Abstract
The non-enzymatic synthesis of N-benzyloxycarbonyl-L-phenylalanyl-L-leucine (Cbz-Phe-Leu) from lipophilic N-benzyloxycarbonyl-L-phenylalanine (Cbz-Phe) and hydrophilic L-leucine (Leu), by N, N’-dicyclohexylcarbodiimide (DCC) as a condensing agent, was carried out using a reversed micellar system composed of bis(2-ethylhexyl) sodium sulfosuccinate (AOT) as a surfactant and isooctane. We successfully synthesized Cbz-Phe-Leu in a short time and investigated the effects of its operational conditions, the DCC concentration, w0, and the pH on the kinetic parameters and the maximum yields. For dipeptide synthesis, we had to add an excess of DCC with the substrates because of the side reactions of Cbz-Phe. From the pH dependency of the reactivity, a partially cationic form of Leu was better for a synthesis reaction because of the enrichment of Leu at the interface by anionic AOT. The optimum water content on the dipeptide synthesis was w0 = 28 due to the competition of the peptide synthesis and the side reactions. The maximum yield of Cbz-Phe-Leu was 0.565 at 80 h under optimum experimental conditions.
1. Introduction
Some kinds of proteins, poly and oligopeptides, and amino acids have demonstrated positive health influences and also provide numerous potential physiological functions. In contrast, L-α-dipeptides (dipeptides) have been poorly investigated [1]. One major reason is their low availability because of a lack of cost-effective production processes. However, knowledge regarding the functions of dipeptides is gradually expanding. Dipeptides syntheses are categorized into three methods: chemical, chemoenzymatic (using an enzyme and at least one protected amino acid), and enzymatic [1]. In an enzymatic synthesis, the expression of enzymes, which catalyze the formation of the peptide bond without protecting the amino acids, are found in gene-modified microbes. However, the yields are often low, and the purification procedures are time-consuming [2]. In chemical synthesis, including solid-phase peptide synthesis, its cost is relatively high and a harmful reagent is sometimes needed, although it has the following advantages: every kind of dipeptide can be synthesized, the yield is usually high, and the procedure is simple [2]. Furthermore, they require fully protected peptides, which are difficult to dissolve into an aqueous solution due to their hydrophobicity. Peptide bond hydrolytic enzymes can also catalyze the formation of peptide bonds. Many studies have investigated the chemoenzymatic method using hydrolytic enzymes [2]. Although this method has advantages, including high stereo-selectivity and mild reaction conditions, it also requires protected amino acids to form desired peptide bonds and suffers from a hydrophobicity problem [1].
Nakanishi et al. [3] reported the enzymatic synthesis of lipophilic N-benzoxycarbonyl (Cbz)-Phe-Phe-OMe from lipophilic-protected amino acids, Cbz-Phe and Phe-OMe, using thermolysin in a biphasic system. The yield in an ethyl acetate/water biphasic system exceeded that in water, although the production rate of the dipeptide in biphasic systems was lower than in water. Organic solvents promoted peptide synthesis with lipophilic substrates that cannot proceed in an aqueous medium because the equilibrium is mostly shifted toward the hydrolysis in an aqueous medium [4]. When one substrate is lipophilic and the other is hydrophilic, enzymatic reactions in an aqueous/organic two-phase system are conceivable.
A simple two-phase system contains bulk water, which is unfavorable for synthesis reactions. Reversed micelles have nanoscopic water droplets in bulk apolar solvents and have been used as nanoreactors in various applications [5,6]. They homogeneously solubilize the biomolecules in the organic phase and possess an enormous contact area (10–100 m2cm−3). Di- and tripeptide synthesis using enzymes solubilized in reversed micelles was reported by Lüthi and Luisi [7] and Shield et al. [8]. The former work [7] studied the synthesis of lipophilic N-benzoxycarbonyl (Cbz)-Ala-Phe-Leu-NH2 from lipophilic Cbz-Ala-Phe-OMe and hydrophilic Leu-NH2 using α-chymotrypsin in a bis(2-ethylhexyl) sodium sulfosuccinate (AOT)/isooctane reversed micelle. Shield et al. [8] also studied the lipophilic dipeptide synthesis of Cbz-Tyr-Gly-NH2 from lipophilic Cbz-Tyr-OMe and hydrophilic Gly-NH2 using α-chymotrypsin in a dodecyltrimethylammonium bromide/1-hexanol/octane reversed micelle. However, in a reversed micellar system, as argued by Lüthi and Luisi [7], there is no easy way to recover the expensive enzymes. Though this can be achieved partly using a hollow fiber reactor or an immobilized enzyme [7,9], a simple reaction-separation system is desired. Alternatively, non-enzymatic dipeptide synthesis using reversed micelles was reported. Ranganathan et al. [10] used a condensing agent, like N, N’-dicyclohexylcarbodiimide (DCC) and N, N’-dioctadecylcarbodiimide, to form a peptide of Cbz-AA1-AA2-OMe (AA1 = Trp, Phe, Leu, Pro, AA2 = Trp, Leu, Pro, Phe) from protected amino acids, Cbz-AA1 and AA2-OMe, in a reversed micelle composed of AOT and isooctane. After stirring for 2 days, the yields of the dipeptides were 25–67%, depending on the hydrophobicity of the amino acids. Dias et al. [11] reported a spontaneous dipeptide synthesis, AcPheIleNH2, from AcPheOEt and IleNH2 in tetradecyltrimethylammonium bromide/heptane/octanol reversed micelles. Unfortunately, it required 20 days to obtain a dipeptide yield higher than 87%. These non-enzymatic methods are attractive since they avoid the problem of enzyme recovery. However, both chemical syntheses using reversed micelles required long reaction times.
In this paper, we synthesized non-enzymatically lipophilic Cbz-Phe-Leu from lipophilic protected amino acid, Cbz-Phe, and hydrophilic free amino acid, Leu, or protected amino acid, Leu-NH2 using a reversed micellar system composed of DCC, AOT, and isooctane in a short time by replacing Leu-OMe with Leu and Leu-NH2 and its kinetic behaviors were investigated. We examined the effect of the operational conditions, the molar ratio, w0, of water solubilized within the reversed micelles to the amount of the surfactant present, the pH of the water core, and the DCC concentration in the dipeptide synthesis on the kinetic parameters and the maximum yields. Figure 1 shows the schematic representation of the Cbz-Phe-Leu synthesis within reversed micelles.
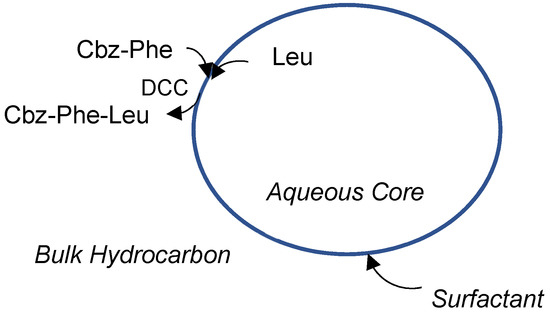
Figure 1.
Schematic representation of dipeptide synthesis using a reversed micelle.
2. Materials and Methods
2.1. Chemicals
Bis(2-ethylhexyl) sodium sulfosuccinate (AOT), isooctane and N, N’-dicyclohexylcarbodiimide (DCC), N-benzoxycarbonyl-L-phenylalanine (Cbz-Phe), L-leucine (Leu) and L-leucineamide (Leu-NH2) were purchased from Wako Pure Chemical Industries, Ltd., and N-benzoxycarbonyl-L-phenylalanyl-L-leucine (Cbz-Phe-Leu) was purchased from Sigma Chem. Co. These products were used as received. All the remaining reagents were of analytical grade and used without further purification.
2.2. Dipeptide Synthesis in Reversed Micelles
The synthesis of Cbz-Phe-Leu using DCC in a reversed micellar solution was carried out in a stirred tank thermostated at 298 K. The micellar solution was prepared by mixing 10 cm3 of aqueous leucine solution (1 mol/m3) and 150 cm3 of isooctane solution containing AOT (100 mol/m3) and Cbz-Phe (1 mol/m3). The pH of the aqueous solution was adjusted by a phosphate buffer. After 40 cm3 of isooctane solution containing DCC was added to the reactor and stirred at 2 s−1, the reaction started. Our preliminary experiment confirmed that the order of adding the substrate and DCC barely affected the reaction rate. Samples were withdrawn at proper time intervals. Concentrations are based on the whole reaction volume.
2.3. Analysis
Standard Cbz-Phe and Cbz-Phe-Leu solutions were used for preparing the calibration curves. The concentrations of Cbz-Phe and Cbz-Phe-Leu were determined by HPLC (Shimadzu LC-10ADvp) with an ODS column (Simpack CLC-ODS, Shimadzu, Kyoto, Japan) and a mixture of 2 g/L triethylamine solution (45%) of pH 2.3 that was adjusted by phosphoric acid and acetonitrile (55%). Protected peptides and amino acids were detected with a UV detector at 260 nm (Shimadzu SPD10AV).
3. Results and Discussion
Figure 2 shows the time courses of the Cbz-Phe, Cbz-Phe-Leu concentrations and the yield from the Cbz-Phe to the Cbz-Phe-Leu (w0 = 28, pH 5, initial concentrations Cbz-Phe and Leu = 1 mol/m3). Immediately after the reaction started, Cbz-Phe-Leu was observed. Conversely, when we used Leu-NH2 as a substrate, no new product peaks were found within such a quick reaction time. As described previously [10], it may take longer to form Cbz-Phe-Leu-NH2 because cationic Leu-NH2 in an aqueous core electrostatically interacts with the anionic surfactant AOT at the interface. Although the net charge of Leu (pI = 5.98) in a water core of pH 5 was partially positive, the electrostatic interaction of Leu may not be as strong as that of Leu-NH2. We successfully prepared dipeptide using a non-enzymatic reversed micellar system in a short time.
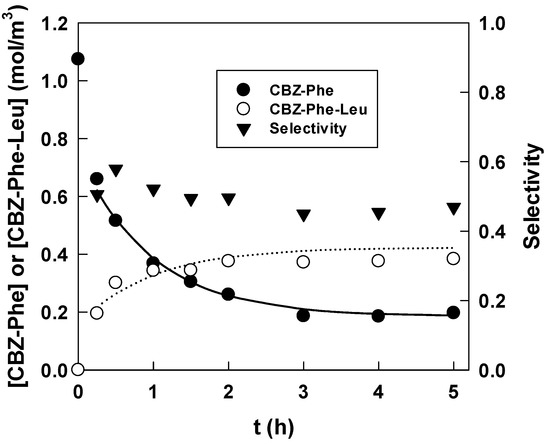
Figure 2.
Time courses of Cbz-Phe and Cbz-Phe-Leu concentrations, and selectivity (w0 = 28, pH 5, DCC = 7 mol/m3). Solid and dotted lines were calculated from the rate constants and selectivity.
Figure 2 also shows a decrease in the Cbz-Phe concentration and an increase in the Cbz-Phe-Leu concentration in time, and after 3 h, both concentrations reached a steady state, suggesting that the dipeptide formation was a reversible reaction. However, the decrease in the Cbz-Phe concentration did not correspond to the increase in the Cbz-Phe-Leu concentration. Selectivity S, which is defined by the ratio of the Cbz-Phe-Leu produced to the Cbz-Phe reacted, is also shown in Figure 2. During the experiment, the selectivity was almost constant, 0.46, suggesting that the consumption of Cbz-Phe by the side reactions occurred at a constant ratio. To confirm this, the reaction behavior without Leu in an aqueous core was investigated, and the concentration changes of Cbz-Phe in the presence and absence of Leu are shown in Figure 3. Despite the absence of Leu, the concentration of Cbz-Phe decreased with time. The difference in both lines approximately corresponds to the production of Cbz-Phe-Leu. Scheme 1 shows the reaction scheme of dipeptide synthesis using DCC in an anhydrous homogeneous medium [12]. Although it is expected that the main by-product will be N-acylurea, identification of by-products in a reversed micellar system is a subject for future analysis.
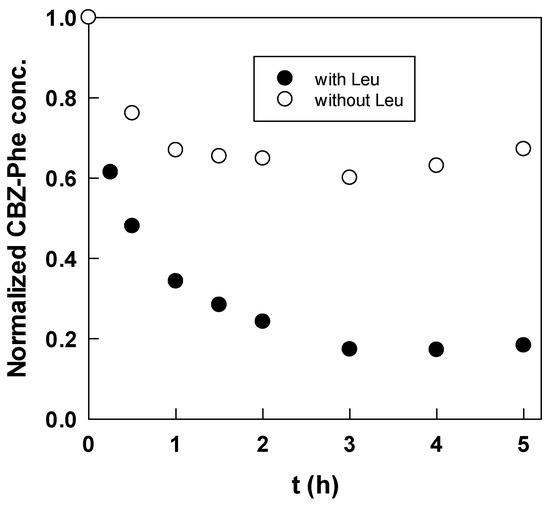
Figure 3.
Time courses of Cbz-Phe concentrations in presence and absence of Leu in aqueous core (w0 = 28, pH 5, DCC = 7 mol/m3).
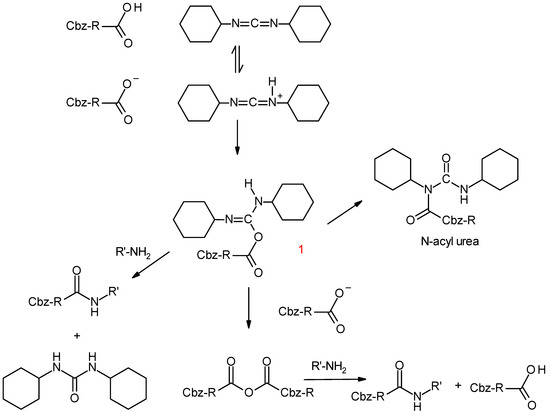
Scheme 1.
Reaction scheme of dipeptide synthesis using a condensing agent DCC.
This dipeptide formation is apparently expressed as Equation (1) based on the results shown in Figure 2:
Cbz-Phe + Leu ⇌ Cbz-Phe-Leu + H2O
We conducted an experiment with Cbz-Phe and Leu = 1 mol/m3, whose concentrations are based on the whole reaction volume. Because Leu dissolved in the water core and Cbz-Phe dissolved in an isooctane solution, the Leu concentration in the water core was enriched by 20 times. Therefore, an excessive amount of Leu exists at the interface between the water core and the bulk isooctane solution compared to Cbz-Phe. The reaction rate of Equation (1), r, was simplified to the following reversible first-order reaction:
where k and k’ are the apparent forward and reverse reaction rate constants and S is the ratio of Cbz-Phe-Leu produced to the Cbz-Phe reacted.
Equation (3) was derived by integrating Equation (2) in a batch reactor;
where subscripts 0 and e denote the initial and equilibrium states, respectively.
Figure 4 shows the relation with Equation (3), and the plot was almost linear, suggesting that the approximation to Equation (2) was valid.
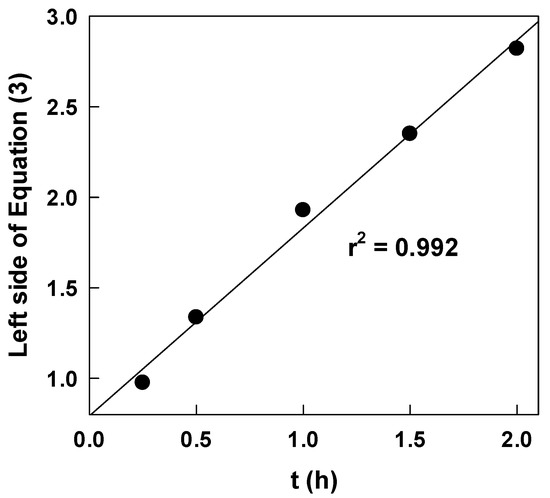
Figure 4.
Plots based on Equation (3) to determine rate constants.
At equilibrium state (r = 0), the following equation was derived.
where K is an apparent equilibrium constant of Equation (1).
From Equations (3) and (4), the rate constants were obtained, and the solid and dotted lines in Figure 2 were calculated using the obtained rate constants and selectivity.
3.1. Effect of DCC Concentration
DCC as a condensing agent in an anhydrous homogeneous medium reacts with the carboxylic acid to form O-acylurea (1). This intermediate yields a dipeptide via direct coupling with the amine and, formation of carboxylic acid anhydride, which subsequently yields the amide by reaction with the amine, as shown in Scheme 1. Table 1 lists the effects of DCC concentration. An excess in DCC compared with the substrate concentrations was needed. In addition to the formation of by-products such as N-acylurea, the following other factors are considered. Ranganathan et al. [10] stated that DCC played the role of co-surfactant, therefore, a part of DCC is located at the micellar interface and may be partly hydrated by water not from Cbz-Phe but from the aqueous bulk. The other point is that the formed Cbz-Phe anhydride is hydrolyzed at the micellar interface. The reactions of the Cbz-Phe anhydride with Leu or water are competitive at the interface. These two factors, in addition to the formation of by-products, may cause excess DCC.

Table 1.
Effect of DCC concentration on dipeptide synthesis (w0 = 28, pH = 5).
3.2. Effect of w0
Reversed micelles are characterized by parameter w0, which is the number of water molecules per surfactant molecule: w0 = H2O/AOT. An increase in w0 leads not to more reversed micelles but to a larger average diameter for them [13]. Table 2 lists the effect of w0 on the reaction rate constants, selectivity, equilibrium constants, and maximum yields. The value of w0 was varied by changing the water concentration while maintaining an AOT concentration constant at a 100 mol/m3. Yield at w0 = 28 was maximum and, at the lowest w0, the peptide was not synthesized at all. For a reversed micelle of low w0, because most water molecules are involved in the hydration of the head groups of surfactants, the whole aqueous microenvironment is rigid and the micro-viscosity of the micellar water was higher than in the bulk solutions. Therefore, the reactivity in the lowest w0 may be poor. Thermodynamic equilibrium constants, K, and the conversion rate constant of Cbz-Phe, k, increased with the w0 values. However, an increase in k values does not increase the yield, suggesting that an increase in w0 caused an acceleration in the side reactions involving Cbz-Phe. For the results of the competition of peptide synthesis and the side reactions, the yield was maximum at w0 = 28.

Table 2.
Effect of w0 on dipeptide synthesis (pH = 5, DCC = 7 mol/m3).
3.3. Effect of pH
Because the hydrophilic substrate, Leu, is amphoteric, aqueous pH is expected to affect the charge state of Leu, that is, its reactivity. Table 3 lists the effects of pH. The maximum yield was obtained around pH 5. Here the pH values are those of the aqueous buffers with which the micellar solution was prepared. Anionic surfactant, AOT, was reported to be somewhat lower than the pH of the water within reversed micelles [14]. At the optimum pH, we expected that Leu, with a pI of 5.98 and pKa1 = 2.36, could exist as a cationic form to some extent at the micellar interface. As described previously, no fully cationic Leu-NH2 was reacted at all with Cbz-Phe because the cationic Leu-NH2 was bound to the anionic AOT by a strong electrostatic interaction. On the contrary, a partially cationic Leu was enriched in the vicinity of the micellar interface with the aid of the electrostatic interaction. Therefore, for the results of the competition of the enrichment effect and the electrostatic interaction, the yield was maximum around pH 5.

Table 3.
Effect of pH on dipeptide synthesis (w0 = 28, DCC= 7 mol/m3).
Although dipeptide Cbz-Phe-Leu was observed in a short time (~5 h), the reaction was carried out for 80 h. A yield of 0.565 was obtained under the optimum condition (w0 = 28, pH 5, DCC = 7 mol/m3).
Author Contributions
Conceptualization, M.M. and T.H.; methodology, M.M.; formal analysis, M.M.; investigation, M.M.; data curation, M.M..; writing—original draft preparation, M.M.; writing—review and editing, T.H.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Fumio Katagiri for his experimental assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yagasaki, M.; Hashimoto, S. Synthesis and application of dipeptides; current status and perspectives. Appl. Microbiol. Biotechnol. 2008, 81, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, K.; Numata, K. Recent advances in chemoenzymatic peptide synthesis. Molecules 2014, 19, 13755–13774. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Matsuno, R. Kinetics of enzymatic synthesis of peptides in aqueous/organic biphasic systems: Thermolysin-catalyzed synthesis of N-(benzyloxycarbonyl)-L-phenylalanyl-L-phenylalanine methyl ester. Eur. J. Biochem. 1986, 161, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, T.; Watanabe, A.; Kise, H. Peptide synthesis by proteases in organic solvents: Medium effect on substrate specificity. Enzym. Microb. Technol. 1992, 14, 842–847. [Google Scholar] [CrossRef]
- Tonova, K.; Lazarova, Z. Reversed micelle solvents as tools of enzyme purification and enzyme-catalyzed conversion. Biotechnol. Adv. 2008, 26, 516–532. [Google Scholar] [CrossRef]
- Mohd-Setapar, S.H.; Mohamad-Aziz, S.N.; Chuong, C.S.; Yunus, M.A.C.; Zaini, M.A.A.; Kamaruddin, M.J. A review of mixed reversed micelle system for antibiotic recovery. Chem. Eng. Commun. 2014, 201, 1664–1685. [Google Scholar] [CrossRef]
- Lüthi, P.; Luisi, P.L. Enzymatic synthesis of hydrocarbon-soluble peptides with reversed micelles. J. Am. Chem. Soc. 1984, 106, 7285–7286. [Google Scholar] [CrossRef]
- Shield, J.W.; Ferguson, H.D.; Cleason, K.K.; Hatton, T.A. Enzymatic reactions in reversed micelles at low solubilized water concentrations. ACS Symp. Ser. 1989, 392, 90–103. [Google Scholar]
- Fadnavis, N.W.; Luisi, P.L. Immobilized enzyme in reversed micelles: Studies with gel-trapped trypsin and α-chymotrypsin in AOT reversed micelles. Biotechnol. Bioeng. 1989, 33, 1277–1282. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, D.; Singh, G.P.; Ranganathan, S. Peptide bond formation at the micellar interface. J. Am. Chem. Soc. 1989, 111, 1144–1145. [Google Scholar] [CrossRef]
- Dias, A.I.; Feliciano, A.S.; Cabral, J.M.S.; Prazeres, D.M.F. Chemical synthesis and crystallization of the dipeptide AcPheIleNH2 in TTAB/heptane/octanol reversed micelles. J. Colloid Interface Sci. 2007, 305, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Valeur, E.; Bradley, M. Amide bond formation: Beyond the myth of coupling reagents. Chem. Soc. Rev. 2009, 38, 606–631. [Google Scholar] [CrossRef] [PubMed]
- Eskici, G.; Axelsen, P.H. The size of AOT reverse micelle. J. Phys. Chem. B 2016, 120, 11337–11347. [Google Scholar] [CrossRef] [PubMed]
- Shield, J.W.; Ferguson, H.D.; Bommarius, A.S.; Hatton, T.A. Enzymes in reversed micelles as catalysts for organic phase synthesis reaction. Ind. Eng. Chem. Fundam. 1986, 25, 603–612. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).