Docetaxel for Breast Cancer Treatment-Side Effects on Ocular Surface, a Systematic Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Zazzo, A.; Micera, A.; De Piano, M.; Cortes, M.; Bonini, S. Tears and ocular surface disordes:Usefulness of biomarkers. J. Cell. Physiol. 2019, 234, 9982–9993. [Google Scholar] [CrossRef] [PubMed]
- Mansur, C.; Pfeiffer, M.L.; Esmaeli, B. Evaluation and Management of Chemotherapy-Induced Epiphora, Punctual and Canalicular Stenosis and Nasolacrimal Duct Obstruction. Ophthalmic Plast. Reconstr. Surg. 2017, 33, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.Y.; Mackey, J.R. Presentation and management of docetaxel-related adverse effects in patients with breast cancer. Cancer Manag. Res. 2014, 6, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Dumontet, C.; Sikic, B.I. Mechanisms of action of and resistance to antitubulin agents:microtubules dynamics, drug transport, and death. J. Clin. Oncol. 1999, 17, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.D.; Von Minckwitz, G.; Raab, G.; Blohmer, J.U.; Dresel, V.; Eidtmann, H.; Hilfrich, J.; Jackisch, C.; Merkle, E.; Gademann, G.; et al. The role of docetaxel(taxotere) in neoadjuvant chemotherapy of breast cancer. Semin. Oncol. 1999, 26, 24–31. [Google Scholar]
- Esmaeli, B.; Valero, V. Epiphora and Canalicular Stenosis Associated With Adjuvant Docetaxel in Early Breast Cancer: Is Excessive Tearing Clinically Important? J. Clin. Oncol. 2013, 31, 2076–2077. [Google Scholar] [CrossRef]
- Esmaeli, B.; Amin, S.; Valero, V.; Adinin, R.; Arbuckle, R.; Banay, R.; Do, K.A.; Rivera, E. Prospective Study of Incidence and Severity of Epiphora and Canalicular Stenosis in Patients with Metastatic Breast Cancer Receiving Docetaxel. J. Clin. Oncol. 2006, 24, 3619–3622. [Google Scholar] [CrossRef]
- Chen, Y.M.; Shih, J.F.; Perng, R.P.; Tsai, C.M.; Whang-Peng, J. A randomized trial of different docetaxel schedules in non-small cell lung cancer patients who failed previous platinum-based chemotherapy. Chest 2006, 129, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Tannock, I.F.; De Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Théodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Sulkes, A.; Smyth, J.; Sessa, C.; Dirix, L.Y.; Vermorken, J.B.; Kaye, S.; Wanders, J.; Franklin, H.; LeBail, N.; Verweij, J. Docetaxel (Taxotere) in advanced gastric cancer: Results of a phase II clinical trial. Br. J. Cancer 1994, 70, 380–383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kintzel, P.E.; Michaud, L.B.; Lange, M.K. Docetaxel—Associated Epiphora. Pharmacotherapy 2006, 26, 853–867. [Google Scholar] [CrossRef]
- Freifeld, A.G.; Bow, E.J.; Sepkowitz, K.A.; Boeckh, M.J.; Ito, J.I.; Mullen, C.A.; Raad, I.I.; Rolston, K.V.; Young, J.A.H.; Wingard, J.R. Clinical practice quideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infections Diseases Society of America. Clin. Infect. Dis. 2011, 52, e56–e93. [Google Scholar] [CrossRef] [PubMed]
- Spell, D.W.; Estephan, F.F.; Lin, J.T.; Jones, D.V., Jr. Epiphora induced by intermittent docetaxel (Taxotere) in patients with non-small cell lung cancer. Cancer Investig. 2003, 21, 550–552. [Google Scholar] [CrossRef] [PubMed]
- Esmaeli, B.; Hortobagyi, G.N.; Esteva, F.J.; Booser, D.; Ahmadi, M.A.; Rivera, E.; Arbuckle, R.; Delpassand, E.; Guerra, L.; Valero, V. Canalicular stenosis secondary to weekly versus every-3-weeks docetaxel in patients with metastatic breast cancer. Ophthalmology 2002, 109, 1188–1191. [Google Scholar] [CrossRef]
- Baker, S.D.; Zhao, M.; Lee, C.K.; Verweij, J.; Zabelina, Y.; Brahmer, J.R.; Wolff, A.C.; Sparreboom, A.; Carducci, M.A. Comparative pharmacokinetics of weekly and every-three-weeks docetaxel. Clin. Cancer Res. 2004, 10, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Bremer, C.T.; Monahan, B.P. Necrotizing enterocolitis in neutropenia and chemotherapy: A clinical update and old lessons relearned. Curr. Gastroenterol. Rep. 2006, 8, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.Y.; Yang, C.H.; Yang, C.Y.; Hsiao, G.H.; Chiu, H.C. Fixed erythrodysaesthesia plaque due to intravenous injection of docetaxel. Br. J. Dermatol. 2000, 142, 808–811. [Google Scholar] [CrossRef]
- Coviltir, V.; Burcel, M.; Cherecheanu, A.P.; Ionescu, C.; Dascalescu, D.; Potop, V.; Burcea, M. Update on myopia risk factors and microenvironmental changes. J. Ophtalmol. 2019, 2019, 4960852. [Google Scholar] [CrossRef]
- Liu, C.Y.; Francis, J.H.; Brodie, S.E.; Marr, B.; Pulido, J.S.; Marmor, M.F.; Abramson, D.H. Retinal toxicities of cancer therapy drugs:biologics, small molecule inhibitors, and chemotherapies. Retina 2014, 34, 1261–1280. [Google Scholar] [CrossRef] [PubMed]
- Moloney, T.P.; Xu, W.; Rallah-Baker, K.; Oliveira, N.; Woodward, N.; Farrah, J.J. Toxic optic neuropathy in the setting of docetaxel chemotherapy: A case report. BMC Ophtalmol. 2014, 14, 18. [Google Scholar] [CrossRef]
- Skolnick, C.A.; Doughman, D.J. Erosive Conjunctivitis and Docetaxel (Taxotere). Eye Contact Lens 2003, 29, 134–135. [Google Scholar] [CrossRef]
- Lee, J.J.; Swain, S.M. Peripheral neuropathy induced by microtubule-stabilizing agents. J. Clin. Oncol. 2006, 24, 1633–1642. [Google Scholar] [CrossRef]
- Piccart, M.J.; Klijn, J.; Paridaens, R.; Nooij, M.; Mauriac, L.; Coleman, R.; Bontenbal, M.; Awada, A.; Selleslags, J.; Van Vreckem, A.; et al. Corticosteroids significantly delay the onset of docetaxel-induced fluid retention: Final results of a randomized study of the European Organization for Research and Treatment of Cancer Investigational Drug Branch for Breast Cancer. J. Clin. Oncol. 1997, 15, 3149–3155. [Google Scholar] [CrossRef]
- Esmaeli, B.; Hidaji, L.; Adinin, R.B.; Faustina, M.; Coats, C.; Arbuckle, R.; Rivera, E.; Valero, V.; Tu, S.M.; Amir Ahmadi, M. Blockage of the Lacrimal Drainage Apparatus as a Side Effect of Docetaxel Therapy. Cancer 2003, 98, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Esmaeli, B.; Valero, V.; Ahmadi, M.A.; Booser, D. Canalicular stenosis secondary to docetaxel (Taxotere): A newly recognized side effect. Ophthalmology 2001, 108, 994–995. [Google Scholar] [CrossRef]
- Aslam, S.; Emmanuel, P. Formulating a researchable question: A critical step for facilitating good clinical research. Indian J. Sex. Transm. Dis. AIDS 2010, 31, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, Y.; Kawashima, Y.; Maruyama, M.; Kawara, H.; Tokuyama, Y.; Uchiyama, K.; Shimizu, Y. Current status of eye disorders caused by docetaxel administration every 3 weeks:A case-control study in Japanese patients. J. Oncol. Pharm. Pract. 2020, 26, 655–665. [Google Scholar] [CrossRef]
- Chan, A.; Su, C.; de Boer, R.H.; Gajdatsy, A. Prevalence of Excessive Tearing in Women with Early Breast Cancer Receiving Adjuvant Docetaxel-Based Chemotherapy. J. Clin. Oncol. 2013, 31, 2123–2127. [Google Scholar] [CrossRef]
- Tabernero, J.; Climent, M.A.; Lluch, A.; Albanell, J.; Vermorken, J.B.; Barnadas, A.; Anton, A.; Laurent, C.; Mayordomo, J.I.; Estaun, N.; et al. A multicentre, randomised phase II study of weekly or 3-weekly in patients with metastatic breast cancer. Ann. Oncol. 2004, 15, 1358–1365. [Google Scholar] [CrossRef]
- Leyssens, B.; Wildiers, H.; Lobelle, J.P.; Gillis, A.; Paridaens, R.; Mombaerts, I. A double-blind randomized phase II study on the efficacy of topical eye treatment in the prevention of docetaxel-induced dacryostenosis. Ann. Oncol. 2010, 21, 419–423. [Google Scholar] [CrossRef]
- Tsalic, M.; Gilboa, M.; Visel, B.; Miller, B.; Haim, N. Epiphora (Excessive tearing) and other ocular manifestations related to weekly docetaxel. Med. Oncol. 2006, 23, 57–61. [Google Scholar] [CrossRef]
- Noguchi, Y.; Kawashima, Y.; Kawara, H.; Tokuyama, Y.; Tamura, Y.; Uchiyama, K.; Shimizu, Y. A Retrospective Analysis of Epiphora Due to Docetaxel. Cancer Chemother. 2016, 43, 737–741. [Google Scholar]
- Esmaeli, B. Management of Excessive Tearing as a Side Effect of Doceyaxel. Clin. Breast Cancer 2005, 5, 455–457. [Google Scholar] [CrossRef]
- Burris, H.A. Single-agent docetaxel (Taxotere) in randomized phase III trails. Semin. Oncol. 1999, 26, 1–6. [Google Scholar]
- Cortes, J.E.; Pazdur, R. Docetaxel. J. Clin. Oncol. 1995, 13, 2643–2655. [Google Scholar] [CrossRef]
- Bakri, S.J.; Carney, A.S.; Robinson, K.; Jones, N.S.; Downes, R.N. Quality of life outcomes following dacryocystorhinostomy:external and endonasal laser techniques compared. Orbit 1999, 18, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Schröder, C.P.; de Munck, L.; Westermann, A.M.; Smit, W.M.; Creemers, G.J.M.; de Graaf, H.; Stouthard, J.M.; van Deijk, G.; Erjavec, Z.; van Bochove, A.; et al. Weekly docetaxel in metastatic breast cancer patients:no superior benefits compared to three-weekly docetaxel. Eur. J. Cancer 2011, 47, 1355–1362. [Google Scholar] [CrossRef]
- Esmaeli, B.; Hortobagyi, G.N. Canalicular Stenosis as the Underlying Mechanism for Epiphora in Patients Receiving Weekly Docetaxel. Oncologist 2001, 6, 551–552. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Manola, J.; Younger, J.; Parker, L.M.; Bunnell, C.A.; Scheib, R.; Matulonis, U.A.; Garber, J.E.; Clarke, K.D.; Shulman, L.N.; et al. Docetaxel administered on a weekly basis for metastatic breast cancer. J. Clin. Oncol. 2000, 18, 1212–1219. [Google Scholar] [CrossRef]
- Esmaeli, B.; Hortobagyi, G.; Esteva, F.; Valero, V.; Ahmadi, M.A.; Booser, D.; Ibrahim, N.; Delpassand, E.; Arbuckle, R. Canalicular stenosis secondary to weekly docetaxel:a potentially preventable side effect. Ann. Oncol. 2002, 13, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Mănescu, M.R.; Bălăşoiu, M.; Crăiţoiu, M.M.; Crăiţoiu, Ş.; Simionescu, C.E.; Sfredel, V.; Dinescu, S.N.; Niculescu, M.; Burcea, M.; Bălăşoiu, A.T.; et al. Oral mucosa self-graft in a patient with invasive conjunctival melanoma: A case report. Rom. J. Morphol. Embriol. 2014, 55, 1515–1519. [Google Scholar]
- Esmaeli, B.; Ahmadi, M.A.; Rivera, E.; Valero, V.; Hutto, T.; Jackson, D.M.; Newman, R.A. Docetaxel secretion in tears: Association with lacrimal drainage obstruction. Arch. Ophtalmol. 2002, 120, 1180–1182. [Google Scholar] [CrossRef]
- Paúl, L.; Pinto, I.; Vicente, J.M.; Armendariz, A.; Moreno, G.; Baraibar, M.C. Nasolacrimal stents in the treatment of epiphora:long-term results. J. Vasc. Interv. Radiol. 2002, 13, 83–88. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Esmaeli, B. Surgical Treatment of Canalicular Stenosis in Patients Receiving Docetaxel Weekly. Arch. Ophtalmol. 2001, 119, 1802–1804. [Google Scholar] [CrossRef] [PubMed]
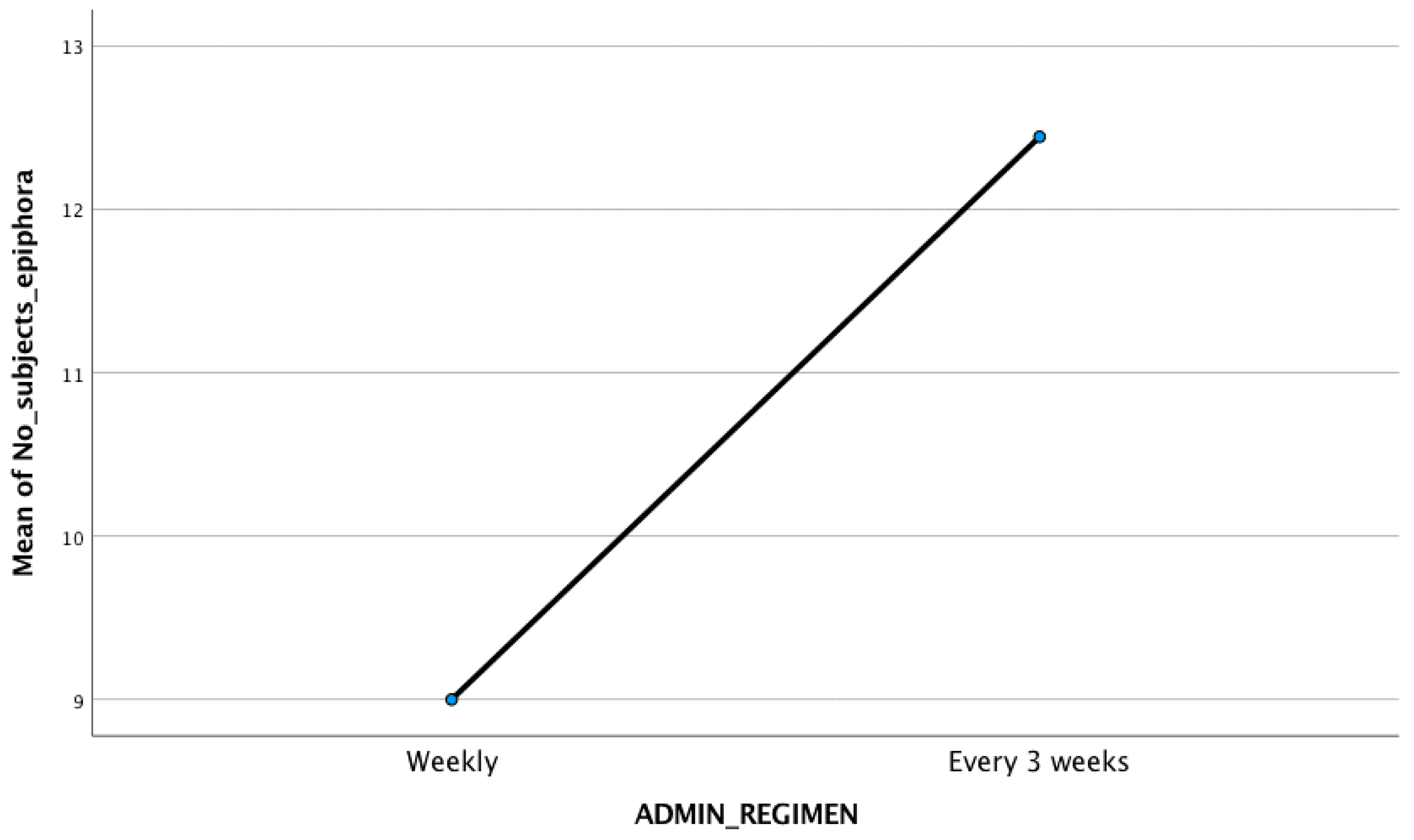
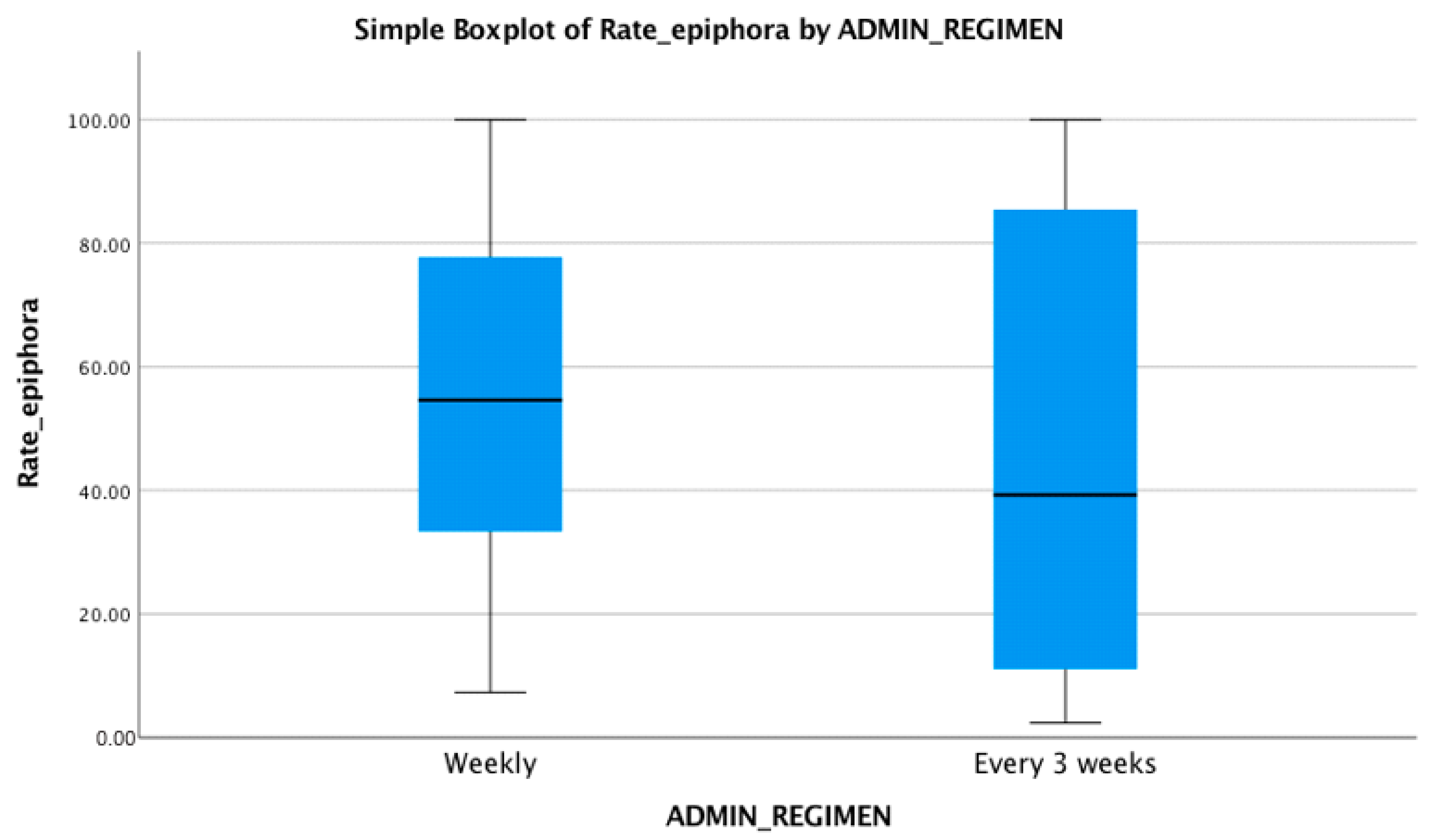

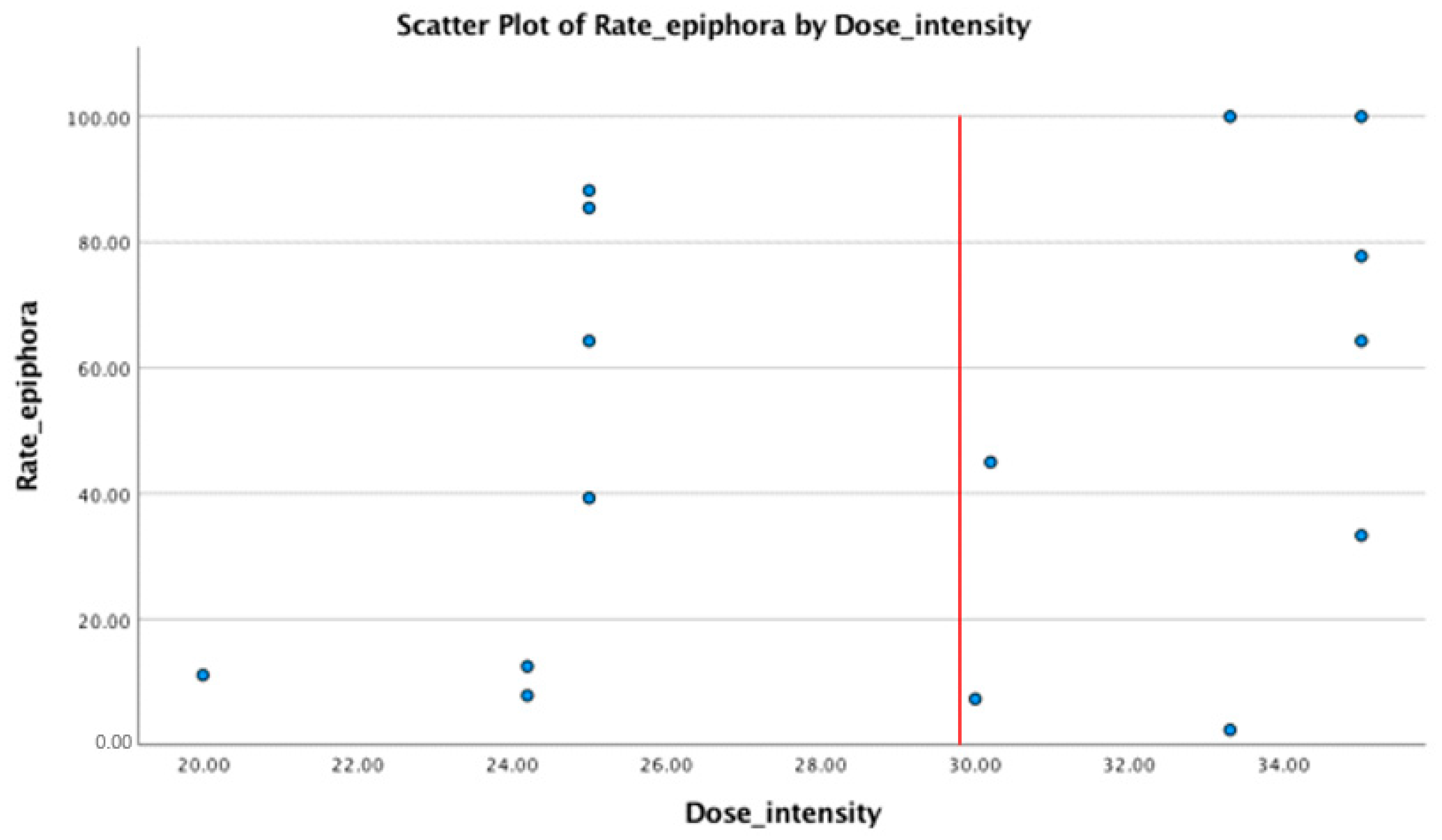
| Study | Study Type | Administration Regimen | Total No. of Subjects | Epiphora |
|---|---|---|---|---|
| Noguchi Y 2019 [27] | Case control | Every 3 weeks TH, TC, TCH | 89 | 7 |
| Leyssens B 2009 [30] | RCT | Weekly | 20 | 9 |
| Esmaeli B 2002 [14] | Retrospective | Weekly TH | 18 | 14 |
| Esmaeli B 2002 [14] | Retrospective | Every 3 weeks TA | 18 | 2 |
| Esmaeli B 2001 [25] | Observational | Weekly T | 3 | 3 |
| Tabernero J 2004 [29] | RCT | Weekly T | 41 | 3 |
| Tabernero J 2004 [29] | RCT | Every 3 weeks T | 42 | 0 |
| Tsalic M 2006 [31] | Prospective | Weekly T | 21 | 7 |
| Esmaeli B 2006 [7] | Prospective | Weekly T | 28 | 18 |
| Esmaeli B 2006 [7] | Prospective | Every 3 weeks T | 28 | 11 |
| Noguchi Y 2016 [32] | Retrospective | Every 3 weeks T | 48 | 6 |
| Chan A 2013 [28] | Prospective | Every 3 weeks FEC-T | 14 | 14 |
| Chan A 2013 [28] | Prospective | Every 3 weeks TCH | 17 | 15 |
| Chan A 2013 [28] | Prospective | Every 3 weeks TAC | 55 | 47 |
| Chan A 2013 [28] | Prospective | Every 3 weeks TC +/− H | 14 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoicescu, E.A.; Burcea, M.; Iancu, R.C.; Zivari, M.; Popa Cherecheanu, A.; Bujor, I.A.; Rastoaca, C.; Iancu, G. Docetaxel for Breast Cancer Treatment-Side Effects on Ocular Surface, a Systematic Review. Processes 2021, 9, 1086. https://doi.org/10.3390/pr9071086
Stoicescu EA, Burcea M, Iancu RC, Zivari M, Popa Cherecheanu A, Bujor IA, Rastoaca C, Iancu G. Docetaxel for Breast Cancer Treatment-Side Effects on Ocular Surface, a Systematic Review. Processes. 2021; 9(7):1086. https://doi.org/10.3390/pr9071086
Chicago/Turabian StyleStoicescu, Elena Andreea, Marian Burcea, Raluca Claudia Iancu, Mirela Zivari, Alina Popa Cherecheanu, Inna Adriana Bujor, Cristina Rastoaca, and George Iancu. 2021. "Docetaxel for Breast Cancer Treatment-Side Effects on Ocular Surface, a Systematic Review" Processes 9, no. 7: 1086. https://doi.org/10.3390/pr9071086
APA StyleStoicescu, E. A., Burcea, M., Iancu, R. C., Zivari, M., Popa Cherecheanu, A., Bujor, I. A., Rastoaca, C., & Iancu, G. (2021). Docetaxel for Breast Cancer Treatment-Side Effects on Ocular Surface, a Systematic Review. Processes, 9(7), 1086. https://doi.org/10.3390/pr9071086






