Preparation of K Modified Three-Dimensionally Ordered Macroporous MnCeOx/Ti0.7Si0.3O2 Catalysts and Their Catalytic Performance for Soot Combustion
Abstract
:1. Introduction
2. Experimental Section
2.1. Catalysts Preparation
2.1.1. Synthesis of Highly Well-Defined PMMA Microspheres
2.1.2. Synthesis of 3DOM Ti0.7Si0.3O2
2.1.3. Synthesis of K Modified 3DOM MnCeOx/Ti0.7Si0.3O2
2.2. Physical and Chemical Characterization
2.3. Catalytic Activity Measurements
3. Result and Discussion
3.1. Structural Features of the Synthesized Catalysts
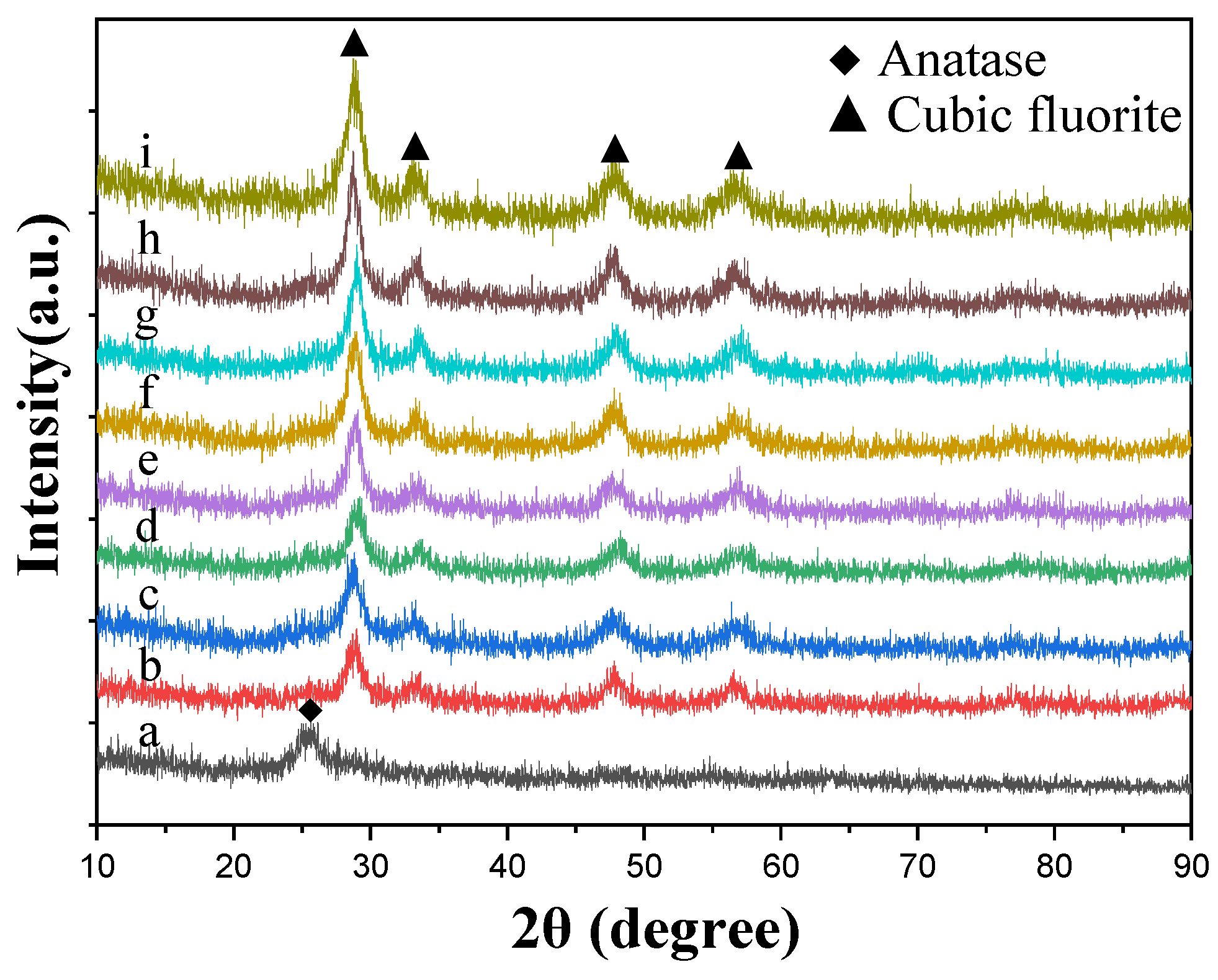
3.1.1. XRD Patterns of the Prepared Catalysts
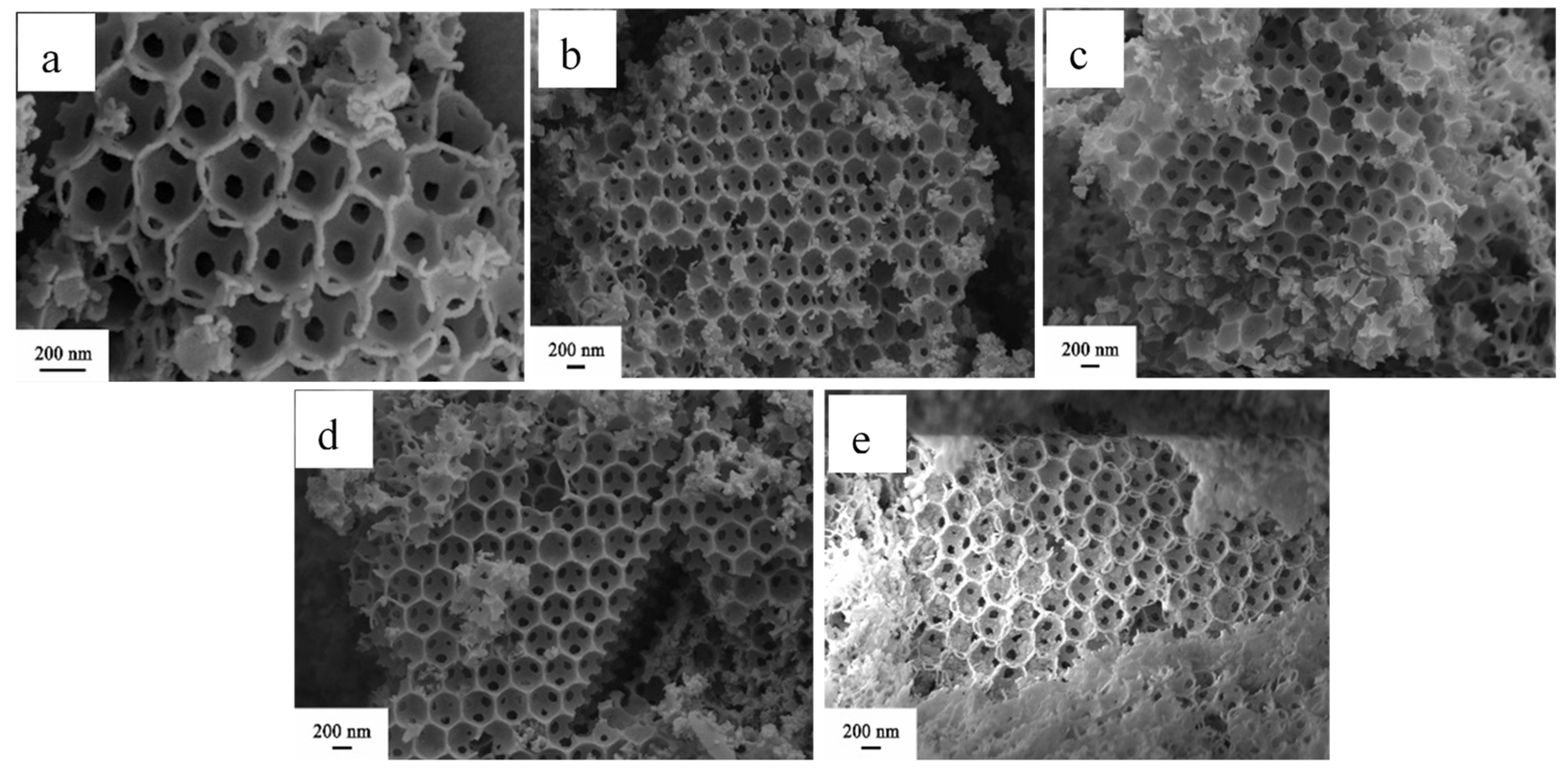
3.1.2. SEM Images of the Catalysts
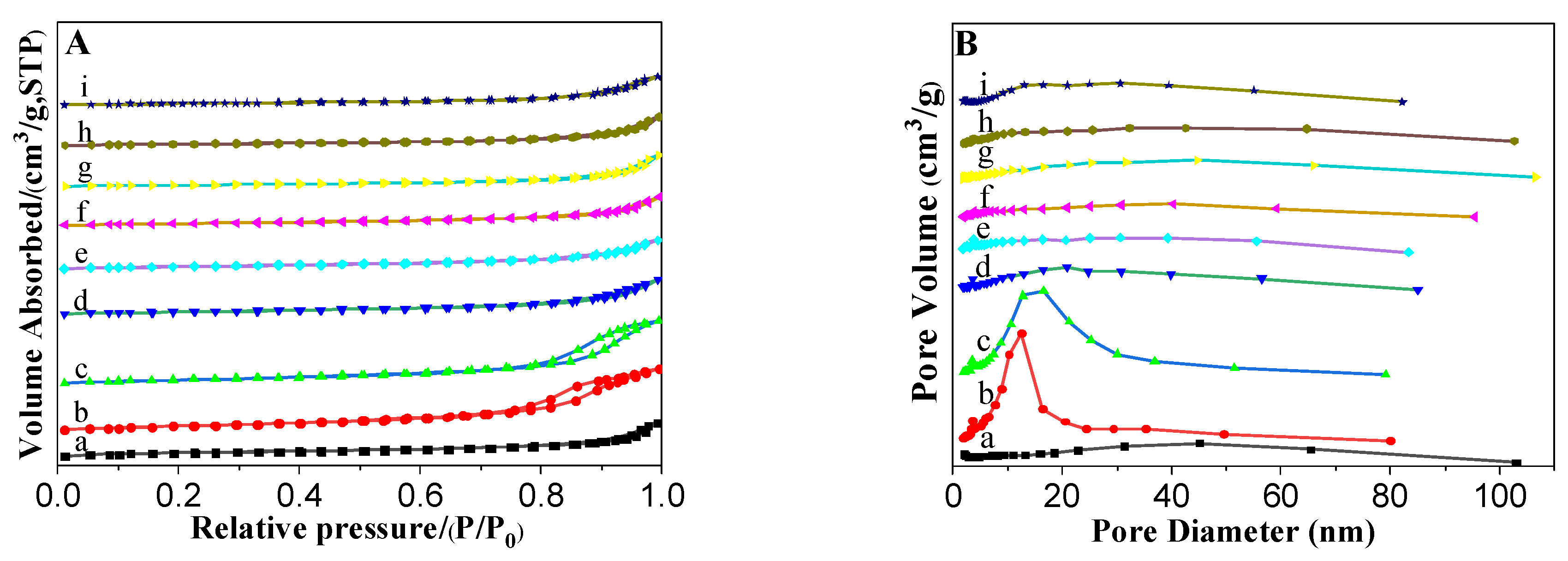
3.1.3. N2 Adsorption–Desorption Isotherms of the Prepared Catalysts
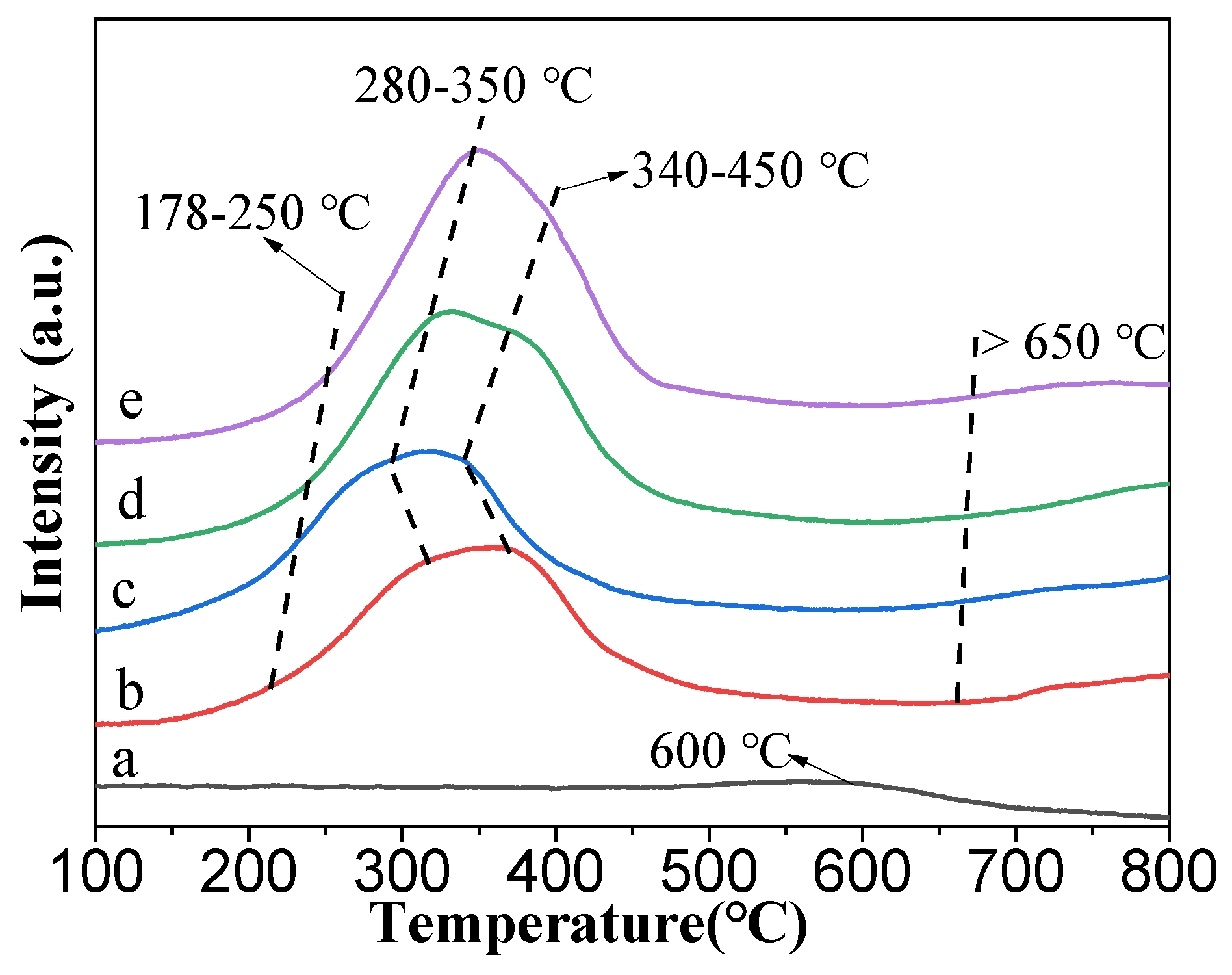
3.1.4. H2-TPR Profiles of the Prepared Catalysts
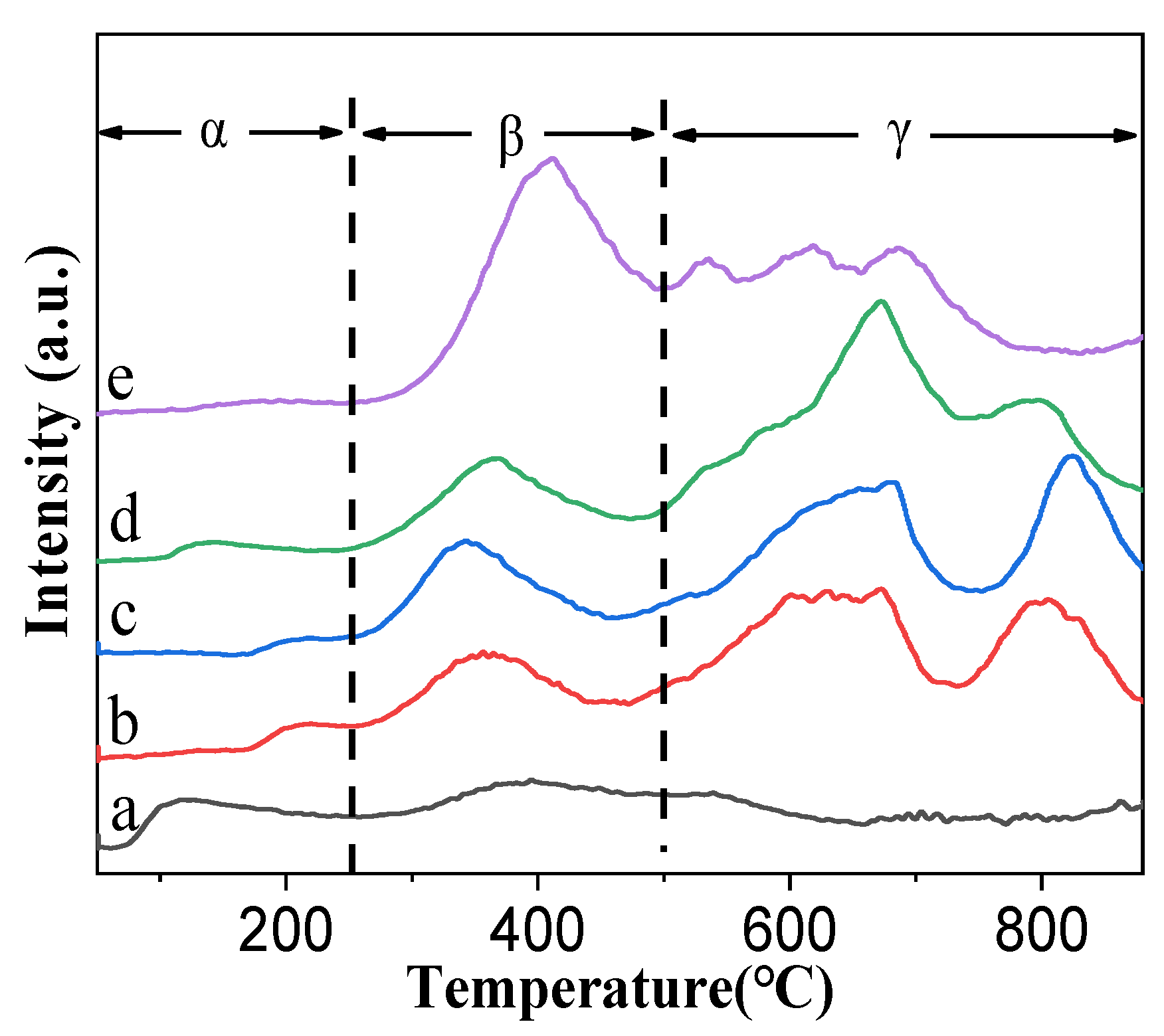
3.1.5. O2-TPD Results of the Prepared Catalysts
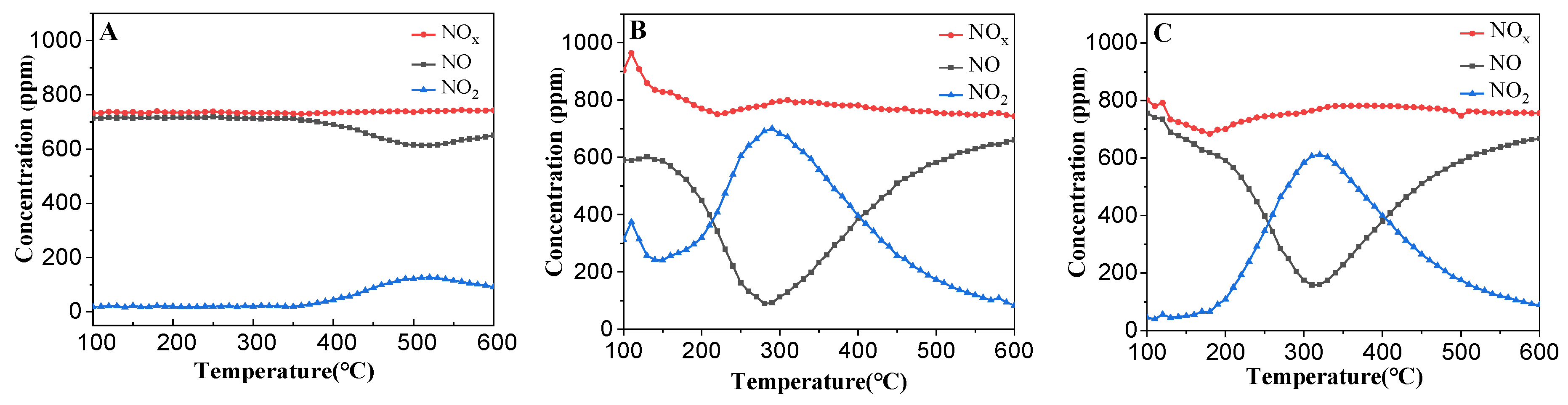
3.1.6. NO-TPO Results of the Prepared Catalysts
3.2. Catalytic Performance in Soot Combustion
3.2.1. Catalytic Activities of the Prepared Catalysts
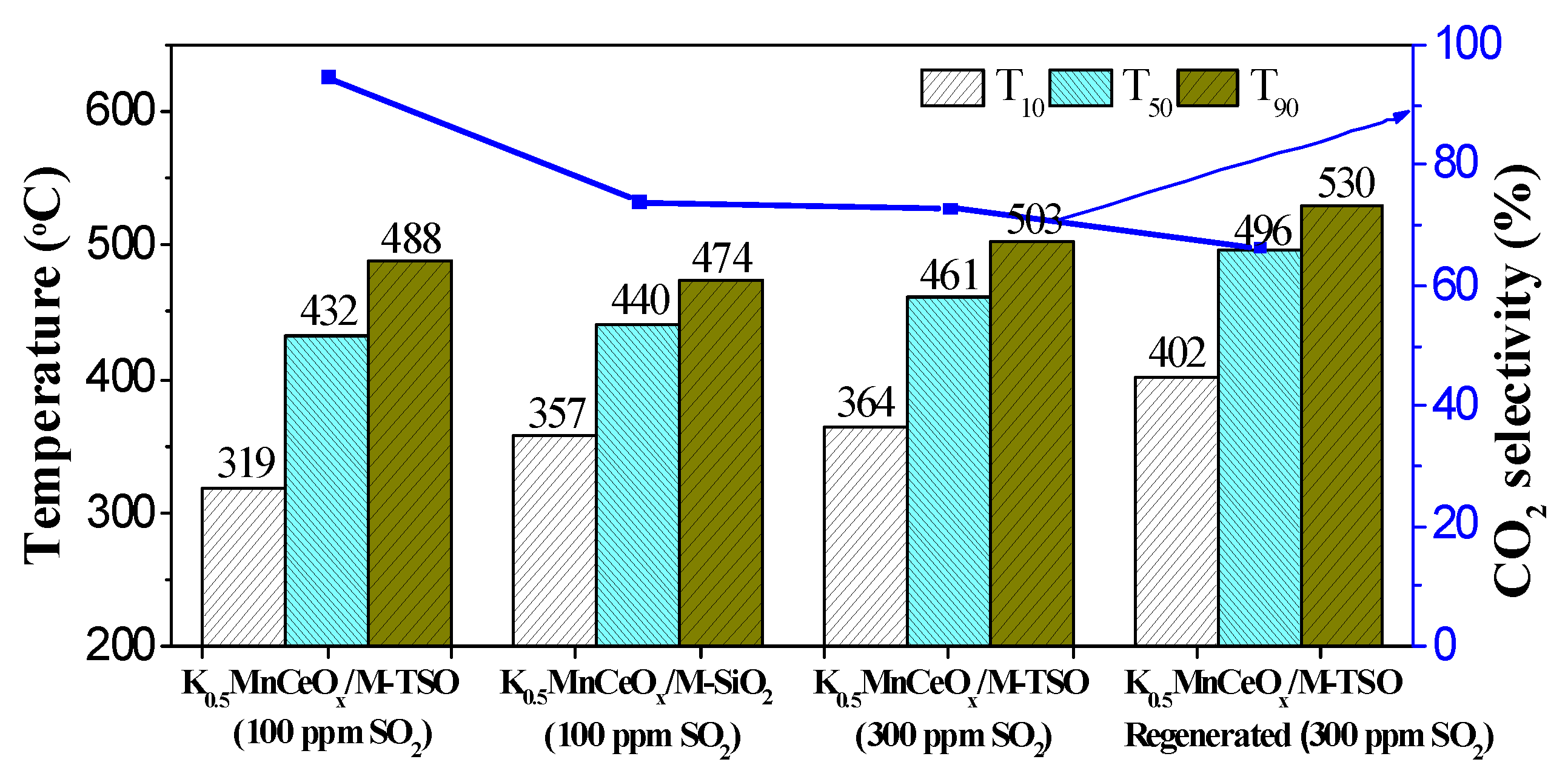
3.2.2. Sulfur Resistances of Prepared Catalysts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xing, L.; Yang, Y.; Cao, C.; Zhao, D.; Gao, Z.; Ren, W.; Tian, Y.; Ding, T.; Li, X. Decorating CeO2 Nanoparticles on Mn2O3 Nanosheets to Improve Catalytic Soot Combustion. ACS Sustain. Chem. Eng. 2018, 6, 16544–16554. [Google Scholar] [CrossRef]
- Fino, D.; Bensaid, S.; Piumetti, M.; Russo, N. A review on the catalytic combustion of soot in diesel particulate filters for automotive applications: From powder catalysts to structured reactors. Appl. Catal. A Gen. 2016, 509, 75–96. [Google Scholar] [CrossRef]
- Cao, C.; Xing, L.; Yang, Y.; Tian, Y.; Ding, T.; Zhang, J.; Hu, T.; Zheng, L.; Li, X. Diesel soot elimination over potassium-promoted Co3O4 nanowires monolithic catalysts under gravitation contact mode. Appl. Catal. B Environ. 2017, 218, 32–45. [Google Scholar] [CrossRef]
- Gentner, D.R.; Jathar, S.H.; Gordon, T.D.; Bahreini, R.; Day, D.; El Haddad, I.; Hayes, P.L.; Pieber, S.M.; Platt, S.; De Gouw, J.; et al. Review of Urban Secondary Organic Aerosol Formation from Gasoline and Diesel Motor Vehicle Emissions. Environ. Sci. Technol. 2017, 51, 1074–1093. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M.P.; Cauqui, M.Á.; Giménez-Mañogil, J.; Martínez-Munuera, J.C.; Muñoz, M.Á.; García-García, A. Catalytic activity of Cu and Co supported on ceria-yttria-zirconia oxides for the diesel soot combustion reaction in the presence of NOx. Chem. Eng. J. 2020, 380, 122370. [Google Scholar] [CrossRef] [Green Version]
- Ren, W.; Ding, T.; Yang, Y.; Xing, L.; Cheng, Q.; Zhao, D.; Zhang, Z.; Li, Q.; Zhang, J.; Zheng, L.; et al. Identifying oxygen activation/oxidation sites for efficient soot combustion over silver catalysts interacted with nanoflower-like hydrotalcite-derived CoAlO metal oxides. ACS Catal. 2019, 9, 8772–8784. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Z.; Jiao, J.; Liu, J.; Duan, A.; Jiang, G. Facile synthesis of three-dimensionally ordered macroporous La-FeO3-supported gold nanoparticle catalysts with high catalytic activity and stability for soot combustion. Catal. Today 2015, 245, 37–45. [Google Scholar] [CrossRef]
- Sui, L.; Wang, Y.; Kang, H.; Dong, H.; Dong, L.; Yu, L. Effect of Cs-Ce-Zr Catalysts/Soot Contact Conditions on Diesel Soot Oxidation. ACS Omega 2017, 2, 6984–6990. [Google Scholar] [CrossRef]
- Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. Nanostructured ceria-based catalysts for soot combustion: Investigations on the surface sensitivity. Appl. Catal. B Environ. 2015, 165, 742–751. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, L.; Jiang, M.; Gao, X.; Huang, M.; Li, Y.; Ren, L.; Yang, Y.; Yang, Z. Controlled synthesis of CeO2 nanorods and their promotional effect on catalytic activity and aging resistibility for diesel soot oxidation. Appl. Surf. Sci. 2020, 510, 145401. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, Y.; Liu, D.; Meng, M. Gravity-Driven Multiple Collision-Enhanced Catalytic Soot Combustion over a Space-Open Array Catalyst Consisting of Ultrathin Ceria Nanobelts. Small 2015, 11, 3659–3664. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ren, J.; Liu, D.; Meng, M. Domain-Confined Multiple Collision Enhanced Catalytic Soot Combustion over a Fe2O3/TiO2–Nanotube Array Catalyst Prepared by Light-Assisted Cyclic Magnetic Adsorption. ACS Catal. 2014, 4, 934–941. [Google Scholar] [CrossRef]
- Lee, C.; Park, J.-I.; Shul, Y.G.; Einaga, H.; Teraoka, Y. Ag supported on electrospun macro-structure CeO2 fibrous mats for diesel soot oxidation. Appl. Catal. B Environ. 2015, 174–175, 185–192. [Google Scholar] [CrossRef]
- Liu, Y.-Z.; Guo, R.-T.; Duan, C.-P.; Wu, G.-L.; Miao, Y.-F.; Gu, J.-W.; Pan, W.-G. Removal of gaseous pollutants by using 3DOM-based catalysts: A review. Chemosphere 2021, 262, 127886. [Google Scholar] [CrossRef]
- Zhao, P.; Feng, N.; Fang, F.; Wan, H.; Guan, G. Surface acid etching for efficient anchoring of potassium on 3DOM La0.8Sr0.2MnO3 catalyst: An integration strategy for boosting soot and NOx simultaneous elimination. J. Hazard. Mater. 2021, 409, 124916. [Google Scholar] [CrossRef]
- Yu, X.; Wang, L.; Zhao, Z.; Fan, X.; Chen, M.; Wei, Y.; Liu, J. 3DOM SiO2-Supported Different Alkali Metals-Modified MnOx Catalysts: Preparation and Catalytic Performance for Soot combustion. ChemistrySelect 2017, 2, 10176–10185. [Google Scholar] [CrossRef]
- Zhai, G.; Wang, J.; Chen, Z.; Yang, S.; Men, Y. Highly enhanced soot oxidation activity over 3DOM Co3O4-CeO2 catalysts by synergistic promoting effect. J. Hazard. Mater. 2019, 363, 214–226. [Google Scholar] [CrossRef]
- Alcalde-Santiago, V.; Davó-Quiñonero, A.; Lozano-Castelló, D.; Bueno-López, A. On the soot combustion mechanism using 3DOM ceria catalysts. Appl. Catal. B Environ. 2018, 234, 187–197. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, G.; Lang, Y.; Chen, R.; Jia, L.; Yue, J.; Shen, M.; Du, C.; Shan, B. Promoting soot combustion efficiency by strengthening the adsorption of NOx on the 3DOM mullite catalyst. J. Catal. 2020, 384, 96–105. [Google Scholar] [CrossRef]
- Alcalde-Santiago, V.; Bailón-García, E.; Davó-Quiñonero, A.; Lozano-Castelló, D.; Bueno-López, A. Three-dimensionally ordered macroporous PrOx: An improved alternative to ceria catalysts for soot combustion. Appl. Catal. B Environ. 2019, 248, 567–572. [Google Scholar] [CrossRef]
- Xiong, J.; Li, Z.; Zhang, P.; Yu, Q.; Li, K.; Zhang, Y.; Zhao, Z.; Liu, J.; Li, J.; Wei, Y. Optimized Pt-MnOx interface in Pt-MnOx/3DOM-Al2O3 catalysts for enhancing catalytic soot combustion. Chin. Chem. Lett. 2021, 32, 1447–1450. [Google Scholar] [CrossRef]
- Jin, B.; Wei, Y.; Zhao, Z.; Liu, J.; Jiang, G.; Duan, A. Effects of Au–Ce strong interactions on catalytic activity of Au/CeO2/3DOM Al2O3 catalyst for soot combustion under loose contact conditions. Chin. J. Catal. 2016, 37, 923–933. [Google Scholar] [CrossRef]
- Xiong, J.; Mei, X.; Liu, J.; Wei, Y.; Zhao, Z.; Xie, Z.; Li, J. Efficiently multifunctional catalysts of 3D ordered meso-macroporous Ce0.3Zr0.7O2-supported PdAu@CeO2 core-shell nanoparticles for soot oxidation: Synergetic effect of Pd-Au-CeO2 ternary components. Appl. Catal. B Environ. 2019, 251, 247–260. [Google Scholar] [CrossRef]
- Yang, Z.; Hu, W.; Zhang, N.; Li, Y.; Liao, Y. Facile synthesis of ceria–zirconia solid solutions with cubic–tetragonal interfaces and their enhanced catalytic performance in diesel soot oxidation. J. Catal. 2019, 377, 98–109. [Google Scholar] [CrossRef]
- Ji, F.; Men, Y.; Wang, J.; Sun, Y.; Wang, Z.; Zhao, B.; Tao, X.; Xu, G. Promoting diesel soot combustion efficiency by tailoring the shapes and crystal facets of nanoscale Mn3O4. Appl. Catal. B Environ. 2019, 242, 227–237. [Google Scholar] [CrossRef]
- Wu, Q.; Jing, M.; Wei, Y.; Zhao, Z.; Zhang, X.; Xiong, J.; Liu, J.; Song, W.; Li, J. High-efficient catalysts of core-shell structured Pt@transition metal oxides (TMOs) supported on 3DOM-Al2O3 for soot oxidation: The effect of strong Pt-TMO interaction. Appl. Catal. B Environ. 2019, 244, 628–640. [Google Scholar] [CrossRef]
- Sacco, N.; Bortolozzi, J.; Milt, V.; Miró, E.; Banús, E. Ce-Mn oxides synthesized with citric acid on ceramic papers used as diesel particulate filters. Catal. Today 2021. [Google Scholar] [CrossRef]
- Zhou, B.; Xi, K.; Fan, L.; Zhou, Y.; Wang, Y.; Zhu, Q.; Lu, H. A comparative study on Ce–Pr and Ce–Mn mixed oxide catalysts toward soot catalytic combustion. Appl. Catal. A Gen. 2018, 562, 1–10. [Google Scholar] [CrossRef]
- Ali, S.; Wu, X.; Zuhra, Z.; Ma, Y.; Abbas, Y.; Jin, B.; Ran, R.; Weng, D. Cu-Mn-Ce mixed oxides catalysts for soot oxidation and their mechanistic chemistry. Appl. Surf. Sci. 2020, 512, 145602. [Google Scholar] [CrossRef]
- Zhao, H.; Li, H.; Pan, Z.; Feng, F.; Gu, Y.; Du, J.; Zhao, Y. Design of CeMnCu ternary mixed oxides as soot combustion catalysts based on optimized Ce/Mn and Mn/Cu ratios in binary mixed oxides. Appl. Catal. B Environ. 2020, 268, 118422. [Google Scholar] [CrossRef]
- Liu, T.; Li, Q.; Xin, Y.; Zhang, Z.; Tang, X.; Zheng, L.; Gao, P.-X. Quasi free K cations confined in hollandite-type tunnels for catalytic solid (catalyst)-solid (reactant) oxidation reactions. Appl. Catal. B Environ. 2018, 232, 108–116. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, Z.; Wei, Y.; Liu, J.; Li, J.; Duan, A.; Jiang, G. Synthesis of K-doped three-dimensionally ordered macroporous Mn0.5Ce0.5Oδ catalysts and their catalytic performances for soot oxidation. Chin. J. Catal. 2015, 36, 1957–1967. [Google Scholar] [CrossRef]
- Mei, X.; Xiong, J.; Wei, Y.; Wang, C.; Wu, Q.; Zhao, Z.; Liu, J. Three-dimensional ordered macroporous perovskite-type La1−xKxNiO3 catalysts with enhanced catalytic activity for soot combustion: The Effect of K-substitution. Chin. J. Catal. 2019, 40, 722–732. [Google Scholar] [CrossRef]
- Yu, X.; Zhen, Z.; Wei, Y.; Zhao, L.; Liu, J. Three-dimensionally ordered macroporous K0.5MnCeOx/SiO2 catalysts: Facile preparation and fine catalytic performances for soot combustion. Catal. Sci. Technol. 2019, 9, 1372–1386. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, X.; Liu, J.; Ye, S. Study on oxidation activity of Ce–Mn–K composite oxides on diesel soot. Sci. Rep. 2020, 10, 10025. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, L.; Chen, M.; Fan, X.; Zhao, Z.; Cheng, K.; Chen, Y.; Sojka, Z.; Wei, Y.; Liu, J. Enhanced activity and sulfur resistance for soot combustion on three-dimensionally ordered macroporous-mesoporous MnxCe1−xOδ/SiO2 catalysts. Appl. Catal. B Environ. 2019, 254, 246–259. [Google Scholar] [CrossRef]
- Yashnik, S.A.; Ismagilov, Z.R. Pt–Pd/MnOx–Al2O3 Oxidation Catalysts: Prospects of Application for Control of the Soot Emission with Diesel Exhaust Gases. Kinet. Catal. 2019, 60, 453–464. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, Q.; Xiong, J.; Li, J.; Liu, J.; Zhao, Z.; Hao, S. Efficient catalysts of supported PtPd nanoparticles on 3D ordered macroporous TiO2 for soot combustion: Synergic effect of Pt-Pd binary components. Catal. Today 2019, 327, 143–153. [Google Scholar] [CrossRef]
- Camposeco, R.; Castillo, S.; Hinojosa-Reyes, M.; Nava, N.; Zanella, R. Manganese promoted TiO2 and ZrO2 nanostructures for soot combustion with boosted efficiency. Surf. Coat. Technol. 2020, 384, 125305. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, Q.; Xiong, J.; Liu, J.; Zhao, Z. Fabrication of ultrafine Pd nanoparticles on 3D ordered macroporous TiO2 for enhanced catalytic activity during diesel soot combustion. Chin. J. Catal. 2018, 39, 606–612. [Google Scholar] [CrossRef]
- Feng, N.; Zhu, Z.; Zhao, P.; Wang, L.; Wan, H.; Guan, G. Facile fabrication of trepang-like CeO2@MnO2 nanocomposite with high catalytic activity for soot removal. Appl. Surf. Sci. 2020, 515, 146013. [Google Scholar] [CrossRef]
- Corroa, G.; Flores, A.; Pacheco-Aguirre, F.; Pal, U.; Bañuelos, F.; Ramirez, A.; Zehe, A. Biodiesel and fossil-fuel diesel soot oxidation activities of Ag/CeO2 catalyst. Fuel 2019, 250, 17–26. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, Z.; Wei, Y.; Liu, J.; Li, J.; Duan, A.; Jiang, G. Three-dimensionally ordered macroporous SiO2 supported transition metal oxide catalysts: Facile synthesis and high catalytic activity for diesel soot combustion. RSC Adv. 2015, 5, 49780–49790. [Google Scholar] [CrossRef]
- Yu, X.; Li, J.; Wei, Y.; Zhao, Z.; Liu, J.; Jin, B.; Duan, A.; Jiang, G. Three-Dimensionally Ordered Macroporous MnxCe1−xOδ and Pt/Mn0.5Ce0.5Oδ Catalysts: Synthesis and Catalytic Performance for Soot Oxidation. Ind. Eng. Chem. Res. 2014, 53, 9653–9664. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems With Special Reference to the Determination of Surface Area and Porosity. IUPAC Stand. Online 2016, 57, 603–619. [Google Scholar] [CrossRef]
- Zhang, P.; Xiong, J.; Wei, Y.; Li, Y.; Zhang, Y.; Tang, J.; Song, W.; Zhao, Z.; Liu, J. Exposed {0 0 1} facet of anatase TiO2 nano-crystals in Ag/TiO2 catalysts for boosting catalytic soot combustion: The facet-dependent activity. J. Catal. 2021, 398, 109–122. [Google Scholar] [CrossRef]
- Pappas, D.K.; Boningari, T.; Boolchand, P.; Smirniotis, P.G. Novel manganese oxide confined interweaved titania nanotubes for the low-temperature Selective Catalytic Reduction (SCR) of NOx by NH3. J. Catal. 2016, 334, 1–13. [Google Scholar] [CrossRef]
- Sun, M.; Yu, L.; Ye, F.; Diao, G.; Yu, Q.; Hao, Z.; Zheng, Y.; Yuan, L. Transition metal doped cryptomelane-type manganese oxide for low-temperature catalytic combustion of dimethyl ether. Chem. Eng. J. 2013, 220, 320–327. [Google Scholar] [CrossRef]
- Chen, M.; Wang, L.; Yu, X.; Zhao, Z. Application of Mn-Based Catalysts for the Catalytic Combustion of Diesel Soot. Prog. Chem. 2019, 31, 723–737. (In Chinese) [Google Scholar]
- Yu, X.; Ren, Y.; Yu, D.; Chen, M.; Wang, L.; Wang, R.; Fan, X.; Zhao, Z.; Cheng, K.; Chen, Y.; et al. Hierarchical Porous K-OMS-2/3DOM-m Ti0.7Si0.3O2 Catalysts for Soot Combustion: Easy Preparation, High Catalytic Activity, and Good Resistance to H2O and SO2. ACS Catal. 2021, 11, 5554–5571. [Google Scholar] [CrossRef]
- Yu, D.; Ren, Y.; Yu, X.; Fan, X.; Wang, L.; Wang, R.; Zhao, Z.; Cheng, K.; Chen, Y.; Sojka, Z.; et al. Facile synthesis of birnessite-type K2Mn4O8 and cryptomelane-type K2−xMn8O16 catalysts and their excellent catalytic performance for soot combustion with high resistance to H2O and SO2. Appl. Catal. B Environ. 2021, 285, 119779. [Google Scholar] [CrossRef]
- Cui, B.; Zhou, L.; Li, K.; Liu, Y.-Q.; Wang, D.; Ye, Y.; Li, S. Holey Co-Ce oxide nanosheets as a highly efficient catalyst for diesel soot combustion. Appl. Catal. B Environ. 2020, 267, 118670. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, Z.; Wei, Y.; Liu, J. Ordered micro/macro porous K-OMS-2/SiO2 nanocatalysts: Facile synthesis, low cost and high catalytic activity for diesel soot combustion. Sci. Rep. 2017, 7, 43894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Catalysts | KNO3/g | Mn(NO3)2/g | Ce(NO3)3/g | 3DOM TiSiO/g | 3DOM SiO2/g |
|---|---|---|---|---|---|
| MnCeOx/M-TSO | 0 | 0.823 | 0.998 | 0.5 | — |
| K0.1MnCeOx/M-TSO | 0.023 | 0.823 | 0.998 | 0.5 | — |
| K0.3MnCeOx/M-TSO | 0.070 | 0.823 | 0.998 | 0.5 | — |
| K0.5MnCeOx/M-TSO | 0.116 | 0.823 | 0.998 | 0.5 | — |
| K0.7MnCeOx/M-TSO | 0.163 | 0.823 | 0.998 | 0.5 | — |
| K0.9MnCeOx/M-TSO | 0.209 | 0.823 | 0.998 | 0.5 | — |
| K1MnCeOx/M-TSO | 0.232 | 0.823 | 0.998 | 0.5 | — |
| K0.5MnCeOx/M-SiO2 | 0.046 | 0.329 | 0.399 | — | 0.2 |
| Catalysts | Surface Area (m2/g) a | Total Pore Volume (m2/g) b | Pore Size (nm) c |
|---|---|---|---|
| M-TSO | 51.3 | 0.123 | 9.9 |
| MnCeOx/M-TSO | 81.1 | 0.218 | 9.4 |
| K0.1MnCeOx/M-TSO | 69.7 | 0.218 | 11.2 |
| K0.3MnCeOx/M-TSO | 45.0 | 0.121 | 10.1 |
| K0.5MnCeOx/M-TSO | 44.2 | 0.105 | 8.9 |
| K0.7MnCeOx/M-TSO | 36.7 | 0.101 | 10.5 |
| K0.9MnCeOx/M-TSO | 31.8 | 0.109 | 13.8 |
| K1MnCeOx/M-TSO | 30.1 | 0.101 | 14.1 |
| K0.5MnCeOx/M-SiO2 | 31.2 | 0.100 | 13.1 |
| Catalysts | T10/℃ | T50/℃ | T90/℃ | Sco2m/% |
|---|---|---|---|---|
| Soot | 457 | 552 | 594 | 41% |
| M-TSO | 376 | 517 | 565 | 50.5% |
| MnCeOx/M-TSO | 284 | 354 | 393 | 99.3% |
| K0.1MnCeOx/M-TSO | 286 | 346 | 380 | 99.4% |
| K0.3MnCeOx/M-TSO | 285 | 338 | 379 | 98.3% |
| K0.5MnCeOx/M-TSO | 287 | 336 | 367 | 98.9% |
| K0.7MnCeOx/M-TSO | 293 | 341 | 379 | 97.7% |
| K0.9MnCeOx/M-TSO | 295 | 345 | 379 | 97.3% |
| K1MnCeOx/M-TSO | 300 | 349 | 384 | 96.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Yu, D.; Peng, C.; Wang, L.; Fan, X.; Yu, X.; Zhao, Z. Preparation of K Modified Three-Dimensionally Ordered Macroporous MnCeOx/Ti0.7Si0.3O2 Catalysts and Their Catalytic Performance for Soot Combustion. Processes 2021, 9, 1149. https://doi.org/10.3390/pr9071149
Zhang C, Yu D, Peng C, Wang L, Fan X, Yu X, Zhao Z. Preparation of K Modified Three-Dimensionally Ordered Macroporous MnCeOx/Ti0.7Si0.3O2 Catalysts and Their Catalytic Performance for Soot Combustion. Processes. 2021; 9(7):1149. https://doi.org/10.3390/pr9071149
Chicago/Turabian StyleZhang, Chunlei, Di Yu, Chao Peng, Lanyi Wang, Xiaoqiang Fan, Xuehua Yu, and Zhen Zhao. 2021. "Preparation of K Modified Three-Dimensionally Ordered Macroporous MnCeOx/Ti0.7Si0.3O2 Catalysts and Their Catalytic Performance for Soot Combustion" Processes 9, no. 7: 1149. https://doi.org/10.3390/pr9071149
APA StyleZhang, C., Yu, D., Peng, C., Wang, L., Fan, X., Yu, X., & Zhao, Z. (2021). Preparation of K Modified Three-Dimensionally Ordered Macroporous MnCeOx/Ti0.7Si0.3O2 Catalysts and Their Catalytic Performance for Soot Combustion. Processes, 9(7), 1149. https://doi.org/10.3390/pr9071149








