogenesis and alcoholic fermentation using immobilized cultures on γ-alumina. Energy Convers. Manag. 2017, 135, 256–260. [Google Scholar] [CrossRef]
- Dima, A.; Boura, K.; Bekatorou, A.; Stergiou, P.-Y.; Foukis, A.; Gkini, O.A.; Kandylis, P.; Pissaridi, K.; Kanellaki, M.; Papamichael, E.M.; et al. Scale-up for esters production from straw whiskers for biofuel applications. Bioresour. Technol. 2017, 242, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Suarez, M.; Zhang, Y.; Outram, V. Current perspectives on acidogenic fermentation to produce volatile fatty acids from waste. Rev. Environ. Sci. Bio/Technol. 2021, 1–40. [Google Scholar] [CrossRef]
- Al-Amshawee, S.; Yunus, M.Y.B.M. Geometry of biofilm carriers: A systematic review deciding the best shape and pore size. Groundw. Sustain. Dev. 2020, 12, 100520. [Google Scholar] [CrossRef]
- Lu, J.; Peng, W.; Lv, Y.; Jiang, Y.; Xu, B.; Zhang, W.; Zhou, J.; Dong, W.; Xin, F.; Jiang, M. Application of cell immobilization technology in microbial cocultivation systems for biochemicals production. Ind. Eng. Chem. Res. 2020, 59, 17026–17034. [Google Scholar] [CrossRef]
- Breig, S.J.M.; Luti, K.J.K. Response surface methodology: A review on its applications and challenges in microbial cultures. Mater. Today Proc. 2021, 42, 2277–2284. [Google Scholar] [CrossRef]
- Adav, S.S.; Lee, D.-J.; Wang, A.; Ren, N. Functional consortium for hydrogen production from cellobiose: Concentration-to-extinction approach. Bioresour. Technol. 2009, 100, 2546–2550. [Google Scholar] [CrossRef]
- Varanasi, J.L.; Roy, S.; Pandit, S.; Das, D. Improvement of energy recovery from cellobiose by thermophillic dark fermentative hydrogen production followed by microbial fuel cell. Int. J. Hydrog. Energy 2015, 40, 8311–8321. [Google Scholar] [CrossRef]
- Buendia-Kandia, F.; Rondags, E.; Framboisier, X.; Mauviel, G.; Dufour, A.; Guedon, E. Diauxic growth of Clostridium acetobutylicum ATCC 824 when grown on mixtures of glucose and cellobiose. AMB Express 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, J.; Idris, A.; Aziz, R.A. Recent advances in production of succinic acid from lignocellulosic biomass. Appl. Microbiol. Biotechnol. 2014, 98, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Fang, L.; Xu, Y.; Dong, J.-J.; Ni, Y.; Sun, Z.-H. Succinic acid production from corn stover by simultaneous saccharification and fermentation using Actinobacillus succinogenes. Bioresour. Technol. 2010, 101, 7889–7894. [Google Scholar] [CrossRef] [PubMed]



| Independent Variables | Units | Symbol | Coded Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | 1 | |||
| Temperature | °C | X1 | 27 | 37 | 47 |
| Initial pH | X2 | 5 | 7 | 9 | |
| Cellobiose | g/L | X3 | 30 | 50 | 70 |
| Run Order | Variables | Total Organic Acids | |||||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Experimental Results (g/L) | Prediction (g/L) | |||
| 1 | −1 | −1 | −1 | 7.03 a | ± | 0.1 | 6.95 |
| 2 | 1 | −1 | −1 | 7.91 b | ± | 0.13 | 7.52 |
| 3 | −1 | 1 | −1 | 7.06 a | ± | 0.27 | 6.95 |
| 4 | 1 | 1 | −1 | 7.56 c | ± | 0.12 | 7.64 |
| 5 | −1 | −1 | 1 | 9.4 c | ± | 0.11 | 9.06 |
| 6 | 1 | −1 | 1 | 9.57 d | ± | 0.03 | 9.42 |
| 7 | −1 | 1 | 1 | 9.08 e | ± | 0.33 | 9.22 |
| 8 | 1 | 1 | 1 | 9.89 e | ± | 0.11 | 9.71 |
| 9 | −1 | 0 | 0 | 11.01 f | ± | 0.03 | 11.41 |
| 10 | 1 | 0 | 0 | 11.3 f,g | ± | 0.16 | 11.94 |
| 11 | 0 | −1 | 0 | 12.8 h | ± | 0.13 | 13.76 |
| 12 | 0 | 1 | 0 | 13.83 h | ± | 0.1 | 13.90 |
| 13 | 0 | 0 | −1 | 13.63 g | ± | 0.22 | 14.13 |
| 14 | 0 | 0 | 1 | 15.69 g,h | ± | 0.14 | 16.22 |
| 15 | 0 | 0 | 0 | 16.16 g | ± | 0.08 | 16.19 |
| 16 | 0 | 0 | 0 | 16.97 g | ± | 0.23 | 16.19 |
| 17 | 0 | 0 | 0 | 16.92 g,h | ± | 0.14 | 16.19 |
| 18 | 0 | 0 | 0 | 16.3 g | ± | 0.15 | 16.19 |
| 19 | 0 | 0 | 0 | 16.6 g,h | ± | 0.17 | 16.19 |
| 20 | 0 | 0 | 0 | 16.24 g | ± | 0.1 | 16.19 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | Prob (P) > F |
|---|---|---|---|---|---|
| Model | 251.8004 | 9 | 27.97782 | 74.87511 | <0.0001 |
| Residual | 3.736598 | 10 | 0.37366 | ||
| Lack of Fit | 3.112115 | 5 | 0.622423 | 4.983503 | 0.0513 |
| Pure Error | 0.624483 | 5 | 0.124897 | ||
| Total | 255.537 | 19 | |||
| R2 = 0.98, CV = 4.99%, RAdj2 = 0.97 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsafrakidou, P.; Tsigkou, K.; Bekatorou, A.; Kanellaki, M.; Koutinas, A.A. Anaerobic Acidogenic Fermentation of Cellobiose by Immobilized Cells: Prediction of Organic Acids Production by Response Surface Methodology. Processes 2021, 9, 1441. https://doi.org/10.3390/pr9081441
Tsafrakidou P, Tsigkou K, Bekatorou A, Kanellaki M, Koutinas AA. Anaerobic Acidogenic Fermentation of Cellobiose by Immobilized Cells: Prediction of Organic Acids Production by Response Surface Methodology. Processes. 2021; 9(8):1441. https://doi.org/10.3390/pr9081441
Chicago/Turabian StyleTsafrakidou, Panagiota, Konstantina Tsigkou, Argyro Bekatorou, Maria Kanellaki, and Athanasios A. Koutinas. 2021. "Anaerobic Acidogenic Fermentation of Cellobiose by Immobilized Cells: Prediction of Organic Acids Production by Response Surface Methodology" Processes 9, no. 8: 1441. https://doi.org/10.3390/pr9081441
APA StyleTsafrakidou, P., Tsigkou, K., Bekatorou, A., Kanellaki, M., & Koutinas, A. A. (2021). Anaerobic Acidogenic Fermentation of Cellobiose by Immobilized Cells: Prediction of Organic Acids Production by Response Surface Methodology. Processes, 9(8), 1441. https://doi.org/10.3390/pr9081441








