Abstract
The current in vitro diagnostic design process is a combination of methods from engineering disciplines and from government regulatory agencies. The goal of design processes that have been developed is to ensure that a new product meets the user’s expectations and is safe and effective in providing its claimed benefits and proper functioning, otherwise known as the essential design outputs. In order to improve the ability of designers and auditors to ascertain the safety and efficacy of a product, the use of design controls has been adopted that specify a method of evaluating the design process at several key stages. The main objective of this research was to examine the resolution and architectural details necessary to build an adequate manufacturing control system to assure the EDO outputs in large IVD instruments in the company under study. The control system is the defined inspections and test processes to delineate between acceptable and unacceptable product before release for sale. The authors reviewed current design control regulatory requirements within the IVD industry, as well as design controls in other regulated industries. This research was completed to determine what opportunities could be transferred to large in-vitro IVD instruments using an IVD manufacturer as a case study. In conclusion, the research identified three areas where a properly configured EDO can add value within IVD instrument design and manufacture, namely: (1) development of a control system which is fit for purpose; (2) a mechanism to manage and proliferate key design knowledge within the organisation and thereby manage outsourced services; and (3) implementing a scaled engineering change process because changes impacting EDO naturally require extra regulatory and engineering oversight.
1. Introduction
In vitro diagnostics (IVD) is Latin for in-glass testing, meaning to test outside the body using test tubes. IVD instrumentation works in combination with the reagents, calibrators, control materials, kits, and software. They are used in hospital laboratory settings to detect infection, identify a medical condition and monitor drug therapies. Large IVD instruments cover a myriad of technologies. Large IVD instruments contain thousands of design outputs (i.e., functional specifications, drawings, build processes, acceptance criteria, packaging, labelling etc.), where a subset are essential for the safe and proper functioning of the device. Geographically dispersed designers, all working on the same project, can find it difficult to agree on the essential design output resolution i.e., formally identifying those that are essential for the “safe and proper functioning” of the device, thereby making essential design outputs (EDO) resolution and standardisation difficult. It is natural for an engineer to believe that their aspects of the sub-system design are “essential” for the safe and proper functioning of the device. EDO should be limited to design outputs pertaining to clinical results only, including clinical safety. User safety elements are covered and controlled via separated mechanisms (e.g., Machinery Directive) and should be out of scope in relation to EDO branding.
The FDA describe the essential design output (EDO) as “… those design outputs that are essential for the proper functioning of the device are identified” [1]. Whereas ISO13485 Section 7.3.4(d) states that “design and development outputs… specify the characteristics of the product that are essential for its safe and proper use” [2]. EDOs are more discernible and more easily explainable for a smaller, less complex device, than for a large, complex IVD instrument.
Control System
A control system is the testing regime to ensure the release of products (component, subassembly, module and instrument) that meet approved design specifications and quality requirements. It is the means to test a product throughout its build process, such that only good product is moved to the next stage, until the final instrument is accepted at final acceptance test (FAT). This appears to be a relatively simple construct; however, all acceptance tests require qualification (verification or validation) to ensure it consistently meets its intended purpose (i.e., discern good versus bad product). For efficiency, the test should not stop good product, i.e., the test being too restrictive, nor should testing of the attribute be repeated at subsequent stages if unperturbed by subsequent process. Also, test processes for sub-assemblies need a level of robustness to accommodate parts released from the production process as service spare parts. Elements of a manufacturing control system typically include test protocols which may require test software, test fixtures and other specialised test equipment. It is performed throughout the manufacturing process from the tiered suppliers to purchased material inspection (PMI), in-process and later final acceptance testing (FAT) at the end-manufacturing facility.
There are long supply chains in many manufacturing processes with multiple tiered suppliers. Lean approaches are paramount when selecting the location in the control system to perform necessary testing. Test coverage robustness is also necessary to eliminate faults going unnoticed from one stage to the next. In an end-to-end control system, there are other items for its design that bear consideration, e.g., earliest point to identify acceptance of the part, where the acceptance record will reside, bill of material (BOM) breakpoints for sub-assemblies that will be released for service spares, customer versus field service installation methodologies, etc. These areas are all contributors in the effectiveness of the control system and need to be managed within the economic confines of the exercise. Furthermore, risk level needs to have a commensurate means of control. Although guidance are provided, e.g., ‘Design Control Guidance for Medical Device Manufacturers’ [3], few studies have examined the actual resolution and details necessary to build an adequate manufacturing control system to assure the IVD’s essential design outputs in large IVD instruments.
Outside of control systems, there are controls on all aspects from the initial concept, released design and design updates, e.g., obsolescence, improved reliability, safety, corrections and manufacturing process changes. In addition to the control of parts and the sub-systems working together in unison, there are controls for direct activities such as build, test and release processes. Indirect activities include supplier control, customer feedback, complaints and non-conformance processes, to name just a few.
This research paper is concerned with test-based control systems and how they can be implemented, primarily the aspects that impacts the essential clinical performance of the instrument (i.e., how do we know that we are releasing ‘good product’, stopping all ‘bad products’, avoiding unnecessary additional testing, meeting regulatory requirements etc.).
The research questions are:
- How to develop a control system which is fit for purpose;
- What mechanism can manage and proliferate key design knowledge within the organisation and thereby manage outsourced services; and
- How to implement a scaled engineering change process as changes impacting EDO naturally require extra regulatory and engineering oversight.
2. Literature Review of Essential Design Outputs
There was much cooperation between the EU and the FDA at the time of the Code of Federal Regulations (CFR) updates in the 1990s to incorporate design controls. Former long-time US FDA official Kim Trautman [4] described how the US FDA was partnering with the Technical Committee 210 [5] who were one of the authors of ISO 13485 (the Quality Management standard for medical device manufacturers) and worked with the Global Harmonisation Task Force (GHTF). Although the FDA published their update before ISO 13485 was eventually published, similarities were evident because of their cooperation. Each subsequent publication of the ISO 13485 standard has brought more and more convergence and aligned thinking between the two systems. Furthermore, the US FDA being a partner to the Medical Device Single Audit Program (MDSAP) [6] means that there is an aligned thinking on how design controls are being implemented and interpreted. The FDA Design Control Guidance for Medical Device Manufacturers published in 1997 [3] describes the relationship and linkages between the FDA’s design control guidance and the ISO 13485 standard as being “cross-referenced” to each other. The aim of this research is to develop a better understanding regarding the motivations behind these initiatives in the context of essential design outputs (EDO).
2.1. Area #1—EDO Standards & Regulations
The regulations and papers published around EDO was evaluated and the analysis is presented in the following sub-sections.
2.1.1. FDA & ISO: Essential Design Outputs
Within the context of this paper, the “design output includes, among other things, the specifications for the manufacturing process, the quality assurance testing and the device labeling and packaging” [7]. Also described as “the total finished design output consists of the device, its packaging and labeling and the device master record” [8]. It is the output from the design process that enables a manufacturer to create a product. Within that paradigm, the subset of “essential” design outputs is a concept used by both the FDA and ISO13485 as follows:
“Design output procedures shall contain or make reference to acceptance criteria and shall ensure that those design outputs that are essential for the proper functioning of the device are identified” [9]. In the more recent publication there is a similar extract for ISO whereby they state that “design and development outputs shall… specify the characteristics of the product that are essential for its safe and proper use” [2].
The intent of both regimes is that there is a subset of design output that is considered ‘essential’ for the safe and proper use of the device. In this case, the ISO 13485:2016 standard being a more recent publication, have included ‘safe’, but it is recognised that the intent of the FDA regulation included “safe” within their term “proper”. The FDA have stated that the design output “should identify the characteristics of the design that are crucial to the safety and proper functioning of the device” [3]. However, there is very little information on how to scope this aspect of the requirement.
2.1.2. Essential Performance & Basic Safety
Large IVD instruments (e.g., Class I) are diagnostic laboratory equipment and are required to comply with the IEC 61010 series of safety standards [10]. The International Electrotechnical Commission is an international standards organization that prepares and publishes international standards for all electrical, electronic and related technologies—collectively known as “electrotechnology”. However, a distinction needs to be made with Medical Electrical (ME) equipment used to detect or transfer energy to or from a patient and therefore subject to the more stringent IEC 60601 series of standards. These two standards, which can be confusing, are presented in Table 1 below.

Table 1.
ME Safety Standard & Electrical Safety Standard.
Large IVD laboratory equipment does not need to comply with IEC 60601 because it has its own set of standards to comply with, i.e., IEC 61010. Both sets of standards work in unison whereby the ME standard IEC 60601 is referenced within the laboratory equipment standard IEC 61010. Therefore IEC 60601 can be considered an umbrella standard for medical devices which includes IVD. Two important definitions in IEC 60601 are functional safety and essential performance (EP).
The IEC state that “Functional safety ensures that a given apparatus functions correctly in response to inputs. For example, if an infusion pump malfunctions, functional safety protocols will ensure that alarms are activated to signal the malfunction and if relevant that the pump is deactivated to protect the patient from harm through over-dosing” [11].
As demonstrated in Figure 1 EP is a limited area, which can be envisaged as showing the intersection between the clinical function of the instrument and the unacceptable risk associated with that instrument. Whereas this definition is particular to the IEC 60601 standards, it is not specifically called out in the IVD family of standards IEC 61010. This does not mean that the area is not applicable to IVD, but rather an alternate albeit slightly different approach is taken therein. Table 2 presents the definitions for the terms deployed in Figure 1, for ‘Basic Safety’, ‘Essential Performance’ and for ‘Functional Safety’.
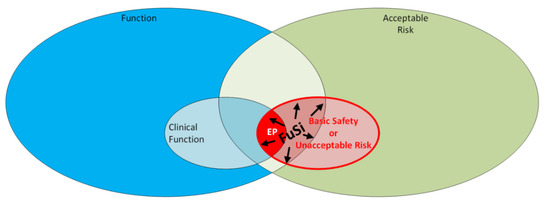
Figure 1.
A Venn Diagram—Essential Performance (EP) & Functional Safety (FuSi) demonstrating the intersection between the clinical function of the instrument and the unacceptable risk associated with that instrument. Source: Authors own.

Table 2.
Definitions—Basic Safety, Essential Performance & Functional Safety.
- Essential Performance (EP): a Venn diagram analysis of IEC 60601-1 is presented in Figure 1—which shows their definition of EP as being that area that intersects clinical function and unacceptable risk (or basic safety).
- Functional Safety (FuSi): the same Venn diagram in Figure 1 identifies the Functional Safety (FuSi) area as the intersection of the instrument function (which includes clinical function) with unacceptable risk. As presented, this incorporates EP and is a bigger area than the EP alone. Functional Safety is more closely aligned with EDO. As a reminder the working definition for EDO is design outputs that are essential for the safe and proper functioning of the device. So, the design team must ensure the EP is maintained while eliminating the red area where functional safety and unacceptable risk may overall to affect functioning.
2.1.3. Changes in 1997 to FDA Medical Device Good Manufacturing Practice (GMP)
Back in 1997 the FDA introduced an update to the Good Manufacturing Practices (GMP) whereby they recognised that “design controls must be enacted that increase the safety and effectiveness of medical devices” [15]. Although totally new concepts were not presented; the FDA’s revised approach was that of incorporating risk and aspects, from production and development, in line with ISO 9001 into their new rules. These new rules at that time were a significant step forward.
At the time, the FDA did not change the structure or content, but they did “emphasize the need for the engineering community to define the development and design processes and follow them” [15]. At this time the FDA identified that “44% of voluntary recalls from October 1983 through September 1989 may have been prevented by adequate design controls… the most frequent causes for recalls are related to Design, Software, and Non-Conforming Materials/Components” [16]. Farrell [15] identified the FDA findings in this area as: (1) Failure to properly identify and establish adequate physical and performance requirements for device production, (2) Failure to verify that the device met the physical and performance requirements before production, (3) Failure to ensure that the device components functioned properly in conjunction with other components, (4) Failure to ensure that the environment would not adversely affect the components and (5) Failure to select adequate packaging materials.
The requirements for Good Manufacturing Practice (GMP) as outlined by the FDA in 1997 are all encompassing in its demands for processes and procedures from pre-production, manufacturing, to packaging, storage and installation of devices to conform to GMP to assure that the device will be safe and effective and in compliance [15]. The 1997 GMP requirements places more importance on the design controls aspect and places more emphasis on production processes. One of the most significant changes was the incorporation of the Quality System Regulation (QSR) in lieu of just Quality Assurance. In the QSR Manual, the area of particular interest in this paper is criteria #2, which advises the manufacturer to “ensure that those design outputs that are essential for the proper functioning of the device are identified” [17]. The QSR manual goes on to show what the FDA expect in terms of demonstration compliance with the design output requirements by documenting design outputs and gaining design output approval.
2.1.4. Design Control Guidance for Medical Device Manufacturers
The following class I devices are subject to design controls: (i) Devices automated with computer software [9]. At around the same time in 1997 that the FDA updated their GMP requirements they also published their guidance document “Design Control Guidance for Medical Device Manufacturers” [3]. This publication addressed the entire area of design controls in a “waterfall” iterative format from design and development planning, design input, design output, design reviews and design transfer and then design changes, verification & validation and finally the design history file.
The design process is intended to assist manufacturers with their quality management system (QMS) requirements in relation to design controls. In effect, it was intended to interpret the language of the QSR and to explain the underlying concepts in practical terms. “Design controls increase the likelihood that the design transferred to production will translate into a device that is appropriate for its intended use” [3]. They are the interrelated set of practices and procedures that are embedded into the design and development process. It provides managers with better visibility of the design process itself, thereby enabling them to identify opportunities earlier in the process, thus avoiding costly errors later in the cycle.
The medical devices industry encompasses a great range of devices from simple tongue depressors to complex IVD instrumentation, which is the reason that regulatory agencies do not prescribe how to bring a product through the development cycle. The product’s complexity as well as the organisation structure has a greater impact on how design controls are applied. The FDA demand that there is a QMS in place commensurate with both the product complexity and the risk to the patient. While not prescribing “how” to achieve the quality system requirements (QSR), the organisation itself is required to document a means that works for their situation on how best to achieve and maintain compliance with the QSR. There are similar requirements in the ISO 13485.
Essential design outputs (EDO) are a subset of the design output stage. In fact, there are two areas of interest in this stage highlighted by the FDA: “Design output should be expressed in terms that allow adequate assessment of conformance to design input requirements and should identify the characteristics of the design that are crucial to the safety and proper functioning of the device” [3]. The guidance in [3] goes on to identify two areas of note as follows:
- What constitutes design output?
- Are the form and content of the design output suitable?
The first item is important because of the volumes of information that is created as a result of product development. Typically, every piece of work output, i.e., deliverable item, created as part of the design effort is design output, and the design output from one stage is often the design input for the next. The FDA define design output as “the design specifications which should meet design input requirements, as confirmed during design verification and validation and ensured during design review. The output includes the device, its labeling and packaging, associated specifications and drawings, and production and quality assurance specifications and procedures. These documents are the basis for the DMR. The total finished design output consists of the device, its labeling and packaging, and the DMR”. This design output can include a lot of material up to and including sample product built during the pre-transfer stage.
The second item is equally important because it delineates the aspects that are necessary for the device master record (DMR). There will be some aspects of the design output necessary to establish conformance to design input requirements, but some of these aspects are not necessary for production, e.g., results of verification activities, risk analysis, software source code etc. In addition, there are the aspects of design output necessary for the DMR, e.g., “production specifications include drawings and documents used to procure components, fabricate, test, inspect, install, maintain, and service the device” [3]. In fact, the FDA contend that “conformance with the quality system requirements concerning design output generally requires no ‘extra’ effort on the part of the manufacturer, but simply the application of some commonsense procedures during the planning, execution, and review of design tasks” [3].
2.2. Area #2—EDO Architecture & Audit Readiness
The International Medical Device Regulators Forum (IMDRF) developed a global approach to auditing the manufacturing of medical devices to ensure their safety. The programme was developed with the input from following countries: Australia (TGA), Brazil (ANVISA), Canada (Health Canada), USA (FDA) and Japan (MHLW) and (PMDA). It enables regulatory oversight of manufacturers’ quality management systems while minimising the regulatory burden on industry. The audit programme is based on an annual audit and full re-certification audit every three years. It is a more prescriptive programme than the QSIT programme. For the purpose of this paper, there is a focus on the Design Outputs and Essential Design Outputs (EDO). The EDO is presented in the MDSAP Companion Document [18] as “Outputs that are essential for the proper functioning of the device must be identified. Typically, an organization can use a risk management tool to determine the essential outputs”. An example is further given as “the establishment of manufacturing process tolerances, the degree of purchasing controls and acceptance activities applied to a supplier or the priority and depth of a failure investigation may be influenced by whether or not the component (assembly, material, etc.) is considered an output essential for the proper functioning of the device”.
With respect to design transfer into production, the MDSAP Companion Document advises the auditors to base their reviews on the “organization’s identification of essential outputs and risk management activities” [18]. The intention being that the MDSAP audit is conducted based on “characteristics of the product that are essential for its safe and proper use” [2]. An area demanding attention is around the control system for acceptance activities. Processes that require validation to demonstrate compliance, and especially processes necessary to assure parts “where routine inspection and/or testing does not examine quality attributes essential to the proper functioning of the finished device” [18]. In these areas, the auditors are directed to identify attributes essential to the proper functioning of the finished device and then to ensure that there are validated processes in place to assure the quality of the output, e.g., bonding, gluing, etc. “When validating processes, organizations must take into account the current thinking of experts where published information is available” [18].
The degree of acceptance activities necessary and their extent should be based on the potential effect the supplied product poses to the essential design outputs. This central tenet on impact to EDO is present throughout the MDSAP Companion Document [18], and acts as a guidance to the inspector on the degree of review warranted in any particular situation. It is ever-present from the opening interview whereby the inspectors are trying to ascertain how many of the processes essential for the proper functioning of the finished medical device are outsourced. The theme is taken through the purchasing controls, and then the manufacturing controls. EDO are the more focused, risk-based approach deployed by previous audit programmes, and to a large extent are a more-clear means of bounding the audits.
2.3. Area #3—Design Process Opportunities from other Industries
This section of the literature review is focused on design and development planning in both the medical devices industry and also in other non-related industries to determine which practices can be incorporated, especially in the area of design output. “The development of devices, … relies on the application of technology to realise a product that meets an identified need. Thus, good design processes are central to the creation of devices that are capable of satisfying user needs and regulatory requirements” [19]. To that end, a Quality Management System (QMS) is mandated by the Code of Federal Regulations for US bound devices; while in the EU, a QMS is equally expected and generally the ISO13485:2016 is deployed. In many respects ISO 13485 Section 7.3 Design and Development is very similar to the Part 820.30 Design Controls. In each case the QMS is necessary, to minimise product failure at the earliest stage in the product lifecycle and also to reduce associated costs.
There is a requirement on the manufacturer to develop plans that describe the design and development activities on a given project. These plans identify the responsibilities and authorities for the delivery of the design, i.e., what needs be done, and who does it.
2.3.1. Agile Project Management
Agile project management is a means used by companies such as Toyota and Honda for years. It is an alternative approach towards project management whereby there is an increased emphasis on cross-functional project teams working on highly complex products, coping with both mechanical and software related issues, which in our case also includes plastics, fluidics and reagents. In addition to pressures on both time and cost, complex new product development brings high levels of uncertainty. Highsmith [20] introduces the core agile values as stated in the Agile Manifesto [21]. Although these are based on software development, the origins of agile is from the Japanese industries as mentioned earlier. These principals, which Highsmith considers mandatory, establish a culture which is necessary for success and helps to form the positive belief system which is the basis of agile project management.
2.3.2. Set-Based Concurrent Engineering (SBCE)
“A new paradigm in engineering design, known as set-based concurrent engineering (SBCE), has been proposed which seems to offer advantages over more traditional techniques” [22]. The traditional approach relied upon defining the requirements early, converging on a design concept and then iteratively improving that design with gradual improvements until all requirements are met. In contrast to this approach, set-based design relies on delaying the setting of requirements, and encourages the development of a series of sets to meet the not-yet-finalised requirements. Then, over time as the customer’s needs are better understood, there is the flexibility to refine the input requirements later in the design cycle. “Set-based approaches to design seem to offer advantages over other methods in terms of improved design quality, reduced development risk and shorter cycle times” [22]. As described by Bernstein, while set-based concurrent engineering consists of a wide variety of design techniques, the basic concepts can be consolidated into two basic principles:
Many alternative designs should be considered i.e., sets of designs, and then gradually narrow down to the eventual chosen design.
In a multidisciplinary approach, engineers should review a series of designs from their own perspective, identify areas of overlap and then gradually develop an integrated solution.
Hasso-Plattner Institute of Design at Stanford (the “d.school”) presents the five key steps ‘Design Thinking’ which is re-emerging as an important area of consideration. ‘Design Thinking’ is centered around the person rather than incremental improvements to existing products. The five stages of design need to be understood as “different modes which contribute to the entire design project, rather than sequential steps” [23]. Dam and Teo [24] describe the five steps as follows:
- Empathising: Understanding the human needs involved.
- Defining: Re-framing and defining the problem in human-centric ways.
- Ideating: Creating many ideas in ideation sessions.
- Prototyping: Adopting a hands-on approach in prototyping.
- Testing: Developing a prototype/solution to the problem
The alternate design approach is iterative where there is a lot of over and back which is the opposite to the traditional serial Stage-Gate© Approach. It lends itself to maximum flexibility and keeping options open until the last possible moment, thereby reducing costly reworks, re-designs and overall project delays.
2.3.3. Design Constraints in the Automotive Industry
The “Toyota Way” as described by Usda [25] is where the “company philosophy models cultivated by development engineers during their daily job” has elements that includes ‘‘Continuous Improvement’’, ‘‘Challenge’’, ‘‘Respect for People’’, ‘‘Genchi Gembutsu’’ [go and see for yourself] and ‘‘Teamwork’’. In recent years, there have been significant improvements in automotive fuel efficiency. A significant aspect of those improvements were as a result of the use of software improvement in the Engine Control Unit (ECU) as well as other complementing technology.
In many ways, the constraints and pressures on the development of automobiles reflects similar constraints in the large IVD industry. The automotive designers drive improvement in fuel consumption, reduces emissions, improved road handling and active safety systems. Thus, their role is assured for the foreseeable future; however, this area is time hungry in an industry that is driving for ever shorter development cycles. Given that this design area is inevitably on the critical path, there is a need to “deliver more complex systems with quality and improved productivity” [26].
2.3.4. Set-Based Design—Opportunities for Naval Ship Design
“Set-Based Design (SBD) is a complex design method that requires a shift in how one thinks about and manages design … it allows more of the design effort to proceed concurrently and defers detailed specifications until trade-offs are more fully understood” [27]. They go on to describe how traditional design processes have failed in large-scale product design due to their inherent complexity, e.g., naval vessels. In addition, the use of automation—removing the human interaction with the process—does not work either. So, there is a growing recognition that designing these large complex projects requires human interaction, however their complexity necessitates a new approach to design. They described the development of the (Landing Platform Dock) LDP17 series of ships as having cross-functional design teams co-located or connected in a virtual environment to perform the overall design task.
This approach was deployed for several reasons. Among them, a bereft of experienced staff to perform the design activities due to attrition of experienced personnel. This resulted in having younger and less experienced engineers taking the role of design managers, whereas in the past this would have been with older and more “practiced” engineers. This transition to younger and less experienced necessitated a different approach towards design communication, negotiation and information transfer. Set-based design was one of the newer means deployed to bridge this gap.
The traditional approach to communicating the ship design was the Evans Design Spiral [27]. The model emphasises that the ship design of hull geometry, resistance, power, weight, stability, etc., interact, and that these can be considered in sequence with increasing detail at each pass around the spiral. This is done until there is an optimised design.
This approach can be classed as point-based design because each iteration attempts to meet the inputs of that stage. The disadvantages with this approach are that generally it will not produce an optimum design, and the number of times around the spiral is limited to the design budget and timelines rather than achieving the best outcome.
“In Concurrent Engineering the point-based design approach is still implemented but engineers analyze in parallel a specific design based on a request for analysis” [27]. The major improvement with concurrent engineering is the enhanced communications enabled by the collaboration. This improved communication minimises errors and speeds up development time. This SBCE process is used for many different types of complex design projects. It is being used, to recognised good effect by Toyota Motor Company. “Toyota considered a broader range of designs and delays certain decisions longer than any other automotive companies yet has what may be the fastest and most efficient vehicle development cycle in the industry” [28]. They spend longer in the earlier stages to define possible solutions, and then more quickly converge on the eventual solution in the latter stages.
3. Implementation & Results
3.1. Methodology
The research that was undertaken was an action-based case study in a large IVD manufacturing company. The research was caried out with a large design team understand, interpret and implement the regulation around EDO. Solutions were brainstormed and applied and implemented in order to apply solutions to meet the research objectives. The following sections will discuss and document the actions, methods and findings.
The authors consolidated and reviewed learnings from the literature review and regulations review to establish how these learnings could be applied to refine the design control and EDO process for the company under case study—a large IVD manufacturer.
The Essential Design Outputs are required in the FDA CFRs and are equally required by MDSAP auditors as an output of the ISO 13485. The regulators do not prescribe the level of detail warranted nor the resolution level expected of these EDO. From an IVD instrument perspective, there is no standard specifically set out to provide necessary guidance or to act as a reference. However, when an investigation is performed in the more regulated medical electrical equipment, some insights can be gained from how they view essential performance and functional safety:
- Essential performance (EP): a Venn diagram analysis of IEC 60601-1 is presented in Figure 1 which shows their definition of EP as being that area that intersects clinical function and unacceptable risk (or basic safety).
- Functional safety (FuSi): the same Venn diagram in Figure 1 identifies the FuSi area as the intersection of the instrument function (which includes clinical function) with unacceptable risk. As presented, this incorporates EP and is a bigger area than the EP alone. Functional Safety is more closely aligned with EDO. As a reminder, the working definition for EDO is design outputs that are essential for the safe and proper functioning of the device.
IVD instrument is laboratory equipment, and therefore has its own suite of safety standards. Whereas the IVD instrument range are not within the scope of medical electrical (ME) equipment there are opportunities to bring some of their processes across to the IVD.
3.2. Literature Review—Summary Findings
The literature review informed the following key lessons in relation to this paper which informed how EDO was managed in the company under study.
- EDO are not a new phenomenon
They were first described by the FDA in the early 1990s as part of the design controls updates to the QSRs [16]. Later as part of the GHTF the thinking was incorporated into the ISO13845 and later still into the MDSAP [4]. In more recent publications, the EDO thinking has matured, and the FDA has always remained aligned to the later publications from MDSAP [6].
- Essential Performance as defined by IEC 60601-1
“Characteristics related to loss or degradation of the clinical performance of a medical device that can result in unacceptable risk, are sometimes referred to as essential performance” [29], which then refers out to IEC 60601-1. By reviewing these standards, and referenced standards therein, it was possible to gain a wider perspective on how the broader medical electrical (ME) equipment industry addresses this area.
- EDO Identification Methodology
Bijan Elahi [30] presented a formulaic approach towards identifying EDO using the FMEA as his foundation. He states that each company should tailor that approach towards their own situation. Whereas ISO 14971 is concerned with medical risk, FMEA is concerned with reliability improvement—similar but different. The scope of FMEA is widened out to accommodate risk impacts, as well as the reliability impacts. As a result, reviewing the FMEA process in the company under study, the following proposal for EDO could be deployed as presented in Figure 2. This matrix is used as a risk acceptability matrix to help the design team evaluate risks to patients or operators of the IVD device. Depending on the risk and its severity and likelihood risks can be deemed as negligible, low, medium or high risk. Anything failing I the medium to high-risk category or low to high-risk category is considered an EDO and needs to be actioned by the design team.
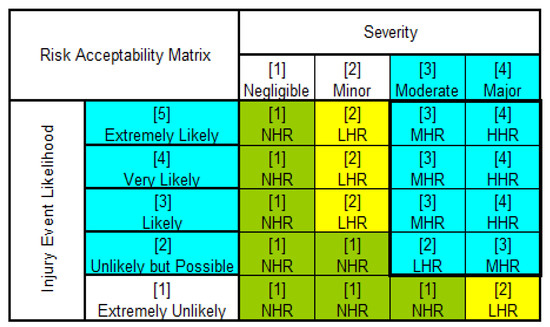
Figure 2.
EDO Assignment to evaluate risk to patient or operator of the IVD device—Authors own work. Legend: (1) NHR: Negligible Risk; (2) LHR: Low Risk; (3) MHR: Medium Risk; (4) HHR: High Risk; EDO: Turquoise Blue. NHR and LHR does not require professional medical intervention.
EDO could be defined as risk mitigations for impact to patient or operator. For the patient, where there are risk to results if nothing is implemented to mitigate, and therefore the mitigation is an “Essential” design output.
3.2.1. Design Control: Stage-Gate© approach
The Waterfall approach [3] lends itself naturally to the traditional Stage-Gate© approach for product development. This approach was suggested by the FDA in their guidance documents, which has resulted in the almost universal deployment within the medical devices industry. This design control model is not the only model available for complex product development, and studies from other industries have demonstrated that it is not the most efficient approach for product development either. An alternate approach known as set based concurrent engineering has many advantages over the traditional approach, achieving better designs, less development risks and better turn-around time [22].
3.2.2. Toyota Development Model
The Toyota product development process is prefaced with their innate ability to delay significant commitment of resources, and then being faster with the latter stages of converting their design inputs to design outputs. “Even though Toyota has shared many details of its manufacturing practices, it has been closed lipped about many of the details of its design process” [27]. As other industries learn about their approach towards product development, their culture and that of their “family” of suppliers makes it very difficult to replicate.
3.2.3. Application of an Alternate Development Model
As demonstrated by the US Navy [27], set-based concurrent engineering philosophies from Toyota Motor Company were tailored and adapted to build the LDP17 series of ships. Conscious attempts were made to adapt continuous feedback loops to delay expensive design stages at the earlier stages of development, and to make better use of a younger less experienced and more geographically dispersed engineering pool. The problems with designing complex projects such as ships are very similar to developing large IVD instrumentation.
3.3. Managing EDO within the Company
The decision taken was that EDO that can be assured by an in-house manufacturing test and would have their EDO criteria managed on a trace matrix (as per Figure 2), which in turn is part of the DMR. This would link the specification drawing attribute criteria to the test identified in the control system, and from there to the location of the test result in the DHR. Individual attributes on the part would be contributors to the EDO, but these attributes need not be separately identifiable for the supplier (as there is nothing extra expected from the supplier, other than to make the part to specification). Later in the production cycle in the manufacturing plant, the assembled part would be presented for testing (as part of the normal manufacturing process). The resultant test in the control system would be designed to assure the identified EDO (which incorporates all contributor attributes).
The process for developing the test follows normal engineering design controls. The research and development (R&D) organisation provide the limit specifications to the tester group. They then build a test solution and validate it prior to release. Thus, the design input requirements (DIR) are traceable to the eventual tester solution. All EDOs have a traceable home in the control system. In this case, the trace is via the trace matrix, which is part of the DMR, and the EDO is not required to be on the specification drawing (because it serves no purpose for the entity producing the individual part).
3.4. Managing EDO at Suppliers
In situations where it is not possible to “catch” the fault within the final manufacturing operation, the control system is designed to have extra inspection required by the supplier as part of their process. In this case, the EDO should be marked onto the specification drawing because there is a purpose for the entity producing the individual part.
There is a recognition that the EDO attribute will be measured at the supplier and consequently the trace matrix mentioned earlier will trace the EDO in the control system to an inspection performed at the supplier. As an aside, within the QMS process, when the manufacturing organisation procures parts from approved suppliers, and these parts will be accepted into inventory using procedures from the QMS in alignment with receiving inspection activities. The FDA QSR requires that defines that “Each manufacturer shall establish and maintain procedures for acceptance of incoming product. Incoming product shall be inspected, tested, or otherwise verified as conforming to specified requirements. Acceptance or rejection shall be documented” [7].
Figure 3 presents a flowchart which was created as a result of this strategic research. It demonstrates the extra oversight within the QMS for EDO parts within the IVD manufacturer. These parts have their acceptance at the earlier stages of the value chain (i.e., at the supplier) and are assured by the supplier’s processes. Included also with the EDO parts are other parts which, for other reasons, require a similar level of oversight, e.g., reliability or customer satisfaction. When a part is identified as a “performance part”, i.e., an EDO part or part that requires similar oversight, the inspection plan at the receiving site is set and presented for review to an Inspection Review Board. This is a cross-functional team that reviews a series of criteria and dynamically adjusts the acceptance plan depending upon a series of QMS metrics and recent non-conformance experience.
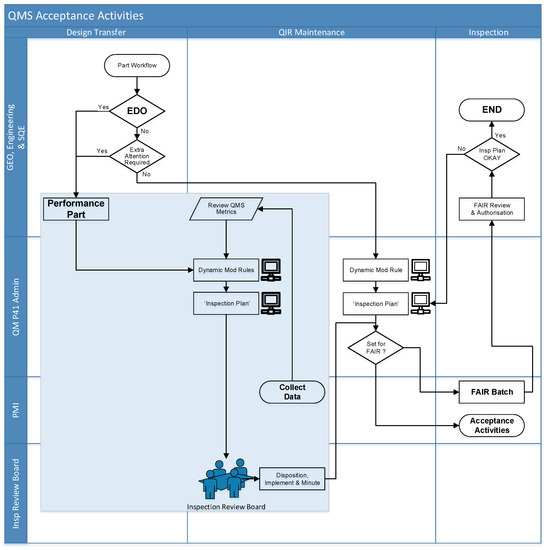
Figure 3.
QMS acceptance activities for performance parts.
In addition, there is an on-going review of integrated metrics, which are an amalgamation of feedback over the entire value chain, from supplier performance to PMI performance, production performance and field performance. In this way, if there is any sensitivity with the part being shown, change to the dynamic inspection plan will be presented to the Inspection Review Board (IRB), and the inspection process can either be tightened or reduced.
3.5. ‘Inspection Review Board’ (IRB) Procedure
A new QMS procedure was written as a result of the strategy described in Section 4.1 and Section 4.2 above. With a clear strategy, and a presentation of this approach in Figure 3, the proposal was presented, and agreed as company policy. By delineating the ‘method of inspection’ as the assurance of the specification into the QMS, it means that the company has more dexterity to dynamically change the inspection plans based upon confidence in the parts from the supplier. In this case the board’s roles and responsibilities are set out very clearly to manage the acceptance activities of performance parts (EDO parts) in a dynamic manner which continuously changes based upon current conditions.
4. Analysis and Discussion
This research has a number of objectives all emanating from essential design outputs (EDO). As a reminder, EDO are a subset of design outputs that are essential for the safe and proper functioning of the device. It is only when an auditor asks for them and the special controls in place for them that their importance comes into focus; especially for a large IVD instrument. Firstly, the objective of the literature review itself was to determine what was published on this very narrow topic of EDO, take a review of these combined sources and determine the relationship between the various sources in order to develop a better understanding of the focus area.
The research centered on design controls, incorporating other industries to determine what lessons could be cross-pollinated back into the medical devices industry. Alternate design approaches were deployed, incorporating aspects of set-based design, and concurrent engineering. However, as the company migrated to a new paradigm with its development processes, it was very difficult to maintain alignment with the associated QMS. Opportunities were identified and implemented within the development cycle otherwise known as ‘New Ways of Working’ (NWoW), but the traditional QMS Stage-Gate© controls were not adequately considered in tandem. A similar paradox was identified by the US Navy with their ship-building processes, and equally needed more attention. In summary, the NWoW promoted SBCE, but it was subsequently recognised that the QMS needed to be updated to maintain alignment.
4.1. Design Transfer
The EDO are the subset that is of interest for this research, and also for the design transfer sub-section. The FDA defined their requirements in the CFR as part of the design output controls. Their requirement is that “those design outputs that are essential for the proper functioning of the device are identified” [9]. In addition, the FDA have stated that the design output “should identify the characteristics of the design that are crucial to the safety and proper functioning of the device” [3]. Likewise, in the MDSAP, which was later published, has similar but more specific requirements to “specify the characteristics of the product that are essential for its safe and proper use” [2]. The term “safe” was not included in the CFR itself but it was detailed in their preamble.
A mixed-method, simple, methodological approach was taken. By working with participants in a real-life work environment, it was possible to take the acquired understanding of the EDOs and ensure that they were applied in a practical, lean manner from the SCM perspective. There is a recognition that the design output is a vast array of authorised documents and reports, and that the essential subset for producing safe and effective devices needs to be separately identified. When this EDO subset is identified, it is expected that they will get extra attention in the control system. As described by Elahi [30], “certain policies should be exercised to provide higher confidence in the implementation and performance of those outputs. This could be in the form of increased process capability requirements, tighter QC inspections, etc.”.
4.2. Tiered Suppliers
From the regulator’s perspective, there is little distinction between the suppliers and the end-manufacturing operation, especially when the responsibilities of the manufacturer are under scrutiny. Since the mid-1990s, more and more of the company’s core activities were outsourced to tiered suppliers, i.e., a series of sub-suppliers feeding into one another until the eventual sub-assembly or module is accepted by the end-manufacturer. This was done primarily to realise the potential from suppliers, who were specialist in their respective fields, to save costs and increase quality. Although this objective may adjust as a result of Covid-19, there remains a need to understand how the manufacturing BOM is de-constructed to exploit the company’s supply chains.
Cross-functional teams were deployed to identify the attributes linked to EDOs. These attributes all have a direct bearing to the safe and proper functioning of the device. Although production processes are required to demonstrate a capability to achieve the requirements of the specification drawing, the location of the DHR information is a strategic imperative. Traditionally, all important testing was performed in-house, and the resultant record was retained in the DHR. In today’s environment, there is a blurring of the lines between in-house manufacture and out-sourced manufacturing. Nevertheless, the attributes that are critical to achieving the EDOs are identifiable in the DMR. In each case, the attribute is traceable to a test within the overall control system, as the means to demonstrate compliance.
‘Specification Definition’ versus ‘Specification Assurance’
This raises the contention between the ‘specification definition’ versus ‘specification assurance’ process. As a member of the review team, the position being espoused by use of this research is that the R&D organisation are responsible for delivering a tolerance specification. Then, with the recognition that the supply of the parts will change over time, there is a need for manufacturing to have the necessary flexibility to adjust the sampling regime when assuring the quality of these EDO attributes. This equates as follows:
- Specification Definition R&D—develop and release
- Specification Assurance SCM—dynamically measure and manage
4.3. Audit Readiness
When clarifying processes, especially in a regulatory area, it is important to be cognizant of the questions that are likely to be addressed during audits. Given the detail provided in both the QSIT by the FDA and the MDSAP for the ISO13485, it is possible to address the touch points that will be raised. In both cases, there was an alignment over the years, and with the involvement of the GHTF this alignment is evident as each new iteration of a standard is produced. It is not the approach of one agency versus another, rather it is the matured thinking of an aligned approach. Suffice to state that the requirements in the area of design controls are aligned, and that the MDSAP auditing programme provides the greatest insight as to how the regulators expect manufacturers to conform. By building an architecture whereby there is an easily understood trace matrix between the EDO and the control system, it should be easier to comply with inspections with little explanations required.
The component parts of control system development in the past was imbued as tacit knowledge within the teams within the IVD manufacturer. As the organisation grew and product development was spread among different sites, this informal process needed to be aligned and made more transparent. EDOs, when properly applied with the use of some commonsense procedures as described in the FDA Guidance [3], have the potential to yield huge benefits for the organisation, in addition to meeting the minimum regulatory requirements for both MDSAP and US FDA.
The following three areas were recognised as key areas for breakthrough when re-searching this research, and at the same time working with this topic in new product de-velopment (NPD). The research questions were also met via the following three areas.
With the current project (i.e., pre-launch), the primary focus was to design and implement a trace matrix to manage the control system as described throughout this research. It afforded the IVD manufacturer the opportunity to put a considered SCM input to the EDO management process, whilst recognising complementary perspectives in both R&D and design quality engineering (DQE). This deeper understanding meant that the SCM position was implemented and fitted within an overall EDO architectural context (in many ways that was still only in development).
4.4. Control Systems
The IVD organisation under study has developed a prescribed means of tracing the EDO to the control system. This trace matrix has facilitated the development of a robust control system which easily demonstrates test coverage for the areas that are essential for the safe and proper functioning of the device. As a by-product, serviced spares were also addressed by this trace matrix, which ensures that they have an adequate test coverage, at the end of production and prior to acceptance at field installation.
SCM deployed a tactical approach whereby the specification definition and specification assurance were separated, with respective oversight under the remit of different review boards. This kept the EDO identification within the responsibility of the R&D, and at the same time affording necessary dexterity to the SCM on how to achieve and maintain product assurance. In effect, from a headquarter (HQ; parent company) perspective, this enables uniform controls in SCM, i.e., accommodating flexibility where necessary, and at the same time keeping appropriate centralised HQ controls.
In summary the first research objective “How to develop a control system which is fit for purpose?” was met via the new prescribed means of tracing the EDO to a control system.
4.5. Knowledge Capture
The EDO is the information that is central to the device itself, i.e., it distinguishes between what is critical for the functioning of the device and the routine engineering areas that do not require the same level of scrutiny. EDO is one of the key foundations for intellectual property (IP). It helps determine aspects of the production process that should be retained in-house, and aspects of the design demanding extra oversight. EDO is also recognised as a means to identify key knowledge for subsequent design changes and for the next generation product development.
As a by-product, personnel having design cognisance in these areas are easily recognised, and succession planning naturally addresses core knowledge. Core knowledge held by individuals can easily exit the company by individuals simply leaving. Aside from the QMS requirements, if designs are identified as EDO, these EDO designs naturally encourage greater detail into the DHF. In many ways, EDO is the ‘glue’ that formally identifies and holds this knowledge together.
By identifying core EDO’s and EDO’s within designs, the second research question “What mechanism can manage and proliferate key design knowledge within the organisation and thereby manage outsourced services?” has been met.
4.6. Scaled Design Change
Similar to the auto industry as mentioned earlier there is a need to “deliver more complex systems with quality and improved productivity” [26]. While this appears like an oxymoron, there is an expectation for faster design turnaround with IVD instrument design, in the face of increased complexity.
When EDO are presented for changes, more extensive verification testing are required because of the potential impact of the change. Design changes are streamed between ‘EDO impacting’ and ‘non-EDO impacting’ but this opportunity is not yet fully exploited. This facility should enable the organisation to develop a scaled approach towards design changes and establish this as a true competitive advantage. At the time of writing, there is a convergence of ideas on exploiting EDO towards this end, but their implementation is slated to happen after the trace matrix (as described earlier) is fully embedded.
This scaled design change with more extensive verification testing has achieved the third research objective “How to implement a scaled engineering change process as changes impacting EDO naturally require extra regulatory and engineering oversight?”.
5. Conclusions
One of the limitations of the research identified was that there is very little published material in this narrow field on EDOs. With smaller IVD products having a limited number of parts, it is very apparent what parts are EDO. However, with the larger IVD instrumentation containing thousands of parts across a wide range of engineering disciplines, this clarity is not so apparent. The fact that the EDO drives the control system and is the means to put extra oversight on aspects of the design only serves to emphasise their importance.
This study has identified that EDO is the vehicle to clearly identify what is essential about the design. It enables R&D to focus on the areas where design cognisance resides. It also directs the organisation to focus extra oversight into areas of importance, thus enabling better controls in key areas such as outside product development, other equipment manufacturers (OEM) and plant-to-plant design transfers. These are all key areas for competitive advantage. Finally, it is hoped that this body of work will serve as a foundation for further research and process development, in these key areas.
Author Contributions
Conceptualization, O.M., B.B.; methodology, O.M., B.B., N.T.K.; investigation, B.B., N.T.K.; software, B.B., N.T.K.; data curation, O.M., B.B.; formal analysis and results discussion, O.M., B.B., N.T.K.; writing—original draft preparation, O.M., B.B.; writing—review and editing, O.M., B.B., N.T.K.; validation, O.M., B.B., N.T.K.; resources, B.B., N.T.K.; visualization, O.M., B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=820 (accessed on 16 April 2021).
- ISO13485 Medical Devices—Quality Management Systems—Requirements for Regulatory Purposes (ISO 13485:2016). Available online: https://eu-i2-saiglobal-com.ezproxy.itsligo.ie/management/display/index/0/176561/-/54deb8da213a8882c3564e274077d76f (accessed on 9 November 2019).
- FDA Guidance Design Control Guidance for Medical Device Manufacturers. 1997.
- Trautman, K. NSF’s Trautman Talks MDSAP, Swapping FDA’s QSR For ISO 13485, Regulatory Convergence, And More. 2018. [Google Scholar]
- ISO/TC 210—Quality Management and Corresponding General Aspects for Medical Devices. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/committee/05/48/54892.html (accessed on 16 April 2021).
- FDA Medical Device Single Audit Program (MDSAP). Available online: https://www.fda.gov/medical-devices/cdrh-international-programs/medical-device-single-audit-program-mdsap (accessed on 11 June 2020).
- FDA CDRH Quality System (QS) Regulation/Medical Device Good Manufacturing Practices. Available online: https://www.fda.gov/medical-devices/postmarket-requirements-devices/quality-system-qs-regulationmedical-device-good-manufacturing-practices (accessed on 16 April 2021).
- IEC IEC—TC 66 Dashboard > Projects / Publications: Work Programme, Publications, Stability Dates, Project Files. Available online: https://www.iec.ch/dyn/www/f?p=103:22:0::::FSP_ORG_ID:1253 (accessed on 16 April 2021).
- 21 C.F.R. § 820 PART 820—Quality System Regulation. 1996.
- I.S. EN 61010-1 Safety Requirements for Electrical Equipment for Measurement, Control, and Laboratory Use—Part 1: General Requirements. Available online: https://eu-i2-saiglobal-com.ezproxy.itsligo.ie/management/display/index/0/370428/-/472e10dd34e3b5339ab204cccedbd900 (accessed on 25 April 2020).
- IEC Briefing Paper: Functional Safety Essential to Overall Safety—En, Ru—IEC Basecamp 2019.
- IEC IEC 60601-1. Available online: https://webstore.iec.ch/searchform&q=60601-1 (accessed on 16 April 2021).
- IEC IEC 60601-1 v3.1 Medical Electrical Equipment—Part 1 General Requirements for Basic Safety and Essential Performance.
- I.S. EN 62061 Safety of Machinery–Functional Safety of Safety-Related Electrical, Electronic and Programmable Electronic Control Systems 2015.
- Farrell, G.N. 6.3.4 FDA good manufacturing practices (GMP) for medical devices. INCOSE Int. Symp. 1995, 5, 84–91. [Google Scholar] [CrossRef]
- Tartal, J. Design Control. Available online: https://www.fda.gov/media/116762/download (accessed on 8 September 2019).
- FDA QSR Manual Medical Device Quality Systems Manual: A Small Entity Compliance Guide. 2005, 373.
- IMDRF MDSAP AU G0002.1.003 Companion Document. 2017.
- Tobin, J.J.; Walsh, G. Medical Product Regulatory Affairs: Pharmaceuticals, Diagnostics, Medical Devices; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Highsmith, J. Agile Project Management: Creating Innovative Products; Addison Wesley: Boston, MA, USA, 2004. [Google Scholar]
- Beck, K.; Beedle, M.; van Bennekum, A.; Cockburn, A.; Cunningham, W.; Fowler, M.; Grenning, J.; Highsmith, J.; Hunt, A.; Jeffries, R.; et al. Principles behind the Agile Manifesto. Available online: http://agilemanifesto.org/principles.html (accessed on 9 May 2020).
- Bernstein, J.I. Design Methods in the Aerospace Industry: Looking for Evidence of Set-Based Practices. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 1998. [Google Scholar]
- Teo, Y.S.; Dam, R.F. What Is Design Thinking? Available online: https://www.interaction-design.org/literature/topics/design-thinking (accessed on 16 May 2020).
- Dam, R.F.; Teo, Y.S. 5 Stages in the Design Thinking Process. Available online: https://www.interaction-design.org/literature/article/5-stages-in-the-design-thinking-process (accessed on 16 May 2020).
- Ueda, H.R. Innovative Development Methodology Based on the Toyota Way; Innovative Entwicklungsmethodik Basierend Auf Dem Toyota Way (Conference)|ETDEWEB. Available online: https://www.osti.gov/etdeweb/biblio/20902750 (accessed on 17 May 2020).
- Ohata, A.; Butts, K.R. Improving Model-based Design for Automotive Control Systems Development. IFAC Proc. Vol. 2008, 41, 1062–1065. [Google Scholar] [CrossRef]
- Singer, D.J.; Doerry, N.; Buckley, M.E. What Is Set-Based Design? Nav. Eng. J. 2009, 121, 31–43. [Google Scholar] [CrossRef]
- Sobek II, D.K.; Ward, A.C.; Liker, J.K. Toyota’s Principles of Set-Based Concurrent Engineering. Sloan Manag. Rev. 1999, 40, 67–83. [Google Scholar]
- ISO14971 Medical Devices—Application of Risk Management to Medical Devices.Pdf 2019.
- Elahi, B. Safety Risk Management for Medical Devices; Elsevier BV: Amsterdam, The Netherlands, 2018. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).