Characterization of Gold Mining Waste for Carbon Sequestration and Utilization as Supplementary Cementitious Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Mine Waste Sampling
2.2. Mineralogical Analysis
2.3. Physicochemical Analysis
2.4. Brick Fabrication for Carbon Capture and Storage
2.5. Carbonation Curing for CO2 Storage
3. Results
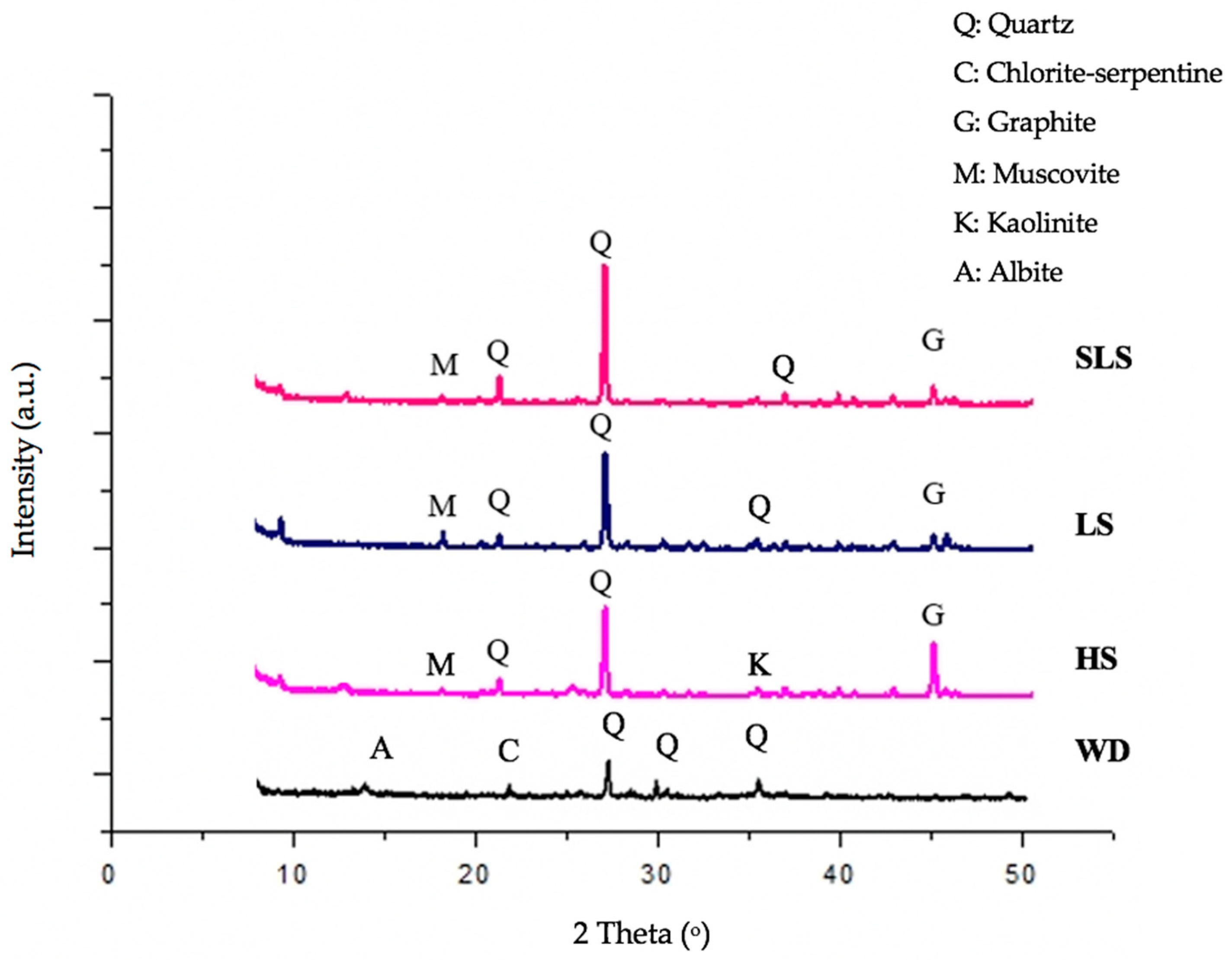
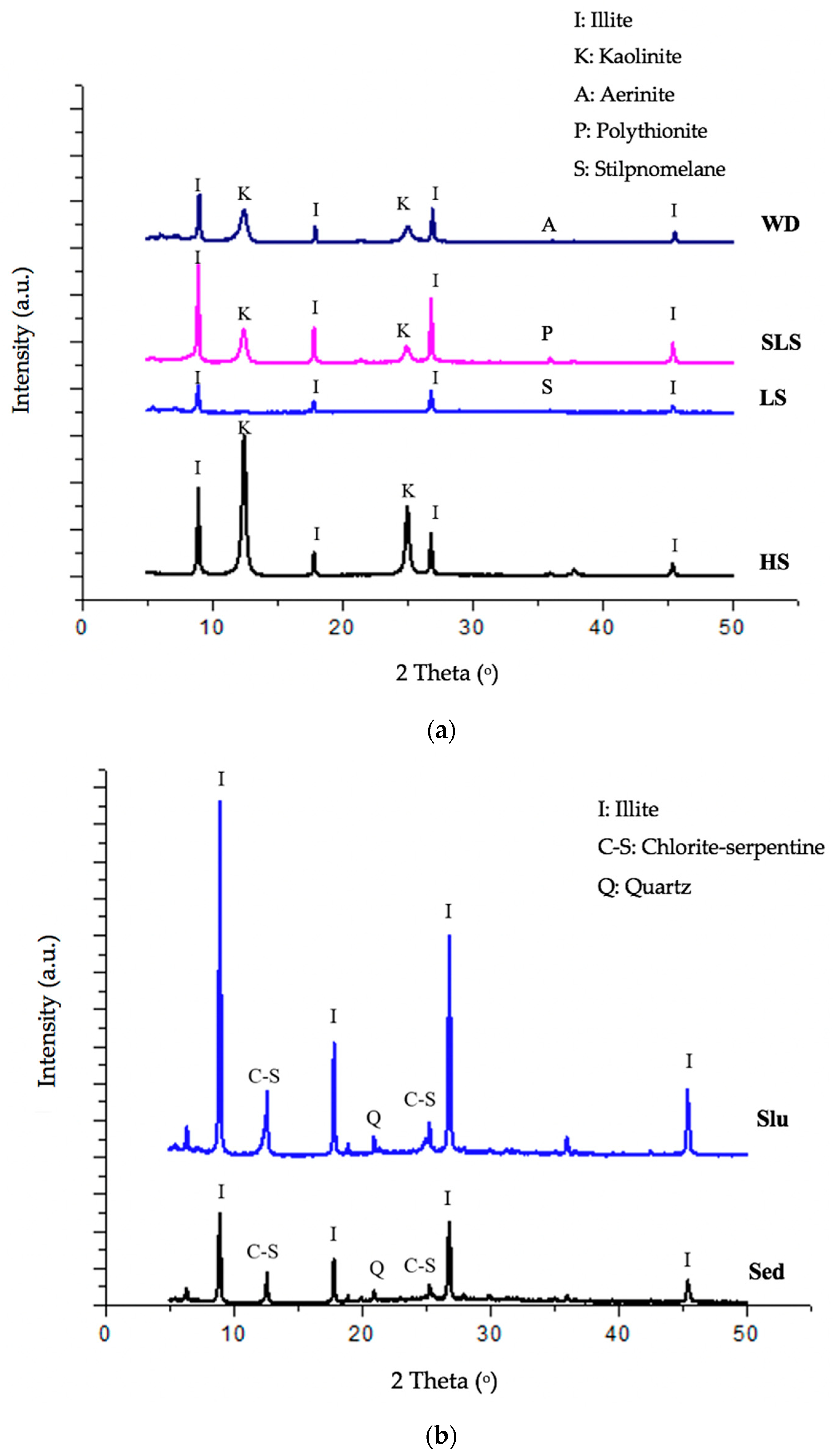
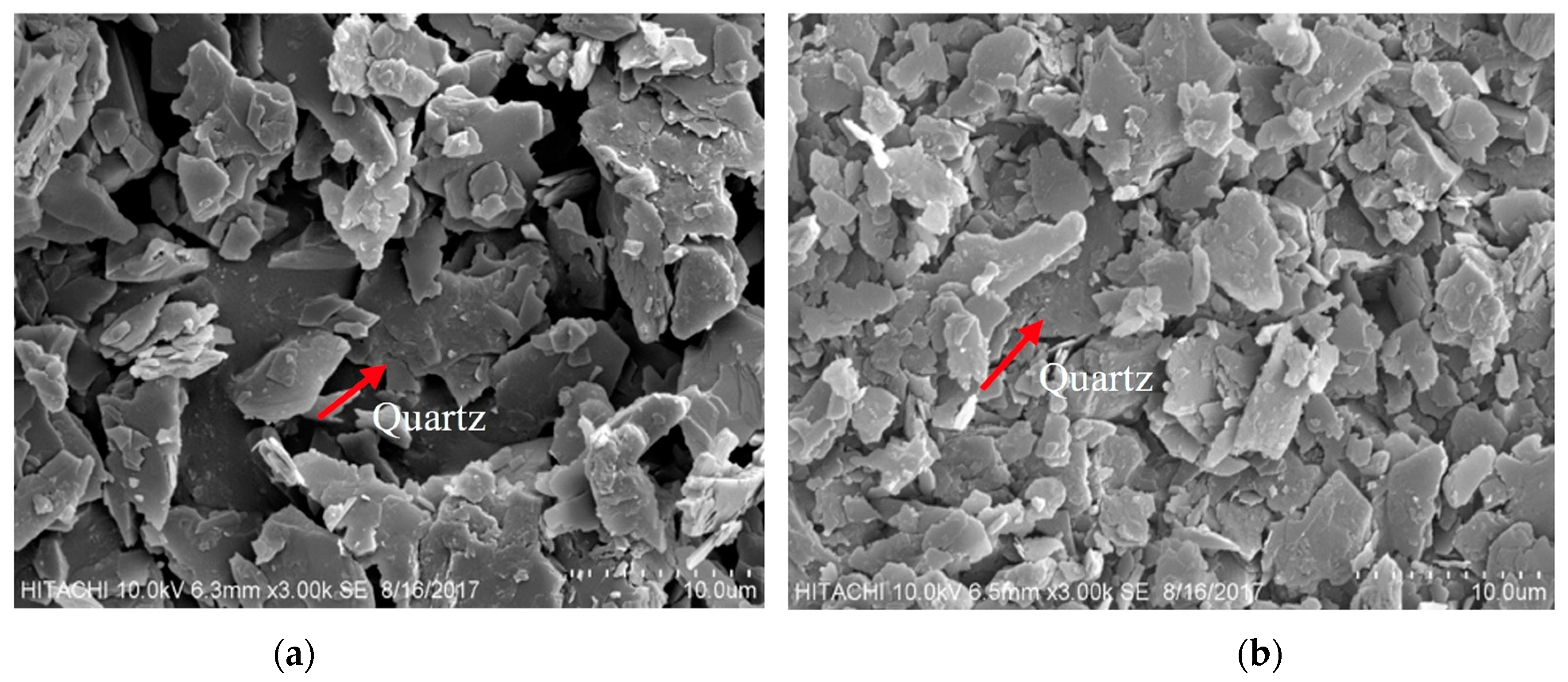
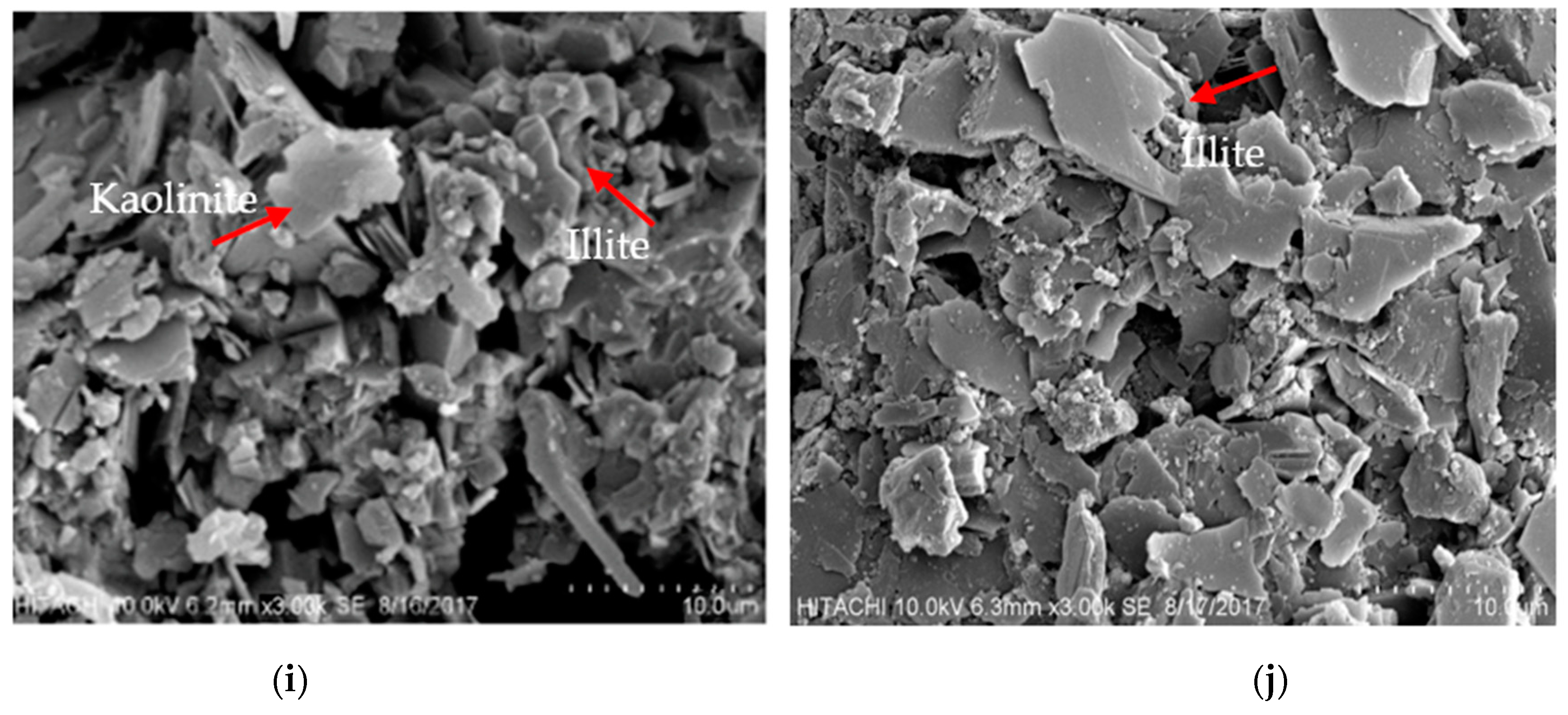
3.1. Mineralogical Characterization of Mine Waste
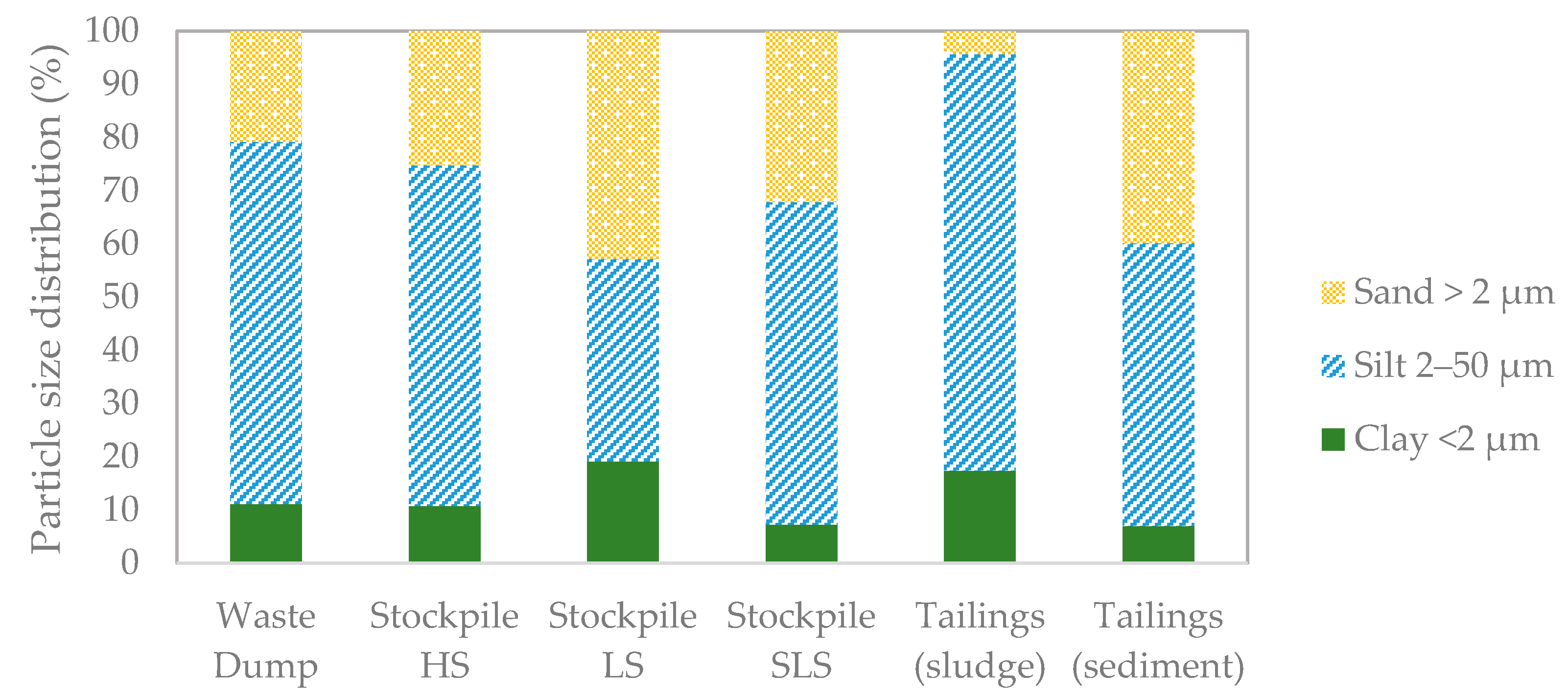
3.2. Physicochemical Composition of Mine Waste
3.3. Mine Waste Utilization as Supplementary Cementitious Material for Carbon Capture
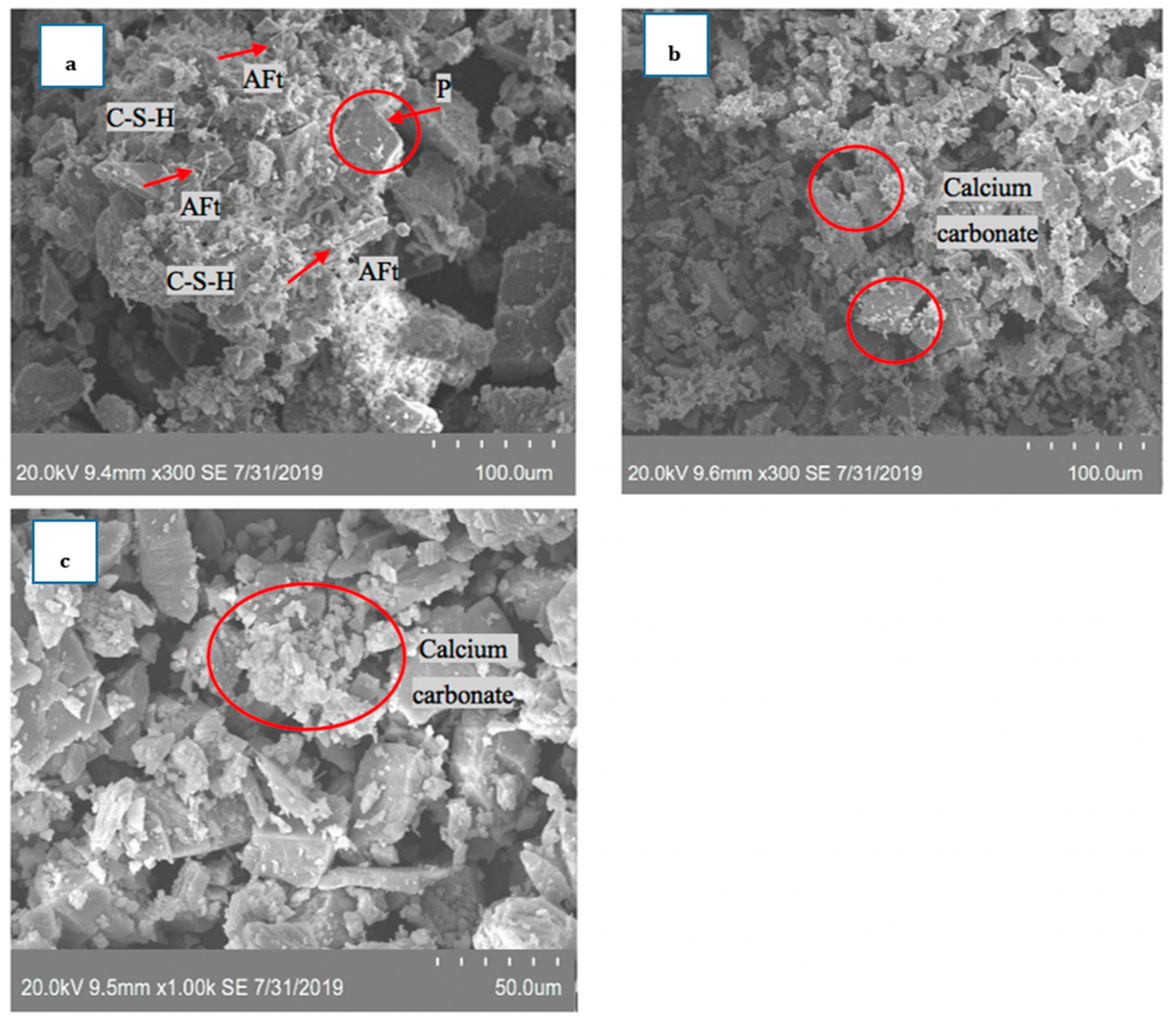
3.4. Carbon Capture and Storage in Cementitious Product
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruhaimi, A.H.; Aziz, M.A.A.; Jalil, A.A. Magnesium oxide-based adsorbents for carbon dioxide capture: Current progress and future opportunities. J. CO2 Util. 2021, 43, 101357. [Google Scholar] [CrossRef]
- IEA. World Energy Outlook; International Energy Agency (IEA): Paris, France, 2019; ISBN 9789264973008. [Google Scholar]
- IEAGHG. CO2 Capture in the Cement Industry; IEAGHG: Cheltenham, UK, 2008. [Google Scholar]
- IPCC. Global Warming of 1.5 C: An IPCC Special Report on the Impacts of Global Warming of 1.5 C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Zhang, D.; Song, J. Mechanisms for geological carbon sequestration. Procedia IUTAm 2014, 10, 319–327. [Google Scholar] [CrossRef]
- Arce, G.L.A.F.; Neto, T.G.S.; Ávila, I.; Luna, C.M.R.; dos Santos, J.C.; Carvalho, J.A. Influence of physicochemical properties of Brazilian serpentinites on the leaching process for indirect CO2 mineral carbonation. Hydrometallurgy 2017, 169, 142–151. [Google Scholar] [CrossRef]
- Gabrielli, P.; Gazzani, M.; Mazzotti, M. The role of carbon capture and utilization, carbon capture and storage, and biomass to enable a net-zero-co2 emissions chemical industry. Ind. Eng. Chem. Res. 2020, 59, 7033–7045. [Google Scholar] [CrossRef]
- Hills, C.D.; Tripathi, N.; Carey, P.J. Mineralization technology for carbon capture, utilization, and storage. Front. Energy Res. 2020, 8, 142. [Google Scholar] [CrossRef]
- Washbourne, C.L.; Lopez-Capel, E.; Renforth, P.; Ascough, P.L.; Manning, D.A.C. Rapid removal of atmospheric CO2 by urban soils. Environ. Sci. Technol. 2015, 49, 5434–5440. [Google Scholar] [CrossRef]
- Jorat, M.E.; Kolosz, B.W.; Goddard, M.A.; Sohi, S.P.; Akgun, N.; Dissanayake, D.; Manning, D.A.C. Geotechnical requirements for capturing CO2 through highways land. Int. J. GEOMATE 2017, 13, 22–27. [Google Scholar] [CrossRef]
- Olajire, A.A. A review of mineral carbonation technology in sequestration of CO2. J. Pet. Sci. Eng. 2013, 109, 364–392. [Google Scholar] [CrossRef]
- Power, I.M.; Dipple, G.M.; Francis, P.S. Assessing the carbon sequestration potential of magnesium oxychloride cement building materials. Cem. Concr. Compos. 2017, 78, 97–107. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; El-Naas, M.H.; Benamor, A.; Al-Sobhi, S.S.; Zhang, Z. Carbon mineralization by reaction with steel-making waste: A review. Processes 2019, 7, 115. [Google Scholar] [CrossRef]
- Plaza, M.G.; Martínez, S.; Rubiera, F. CO2 Capture, Use, and Storage in the Cement Industry: State of the Art and Expectations. Energies 2020, 13, 5692. [Google Scholar] [CrossRef]
- Stopic, S.; Dertmann, C.; Modolo, G.; Kegler, P.; Neumeier, S.; Kremer, D.; Wotruba, H.; Etzold, S.; Telle, R.; Rosani, D.; et al. Synthesis of magnesium carbonate via carbonation under high pressure in an autoclave. Metals 2018, 8, 993. [Google Scholar] [CrossRef]
- Jorat, M.E.; Goddard, M.A.; Manning, P.; Lau, H.K.; Ngeow, S.; Sohi, S.P.; Manning, D.A.C. Passive CO2 removal in urban soils: Evidence from brownfield sites. Sci. Total Environ. 2020, 703, 135573. [Google Scholar] [CrossRef] [PubMed]
- Hitch, M.; Ballantyne, S.M.; Hindle, S.R. Revaluing mine waste rock for carbon capture and storage. Int. J. Min. Reclam. Environ. 2010, 24, 64–69. [Google Scholar] [CrossRef]
- Kusin, F.M.; Awang, N.H.C.; Hasan, S.N.M.S.; Rahim, H.A.A.; Jusop, S.; Kim, K.W. Geoecological evaluation of mineral, major and trace elemental composition in waste rocks, soils and sediments of a gold mining area and potential associated risks. Catena 2019, 183, 104229. [Google Scholar] [CrossRef]
- Power, I.M.; McCutcheon, J.; Harrison, A.L.; Wilson, S.A.; Dipple, G.M.; Kelly, S.; Southam, C.; Southam, G. Strategizing Carbon-Neutral Mines: A Case for Pilot Projects. Minerals 2014, 4, 399–436. [Google Scholar] [CrossRef]
- Wilson, S.A.; Dipple, G.M.; Power, I.M.; Thom, J.M.; Anderson, R.G.; Raudsepp, M.; Gabites, J.E.; Southam, G. Carbon Dioxide Fixation within Mine Wastes of Ultramafic-Hosted Ore Deposits: Examples from the Clinton Creek and Cassiar Chrysotile Deposits Canada. Econ. Geol. 2009, 104, 95–112. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M. Carbon dioxide adsorption isotherm study on mine waste for integrated CO2 capture and sequestration processes. Powder Technol. 2015, 291, 408–413. [Google Scholar] [CrossRef]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusikb, R.; Maroto-Valerac, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef] [PubMed]
- Bobicki, E.R.; Liu, Q.; Xu, Z.; Zeng, H. Carbon capture and storage using alkaline industrial wastes. Prog. Energy Combust. Sci. 2012, 38, 302–320. [Google Scholar] [CrossRef]
- Pan, S.Y.; Chang, E.E.; Chiang, P.C. CO2 capture by accelerated carbonation of alkaline wastes: A review on its principles and applications. Aerosol Air Qual. Res. 2012, 12, 770–791. [Google Scholar] [CrossRef]
- Gras, A.; Beaudoin, G.; Molson, J.; Plante, B.; Bussière, B.; Lemieux, J.M.; Dupont, P.P. Isotopic evidence of passive mineral carbonation in mine wastes from the Dumont Nickel Project (Abitibi, Quebec). Int. J. Greenh. Gas Control 2017, 60, 10–23. [Google Scholar] [CrossRef]
- Guo, Y.; Tan, C.; Wang, P.; Sun, J.; Li, W.; Zhao, C.; Lu, P. Magnesium-based basic mixtures derived from earth-abundant natural minerals for CO2 capture in simulated flue gas. Fuel 2019, 243, 298–305. [Google Scholar] [CrossRef]
- Herzog, H. Carbon Sequestration via Mineral Carbonation: Overview and Assessment; MIT Laboratory for Energy and the Environment: Cambridge, MA, USA, 2002. [Google Scholar]
- Jacobs, A.D.; Hitch, M. Experimental mineral carbonation: Approaches to accelerate CO2 sequestration in mine waste materials. Int. J. Min. Reclam. Environ. 2011, 25, 321–331. [Google Scholar] [CrossRef]
- Manning, D.A.C.; Renforth, P.; Lopez-Capel, E.; Robertson, S.; Ghazireh, N. Carbonate precipitation in artificial soils produced from basaltic quarry fines and composts: An opportunity for passive carbon sequestration. Int. J. Greenh. Gas Conrol 2013, 17, 309–317. [Google Scholar] [CrossRef]
- Lackner, K.S.; Wendt, C.H.; Butt, D.P.; Joyce, E.L.; Sharps, D.H. Carbon dioxide disposal in carbonate minerals. Energy 1995, 20, 1153–1170. [Google Scholar] [CrossRef]
- Vogeli, J.; Reid, D.L.; Becker, M.; Broadhurst, J.; Franzidis, J.P. Investigation of the potential for mineral carbonation of PGM tailings in South Africa. Miner. Eng. 2011, 24, 1348–1356. [Google Scholar] [CrossRef]
- Hasan, S.N.M.S.; Kusin, F.M.; Shamshuddin, J.; Yusuff, F.M. Potential of soil, sludge and sediment for mineral carbonation process in Selinsing gold mine, Malaysia. Minerals 2018, 8, 257. [Google Scholar] [CrossRef]
- Azdarpour, A.; Karaei, A.K.; Hamidi, H.; Mohammadian, E.; Honarvar, B. CO2 sequestration through direct aqueous mineral carbonation of red gypsum. Petroleum 2018, 4, 1–10. [Google Scholar] [CrossRef]
- Mendoza, E.Y.M.; Santos, A.S.; López, E.V.; Drozd, V.; Durygin, A.; Chen, J.; Saxena, S.K. Siderite Formation by Mechanochemical and High Pressure–High Temperature Processes for CO2 Capture Using Iron Ore as the Initial Sorbent. Processes 2019, 7, 735. [Google Scholar] [CrossRef]
- Mendoza, E.Y.M.; Santos, A.S.; López, E.V.; Drozd, V.; Durygin, A.; Chen, J.; Saxena, S.K. Iron oxides as efficient sorbents for CO2 capture. J. Matter. Res. Technol. 2019, 8, 2944–2956. [Google Scholar] [CrossRef]
- Assima, G.P.; Larachi, F.; Molson, J.; Beaudoin, G. Impact of temperature and oxygen availability on the dynamics of ambient CO2 mineral sequestration by nickel mining residues. Chem. Eng. J. 2014, 240, 394–403. [Google Scholar] [CrossRef]
- IEA. Technology Roadmap Low-Carbon Transition in the Cement Industry; IEA: Paris, France, 2018. [Google Scholar]
- Andrew, R.M. Global CO2 emissions from cement production. Earth Syst. Sci. Data 2018, 10, 195–217. [Google Scholar] [CrossRef]
- GCCA. GCCA Launches ‘Innovandi—The Global Cement and Concrete Research Network’. Singapore, 10 October 2019; Global Cement and Concrete Association: London, UK, 2019. [Google Scholar]
- Hepburn, C.; Adlen, E.; Beddington, J.; Carter, E.A.; Fuss, S.; Mac Dowell, N.; Minx, J.C.; Smith, P.; Williams, C.K. The technological and economic prospects for CO2 utilization and removal. Nature 2019, 575, 87–97. [Google Scholar] [CrossRef]
- Tsivilis, S.; Batis, G.; Chaniotakis, E.; Grigoriadis, G.; Theodossis, D. Properties and behavior of limestone cement concrete and mortar. Cem. Concr. Res. 2000, 30, 1679–1683. [Google Scholar] [CrossRef]
- El-Hassan, H.; Shao, Y. Carbon Storage through Concrete Block Carbonation Curing. J. Clean Energy Technol. 2014, 2, 287–291. [Google Scholar] [CrossRef]
- Ashraf, W. Carbonation of cement-based materials: Challenges and opportunities. Constr. Build. Mater. 2016, 120, 558–570. [Google Scholar] [CrossRef]
- Mo, L.; Hao, Y.; Liu, Y.; Wang, F.; Deng, M. Preparation of calcium carbonate binders via CO2 activation of magnesium slag. Cem. Concr. Res. 2019, 121, 81–90. [Google Scholar] [CrossRef]
- He, Z.; Jia, Y.; Wang, S.; Mahoutian, M.; Shao, Y. Maximizing CO2 sequestration in cement-bonded fiberboards through carbonation curing. Constr. Build. Mater. 2019, 213, 51–60. [Google Scholar] [CrossRef]
- Qin, L.; Gao, X.; Chen, T. Influence of mineral admixtures on carbonation curing of cement paste. Constr. Build. Mater. 2019, 212, 653–662. [Google Scholar] [CrossRef]
- Kusin, F.M.; Hasan, S.N.M.S.; Hassim, M.A.; Molahid, V.L.M. Mineral carbonation of sedimentary mine waste for carbon sequestration and potential reutilization as cementitious material. Environ. Sci. Pollut. Res. 2020, 27, 12767–12780. [Google Scholar] [CrossRef]
- Mohd-Isha, N.S.; Kusin, F.M.; Kamal, N.M.A.; Hasan, S.N.M.S.; Molahid, V.L.M. Geochemical and mineralogical assessment of sedimentary limestone mine waste and potential for mineral carbonation. Environ. Geochem. Health 2021, 43, 2065–2080. [Google Scholar] [CrossRef] [PubMed]
- Snowden. Selinsing Gold Sulphide Project-NI 43-101 Technical Report; Project Number AU10173; Snowden: Perth, Australia, 2019; p. 357. [Google Scholar]
- Makoundi, C.; Zaw, K.; Large, R.R.; Meffre, S.; Lai, C.K.; Hoe, T.G. Geology, geochemistry and metallogenesis of the Selinsing gold deposit, Central Malaysia. Gondwana Res. 2013, 26, 241–261. [Google Scholar] [CrossRef]
- Álvarez, R.; Ordóñez, R.; Pérez, A.; Miguel, E.D.; Charlesworth, S. Mineralogical and environmental features of the asturian copper mining district (Spain): A review. Eng. Geol. 2018, 243, 206–217. [Google Scholar] [CrossRef]
- Jusop, S. Methods in Soil Mineralogy; Universiti Putra Malaysia Press: Selangor, Malaysia, 2011. [Google Scholar]
- Malaysian Standard MS 76: 1972. Specification for Bricks and Blocks of Fired Brickearth, Clay or Shale. Part 2: Metric Units; Standards and Industrial Research Institute of Malaysia: Selangor, Malaysia, 1972. [Google Scholar]
- Rivai, T.A.; Yonezu, K.; Syafrizal; Watanabe, K. Mineralogy and geochemistry of host rocks and orebodies at the Anjing Hitam prospect (Dairi, North Sumatera, Indonesia) and their environmental implications. Evergreen 2019, 6, 18–28. [Google Scholar] [CrossRef]
- Hasan, S.N.M.S.; Kusin, F.M.; Shamshuddin, J.; Yusuff, F.M. The mineralogy and chemical properties of sedimentary waste rocks with carbon sequestration potential at Selinsing Gold Mine, Pahang. Pertanika J. Sci. Technol. 2019, 27, 1005–1012. [Google Scholar]
- Busch, A.; Bertier, P.; Gensterblum, Y.; Rother, G.; Spiers, C.J.; Zhang, M.; Wentinck, H.M. On sorption and swelling of CO2 in clays. Geomech. Geophys. Geo Energy Geo Resour. 2016, 2, 111–130. [Google Scholar] [CrossRef]
- Chouikhi, N.; Cecilia, J.A.; Vilarrasa-García, E.; Besghaier, S.; Chlendi, M.; Duro, F.I.F.; Castellon, E.R.; Bagane, M. CO2 Adsorption of Materials Synthesized from Clay Minerals: A Review. Minerals 2019, 9, 514. [Google Scholar] [CrossRef]
- Steinour, H.H. Some effects of carbon dioxide on mortars and concrete-discussion. J. Am. Concr. Inst. 1959, 30, 905–907. [Google Scholar] [CrossRef][Green Version]
- Daud, N.N.N.; Muhammed, A.S.; Md-Yusoff, Z. Geotechnical assessment of palm oil fuel ash (POFA) mixed with granite residual soil for hydraulic barrier purposes. Malays. J. Civil Eng. 2016, 28, 1–9. [Google Scholar] [CrossRef]
- Luukkonen, T.; Heponiemi, A.; Runtti, H.; Pesonen, J.; Yliniemi, J.; Lassi, U. Application of alkali-activated materials for water and wastewater treatment: A review. Rev. Environ. Sci. Biotechnol. 2019, 18, 271–297. [Google Scholar] [CrossRef]
- Hasan, S.N.M.S.; Kusin, F.M. Potential of mining waste from metallic mineral industry for carbon sequestration. In Proceedings of the International Conference on Process Engineering and Advanced Materials (ICPEAM2018), Kuala Lumpur, Malaysia, 13–14 August 2018; Volume 458, p. 012013. [Google Scholar]
- Walker, R.; Pavia, S. Physical properties and reactivity of pozzolans, and their influence on the properties of lime–pozzolan pastes. Mater. Struct. 2011, 44, 1139–1150. [Google Scholar] [CrossRef]
- Resoli, N.; Neuwald, O.A.; Zattera, A.J.; Piazza, D.; Kunst, S.R.; Birriel, E.J. Effect of addition of clay minerals on the properties of epoxy/polyester powder coatings. Polimeros 2018, 28, 355–367. [Google Scholar] [CrossRef]
- Hassouta, L.; Buatier, M.D.; Potdevin, J.L.; Liewig, N. Clay diagenesis in the sandstone reservoir of the Ellon Field (Alwyn, North Sea). Clays Clay Miner. 1999, 47, 269–285. [Google Scholar] [CrossRef]
- Villa, R.V.D.-L.; Frías, M.; García-Giménez, R.; Martínez-Ramirez, S.; Fernández-Carrasco, L. Chemical and mineral transformations that occur in mine waste and washery rejects during pre-utilization calcination. Int. J. Coal Geol. 2014, 132, 123–130. [Google Scholar] [CrossRef]
- Segvic, B.; Benvenuti, A.; Moscariello, A. Illite-smectite-rich clay parageneses from quaternary tunnel valley sediments of the Dutch Southern North Sea—Mineral origin and paleoenvironment implications. Clays Clay Miner. 2016, 64, 608–627. [Google Scholar] [CrossRef]
- Malaysian Standards, MS 1933-1: 2007. Methods of Test for Masonry Units; Standards and Industrial Research Institute of Malaysia: Selangor, Malaysia, 2007. [Google Scholar]
- Ramli, N.A.A.; Kusin, F.M.; Molahid, V.L.M. Influencing factors of the mineral carbonation process of the iron ore mining waste in sequestering atmospheric carbon dioxide. Sustainability 2021, 13, 1866. [Google Scholar] [CrossRef]
- Molahid, V.L.M.; Kusin, F.M.; Kamal, M.N.A.; Hasan, S.N.M.S.; Ramli, N.A.A.; Abdullah, A.M.; Ashaari, Z.H.A. Carbon sequestration of limestone mine waste through mineral carbonation and utilization as supplementary cementitious material. Int. J. Integr. Eng. 2021, 13, 311–320. [Google Scholar] [CrossRef]
- Dal-Pozzo, A.; Armutlulu, A.; Rekhtina, M.; Abdala, P.M.; Müller, C.R. CO2 Uptake and Cyclic Stability of MgO-Based CO2 Sorbents Promoted with Alkali Metal Nitrates and Their Eutectic Mixtures. ACS Appl. Energy Mater. 2019, 2, 1295–1307. [Google Scholar] [CrossRef]
- Jin, S.; Bang, G.; Liu, L.; Lee, C.H. Synthesis of mesoporous MgO–CeO2 composites with enhanced CO2 capture rate via controlled combustion. Microporous Mesoporous Mater. 2019, 288, 109587. [Google Scholar] [CrossRef]
- Xie, H.; Yue, H.; Zhu, J.; Liang, B.; Li, C.; Wang, Y.; Xie, L.; Zhou, X. Scientific and engineering progress in CO2 mineralization using industrial waste and natural minerals. Engineering 2015, 1, 150–157. [Google Scholar] [CrossRef]
- Li, P.; Pan, S.Y.; Pei, S.; Lin, Y.J.; Chiang, P.C. Challenges and perspectives on carbon fixation and utilization technologies: An overview. Aerosol Air Qual. Res. 2016, 16, 1327–1344. [Google Scholar] [CrossRef]
- Panesar, D.K.; Mo, L. Properties of binary and ternary reactive MgO mortar blends subjected to CO2 curing. Cem. Concr. Compos. 2013, 38, 40–49. [Google Scholar] [CrossRef]
- Hasan, S.N.M.S.; Kusin, F.M.; Hassim, M.A.; Molahid, V.L.M. Incorporation of gold and limestone mining waste materials for carbon capture and storage in bricks. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 022046. [Google Scholar] [CrossRef]
- Önel, O.; Tanriverdi, M.; Cicek, T. Utilization of Yatagan power plant fly ash in production of building bricks. IOP Conf. Ser. Earth Environ. Sci. 2017, 95, 042012. [Google Scholar] [CrossRef]
- Rahman, M.E.; Ong, P.J.; Nabinejad, O.; Islam, S.; Khandoker, N.A.N.; Pakrashi, V.; Shorowordi, K.M. Utilization of blended waste materials in bricks. Technologies 2018, 6, 20. [Google Scholar] [CrossRef]
- Kuranchie, F.A.; Shukla, S.K.; Habibi, D.; Mohyeddin, A. Utilisation of iron ore tailings as aggregates in concrete. Cogent Eng. 2015, 2, 1083137. [Google Scholar] [CrossRef]
- Ahmari, S.; Zhang, L. Production of eco-friendly bricks from copper mine tailings through geopolymerization. Constr. Build. Mater. 2012, 29, 323–331. [Google Scholar] [CrossRef]
- Kiventera, J.; Piekkari, K.; Isteri, V.; Ohenoja, K.; Tanskanen, P.; Illikainen, M. Solidification/stabilization of gold mine tailings using calcium sulfoaluminate-belite cement. J. Clean. Prod. 2019, 239, 118008. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Metwally, M.E.A.; Zakey, S.E. Utilizing industrial waste-water as alkali activator in sand-cement kiln dust bricks. Constr. Build. Mater. 2018, 182, 284–289. [Google Scholar] [CrossRef]
- Gonzalez, J.S.; Gayarre, F.L.; Pérez, C.L.; Ros, P.S.; López, M.A.S. Influence of recycled brick aggregates on properties of structural concrete for manufacturing precast prestressed beams. Constr. Build. Mater. 2017, 149, 507–514. [Google Scholar] [CrossRef]
- Mo, L.; Zhang, F.; Deng, M.; Jin, F.; Al-Tabbaa, A.; Wang, A. Accelerated carbonation and performance of concrete made with steel slag as binding materials and aggregates. Cem. Concr. Compos. 2017, 83, 138e145. [Google Scholar] [CrossRef]
- Huang, H.; Wang, T.; Kolosz, B.; Andresen, J.; Garcia, S.; Fang, M.; Maroto-Valer, M.M. Life-cycle assessment of emerging CO2 mineral carbonation-cured concrete blocks: Comparative analysis of CO2 reduction potential and optimization of environmental impacts. J. Clean. Prod. 2019, 241, 118359. [Google Scholar] [CrossRef]
- Kwasny, J.; Basheer, P.A.M.; Russell, M.I.; Doherty, W.; Owens, K.; Ward, N. CO2 Sequestration in Cement-Based Materials during Mixing Process Using Carbonated Water and Gaseous CO2. In Proceedings of the 4th International Conference on the Durability of Concrete Structures (ICDCS), West Lafayette, IN, USA, 24–26 July 2014; Olek, J., Weiss, J., Eds.; Purdue Scholarly Publishing Services: West Lafayette, IN, USA, 2014. [Google Scholar]





| Sampling Location | Type of Sample | Characteristics |
|---|---|---|
| Waste dump (WD) | Waste rock soil | Sedimentary rock, aragonite, volcanic. Highly silicate clay, argillite, kaolinite, serenite, medium to fine size, highly oxidized |
| Stockpile - High-grade (HS) - Low-grade (LS) - Super-low-grade (SLS) | Waste rock soil | Phyllite, conglomerate |
| Mine tailings - Sediment (SED) - Sludge (SLU) | Sediment sludge | Waste from tailing storage facility |
| Brick Type | Mix Design | Cement (%) * | Sand (%) * | Gold Mine Waste (%) * |
|---|---|---|---|---|
| Normal brick (control) | 2:3 | 40 | 60 | - |
| GMW40 | 1.5:1.5:2 | 30 | 30 | 40 |
| GMW50 | 1.5:1:2.5 | 30 | 20 | 50 |
| GMW60 | 1:1:3 | 20 | 20 | 60 |
| Waste Rocks Samples | ||||||
|---|---|---|---|---|---|---|
| Minerals | WD | HS | SLS | LS | ||
| Quartz, SiO2 | +++ | +++ | +++ | ++ | ||
| Kaolinite, Al2Si2O5(OH)4 | - | + | - | - | ||
| Chlorite-serpentine, (Mg,Al)6(Si,Al)4O10(OH)8 | ++ | - | - | - | ||
| Muscovite, K(Mg,Fe)3(AlSi3O10)(OH)2 | - | ++ | ++ | ++ | ||
| Albite, (Na0.84CaO0.16)Al1.16Si2.84O8 | + | - | - | - | ||
| Graphite, C | - | + | + | + | ||
| Soil, sediment and sludge samples | ||||||
| Minerals | WD | HS | SLS | LS | SED | SLU |
| Quartz, SiO2 | - | - | - | - | ++ | ++ |
| Kaolinite, Al2Si2O5(OH)4 | +++ | +++ | +++ | - | - | - |
| Illite, (K,H3O)(Al,Mg,Fe)2(Si,Al)4O10[(OH)2,(H2O)] | +++ | +++ | +++ | +++ | +++ | +++ |
| Chlorite-serpentine, (Mg,Al)6(Si,Al)4O10(OH)8 | - | - | - | - | ++ | ++ |
| Aerinite, [(Fe+2,Fe+3,Al)3Mg3(Ca,Na)4(Si13.5Al4.5O42)(OH)6].12H2O | ++ | - | - | - | - | - |
| Polythionite, K(AlFeLi) (Si3Al)O10(OH)F | - | - | + | - | - | - |
| Stilpnomelane, Fe2Si3O9 | - | - | - | + | - | - |
| WD | HS | LS | SLS | SLU | SED | ||
|---|---|---|---|---|---|---|---|
| Clay | <2 µm | 11.16 | 10.8 | 19.17 | 7.25 | 17.43 | 6.96 |
| Silt | 2–50 µm | 68.06 | 63.98 | 38.08 | 60.65 | 78.23 | 53.21 |
| Sand | >50 µm | 20.66 | 25.17 | 42.77 | 32.01 | 4.28 | 39.74 |
| Total fines (clay + silt) | 79.22 | 74.78 | 57.25 | 67.9 | 95.66 | 60.17 | |
| Soil texture class (USDA) | Silt loam | Silt loam | Silt loam | Silt loam | Silt loam | Silt loam | |
| Percent Composition (wt.%) | ||||||
|---|---|---|---|---|---|---|
| Waste Rocks Samples | ||||||
| Chemical Composition | WD | HS | SLS | LS | ||
| + Fe2O3 | 5.71 | - | - | - | ||
| + MgO | 5.74 | - | - | - | ||
| SiO2 | 57.81 | 70.17 | 71.06 | 64.35 | ||
| SO3 | 0.47 | 4.85 | 3.31 | |||
| Al2O3 | 24.35 | 24.17 | 20.55 | 22.62 | ||
| K2O | 4.94 | 5.01 | 2.60 | 8.91 | ||
| Na2O | 0.53 | - | 0.68 | - | ||
| Pozzolanic oxides ++ | 87.87 | 94.34 | 91.61 | 84.97 | ||
| Percent composition (wt.%) | ||||||
| Soil, sediment and sludge samples | ||||||
| Chemical composition | WD | HS | SLS | LS | SED | SLU |
| + Fe2O3 | 3.60 | 2.91 | 3.15 | 11.79 | 3.04 | 3.20 |
| + MgO | - | 2.13 | - | 2.72 | - | 1.74 |
| SiO2 | 59.53 | 64.61 | 67.9 | 48.46 | 64.7 | 63.39 |
| SO3 | - | 2.53 | - | 8.83 | 4.32 | 4.37 |
| Al2O3 | 29.93 | 24.84 | 22.82 | 20.18 | 19.6 | 19.22 |
| K2O | 6.36 | 3.00 | 5.72 | 7.24 | 6.63 | 7.06 |
| Na2O | 0.48 | 0.1 | 0.31 | 0.68 | 0.48 | 0.45 |
| Pozzolanic oxides ++ | 93.06 | 94.36 | 93.87 | 80.43 | 87.34 | 84.81 |
| Brick Type | Compressive Strength (N/mm2) | Water Absorption (%) | CO2 Uptake (%) | Sequestered CO2 (g CO2/brick) |
|---|---|---|---|---|
| NB | 24.1 | 1.4 | 0.17 | 5.1 |
| GMW40 | 19.5 | 1.7 | 0.30 | 9.0 |
| GMW50 | 23.3 | 1.4 | 0.24 | 7.2 |
| GMW60 | 29.5 | 2.2 | 0.57 | 17.1 |
| Standard specifications | ||||
| Load bearing brick | 7–103.5 N/mm2 | No specific requirements | ||
| Engineering brick | 48.5–69 N/mm2 | 4.5–7.0% | ||
| Chemical Composition | Uncarbonated | 1 h | 3 h |
|---|---|---|---|
| CaO | 32.2 | 41.2 | 46.7 |
| Fe2O3 | 3.6 | 3.9 | 5.4 |
| MgO | 1.3 | 1.9 | 2.8 |
| SiO2 | 43.7 | 36.2 | 29.6 |
| SO3 | 1.5 | 0.9 | 1.1 |
| Al2O3 | 14.7 | 12.5 | 12.3 |
| K2O | 3.5 | 2.8 | 2.7 |
| Na2O | 0.4 | 0.4 | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syed Hasan, S.N.M.; Mohd Kusin, F.; Nik Daud, N.N.; Saadon, M.A.; Mohamat-Yusuff, F.; Ash’aari, Z.H. Characterization of Gold Mining Waste for Carbon Sequestration and Utilization as Supplementary Cementitious Material. Processes 2021, 9, 1384. https://doi.org/10.3390/pr9081384
Syed Hasan SNM, Mohd Kusin F, Nik Daud NN, Saadon MA, Mohamat-Yusuff F, Ash’aari ZH. Characterization of Gold Mining Waste for Carbon Sequestration and Utilization as Supplementary Cementitious Material. Processes. 2021; 9(8):1384. https://doi.org/10.3390/pr9081384
Chicago/Turabian StyleSyed Hasan, Sharifah Nur Munirah, Faradiella Mohd Kusin, Nik Norsyahariati Nik Daud, Muhammad Anwar Saadon, Ferdaus Mohamat-Yusuff, and Zulfa Hanan Ash’aari. 2021. "Characterization of Gold Mining Waste for Carbon Sequestration and Utilization as Supplementary Cementitious Material" Processes 9, no. 8: 1384. https://doi.org/10.3390/pr9081384
APA StyleSyed Hasan, S. N. M., Mohd Kusin, F., Nik Daud, N. N., Saadon, M. A., Mohamat-Yusuff, F., & Ash’aari, Z. H. (2021). Characterization of Gold Mining Waste for Carbon Sequestration and Utilization as Supplementary Cementitious Material. Processes, 9(8), 1384. https://doi.org/10.3390/pr9081384






