Abstract
Since the 1950s, microalgae have been grown commercially in man-made cultivation units and used for biomass production as a source of food and feed supplements, pharmaceuticals, cosmetics and lately biofuels, as well as a means for wastewater treatment and mitigation of atmospheric CO2 build-up. In this work, photosynthesis and growth affecting variables—light intensity, pH, CO2/O2 exchange, nutrient supply, culture turbulence, light/dark cell cycling, biomass density and culture depth (light path)—are reviewed as concerns in microalgae mass cultures. Various photosynthesis monitoring techniques were employed to study photosynthetic performance to optimize the growth of microalgae strains in outdoor cultivation units. The most operative and reliable techniques appeared to be fast-response ones based on chlorophyll fluorescence and oxygen production monitoring, which provide analogous results.
1. Microalgae
Phytoplankton/microalgae are major global contributors to primary productivity, assimilating about half of all carbon dioxide converted annually into organic matter [1]. Microalgae (in applied phycology this term refers to prokaryotic cyanobacteria and eukaryotic algae with a cell size of about 2–50 μm) belong to some of the fastest-growing photosynthetic microorganisms. For about 70 years they have been grown commercially in man-made cultivation units and used as food and feed supplements, pharmaceuticals, cosmetics and lately biofuels, as well as for wastewater treatment and mitigation of atmospheric CO2 build-up [2,3]. These microorganisms produce a spectrum of bioproducts—polysaccharides, proteins, lipids, pigments, antioxidants, vitamins, bioactive compounds and many others [4]. Industrial cultivation of microalgae has developed considerably since the 1990s [2,5,6,7,8,9,10,11]. Current commercial culturing units range from tens of thousands litres in photobioreactors (PBRs) to billions of liters in open ponds [12,13].
Microalgae have several advantages compared to macroalgae and higher plants [14]:
- Possibility of mass cultivation in various environments when necessary variables for their growth are provided;
- A small quantity of microalgae is enough for production scale-up;
- Microalgae cell doubling time is short (when compared to other photosynthesizing organisms);
- Fast growth and minimal nutrient requirements;
- Can grow in wastewater;
- Can produce secondary metabolites under various stress conditions;
- Can be modified for enhanced lipid and carbohydrate production;
- Easy to extract biological compounds from microalgae;
- One-time investment for long-time production of microalgae.
Numerous systems and technologies have been designed and used for phototrophic cultivation of microalgae mass cultures, using either natural or artificial light [3,12,13]. The use of an appropriate culturing system and the set-up of the growth regime must be worked out individually for each strain. In any cultivation system, several basic variables have to be considered: illumination, mixing (circulation) and gas exchange (O2 stripping/supply of CO2) (Figure 1). In microalgae biotechnology two basic systems are used in microalgae mass production: one being open reservoirs (with direct contact of the culture with the environment), which usually covers a large area, while the other represents closed transparent vessels, PBRs, with natural or artificial illumination where the culture has no direct contact with the atmosphere. Generally, biomass production from open-system cultures is cheaper when compared to PBRs, but open ponds are used for a limited number of robust microalgae strains. The production in open ponds with a high culture depth (10–30 cm) results in a low volumetric density and productivity [15]. On the other hand, high biomass concentrations (>20 g DW L−1) can be achieved in thin-layer systems [16,17,18], being among the highest attainable in large-scale microalgae production systems. From a commercial point of view, the value of the final product is often crucial. It is important to analyze the variables affecting culturing of a selected strain and consider possible pros and cons for a particular cultivation system.
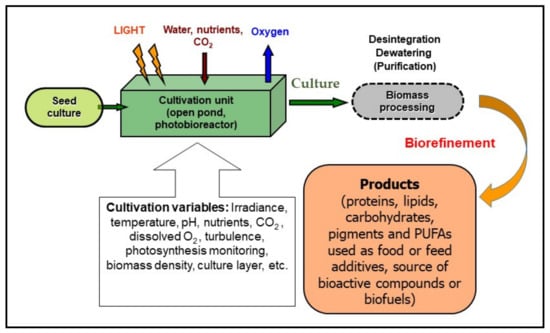
Figure 1.
A schematic diagram of microalgae cultivation (and biomass processing) showing variables affecting the growth of microalgae. They are grown in a cultivation unit using an aqueous mineral medium and are exposed to light. However, a minimum biomass concentration corresponding to about 10 g per square meter (~0.2 g Chl m−2) is recommended to avoid photo-stress. The operation variables—irradiance, pH, CO2 supply, dissolved O2 concentration, turbulence, photosynthetic activity, biomass density—have to be monitored and maintained to optimize the growth of the culture. During processing, the biomass is separated from the medium, disintegrated and dried for use as, e.g., food or feed additive, or as a source of bioactive compounds in pharmacology and cosmetics, or most recently for the production of biofuels, etc. (modified from Masojídek and Torzillo [3]).
2. The Process of Photosynthesis
Oxygenic photosynthesis is an exclusive process of light used as a source of energy for production of organic matter by photoautotrophs. The process emerged in cyanobacteria at least 2.5 billion years ago. Carbohydrate production is based on the simple equation of oxygenic photosynthesis, which shows all the necessary requirements of this biological process:
CO2 + 2 H2O + 8 − 10 photons = [CH2O] + H2O + O2 + waste heat
Virtually all life on Earth depends directly or indirectly on this process as a source of organic matter and energy for its metabolism and growth. Oxygenic photosynthesis (with two photosystems functioning in series) is expressed as a redox reaction driven by light energy (collected by Chl), in which CO2 and water are converted to carbohydrates and oxygen molecules are released (Equation (1)). This process can be divided into two phases, the so-called light reactions and dark reactions (Figure 2) [19]. In the light reactions, which are carried out on photosynthetic membranes, the light energy is converted to chemical energy providing a biochemical reductant NADPH2 and a high-energy compound ATP. In the dark phase, which takes place in the stroma, NADPH2 and ATP are utilised in the biochemical reduction of CO2 to carbohydrates.
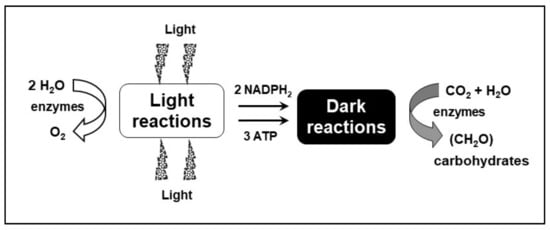
Figure 2.
Major products of the light and dark reactions of photosynthesis. The process of oxygenic photosynthesis is divided into two stages, the so-called light reactions and dark reactions. The light reactions include photobiochemical processes—light absorption, water splitting, transfer of excitons through two photosystems in series (PSII and I), and electron and proton translocation resulting in the production of NADPH2, ATP, and O2. The other phase, the dark reactions that occur in the stroma, represents the reduction of carbon dioxide and the synthesis of carbohydrates using the NADPH2 and ATP produced in the light reactions (modified from Masojídek et al. [21]).
2.1. The Light Reactions
The main role of the light reactions is to supply NADPH2 and ATP for the assimilation of inorganic carbon. The light energy is trapped via two photo-reactions carried out by two pigment–protein systems, Photosystem I (PS I) and II (PS II). The photosystems work in series being connected by a chain of electron carriers that is usually pictured in a so-called ‘Z’ scheme [20].
Upon illumination, two electrons are extracted from water (oxidation; O2 is evolved) and transferred through a chain of electron carriers to produce one molecule of NADPH2. Simultaneously, protons are transported from an external space (stroma) into the intra-thylakoid space (lumen) forming a pH gradient. According to Mitchel’s chemiosmotic hypothesis, the gradient drives ATP synthesis, which is catalysed by the protein complex called ATPase or ATP synthase [22]. Theoretically, the evolution of one oxygen molecule requires the extraction of four electrons from two water molecules. Since two photons are needed to transfer one electron through the photosynthetic electron-transport chain, the minimum quantum requirement is 8 photons per oxygen. According to Equation (1), the evolution of one molecule of oxygen is equivalent to the assimilation of one molecule of CO2 (and the minimum number of photons is eight). However, a ratio of photon/carbon of about 9 or 10 was calculated under low light conditions [23]. The quantum requirement increases according to the macromolecular composition of the produced biomass. The metabolic costs of protein and lipid biosynthesis increase in comparison to carbohydrate. Wilhelm and Torsten [24] reported that in the case of biomass rich in protein or lipids the real quantum requirement can rise up to 28–29.
2.2. The Dark Reactions
The reduction of carbon dioxide happens in the dark reactions involving the NADPH2 and ATP produced in the light phase of photosynthesis. The reaction can be expressed as:
2 NADPH2 + 3 ATP
enzymes
CO2 + 4 H+ + 4 e− -------------------------→ (CH2O) + H2O
enzymes
CO2 + 4 H+ + 4 e− -------------------------→ (CH2O) + H2O
One molecule of CO2 is fixed using two molecules of NADPH2 and three molecules of ATP (i.e., an energy of 52 kJ, about 12.5 kcal). Concerning the quantum efficiency of CO2 fixation, a minimum of eight quanta of absorbed light is required for each CO2 molecule of fixed or O2 evolved [1,21]. The quantum requirement changes with the composition of the biomass [24].
The carbon fixation reactions were worked out by Calvin and Benson in the 1940s and early 1950s (Nobel Prize in 1961). The conversion of CO2 to carbohydrates (or other compounds) occurs via four distinct reaction steps (Figure 3) in the so-called Calvin–Benson cycle (photosynthetic carbon reduction cycle: (i) Carboxylation—a step in which CO2 is combined with the 5-carbon ribulose bisphosphate (Ribulose-bis-P) in order to form two molecules of phosphoglycerate (Glycerate-P). This reaction is catalysed by the enzyme ribulose bisphosphate carboxylase/oxygenase (Rubisco); (ii) In the Reduction step, Glycerate-P is converted to 3-carbon products (Triose-P) in two partial reactions—the phosphorylation of Glycerate-P by ATP (energetic compound) to form diphosphoglycerate (Glycerate-bis-P) followed by the reduction of Glycerate-bis-P to Phosphoglyceraldehyde (Glyceraldehyde-P) by NADPH2; (iii) Regeneration step—ribulose phosphate (Ribulose-P) is regenerated for another CO2 fixation in a series of reactions combining 3-, 4-, 5-, 6- and 7-carbon phosphorylated sugars (not explicitly shown in the diagram); and (iv) the Production phase, where primary end-products of photosynthesis, carbohydrates, are synthesized.
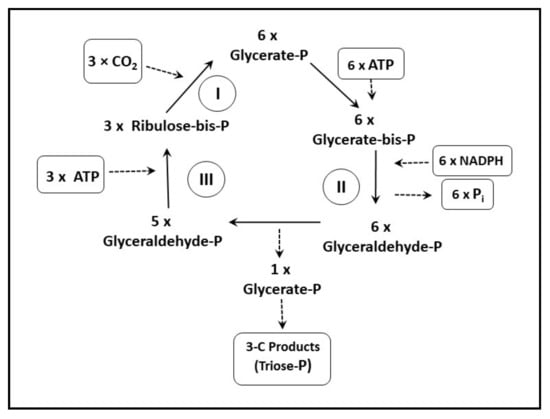
Figure 3.
A scheme of the carbon fixation reactions in photosynthesis—the Calvin–Benson cycle. The fixation of CO2 to the level of carbohydrates occurs in four phases: (i) Carboxylation, (ii) Reduction, (iii) Regeneration (iv) and Production (figure adapted from [25,26]). A more detailed description of individual steps is in the text.
Rubisco plays the main role in the photosynthetic carbon reduction cycle as the enzyme which catalyzes carboxylation of ribulose 1,5-bisphosphate to two molecules of phosphoglycerate (step I). Thus, Rubisco is the key enzyme involved in primary production on Earth [1].
Ribulose-bis-P + CO2 → 6-C intermediate → 2 × phosphoglycerate
All microalgae (including cyanobacteria) rely on the enzyme Rubisco acting in photosynthetic carbon reduction, the so-called Calvin–Benson cycle for assimilation of inorganic carbon to organic matter.
Photorespiration is a competing process to carboxylation in which the organic carbon is converted into CO2 without any metabolic gain. In this process, Rubisco functions as an oxygenase, catalyzing the reaction of ribulose 1,5-bisphosphate with O2 to form phosphoglycolate. Then, glycolate is dephosphorylated and converted, in several steps, to serine, ammonia and CO2 [26].
Ribulose-bis-P + O2 → 5-C intermediate → Phosphoglycerate + Phosphoglycolate
Photorespiration depends on the relative concentrations of O2 and CO2; whereas a high O2/CO2 ratio (i.e., high concentration of O2 and low concentration of CO2) stimulates photorespiration, a low O2/CO2 ratio favors carboxylation. Rubisco shows low affinity to CO2 as its Km (half-saturation) is roughly equal to the level of CO2 in air. Thus, under high irradiance, high O2 levels and reduced CO2 in microalgae culture, the reaction equilibrium is shifted towards photorespiration. Photosynthetic organisms differ significantly in their rates of photorespiration: in some species it may be as high as 50% of net photosynthesis [26].
To achieve optimal yields in microalgae mass cultures, it is necessary to minimize the effects of photorespiration. This can be managed by effective O2 stripping and by CO2 enrichment. Therefore, to reduce photorespiration in microalgae mass cultures, it is desirable to maintain a low ratio O2/CO2 in the PBR.
A knowledge of photosynthesis is essential for microalgae biotechnology as the biological limitations—light absorption, photochemistry, carboxylation and metabolic conversion of primary products into the macromolecules—has be considered [24].
Some oxygen-consuming processes such as photorespiration and chlororespiration are closely associated with photosynthesis [1]. This is also valid for the Mehler reaction in microalgae in which oxygen is reduced to H2O2 [26,27,28]. It involves light-dependent PSI driven O2 uptake in chloroplasts when the rate of CO2 fixation is limited and the NADPH2/NADP ratio is high. In PSI, a significant part of the reductant is oxidized to form H2O2. In the presence of catalase, it undergoes rapid dismutation to H2O and O2. Under active photosynthesis, O2 production exceeds its consumption.
The processes of respiration—often termed “dark respiration”—are not directly associated with photosynthesis or the photosynthetic apparatus, but are denoted as “mitochondrial respiration”, although they also function in the light and their rates are indirectly influenced by irradiance. Broadly speaking, “dark” respiratory rates can be 10–15% of gross photosynthetic rates, though variation between species might be quite large [1]. The respiration rate is important as it significantly reduces the net biomass production. Higher temperatures experienced by microalgae cultures at night usually increase respiratory rates, which result in a greater loss of biomass [27,29]. The rate of respiration at night is also modulated by irradiance levels experienced during the day as high daytime irradiances increase respiration in the microalgae cultures at night [30]. The biomass loss at night has been predominantly ascribed to carbohydrate consumption. High irradiances can also increase dark respiration during the day [31].
3. Variables Affecting Photosynthesis and Growth of Microalgae Cultures
In nature, there exist a number of temporal photosynthetic responses that occur in phytoplankton cells. ‘Short time scale’ responses are considered to be the physiological and biochemical responses within the life span of an individual organism, which are generally called acclimations. These responses are usually related to temporal variations in irradiance, temperature, and nutrient availability [32]. In contrast, ‘long time scale’ responses are referred ecological and evolutionary adaptations of photosynthetic organisms through selection of phenotypic traits.
Cultured microalgae are exposed to a variety of changes in environmental conditions, but they are qualitatively very different from phytoplankton populations. Outdoor mass cultures are grown in constructed units and controlled conditions. Biotechnological production, generally, requires well-defined conditions, but the maintenance of large-scale cultures under outdoor conditions of variable irradiance, temperature, rainfall and other influences presents challenges which are not experienced in the controlled conditions of small-scale laboratory cultures [14,33]. These changes take place in two different timescales. One is the diurnal and seasonal cycle, which causes mostly variation in irradiance intensity and temperature in the day/night cycle. It also varies according to the climatic conditions and geographical location in which the microalgae cultures are grown. In dense microalgae cultures used in microalgae biotechnology, the other, much faster cell cycling is imposed by culture turbulence in the culture layer, which mainly results in an intermittent light–dark regime alternating in seconds or milliseconds as compared to the hours or months for diurnal and seasonal cycles [34].
The necessary cultivation requirements for the growth of microalgae mass cultures are shown in Figure 1. Almost all commercial-scale microalgae production takes place outdoors, exposing the microalgae to diurnal changes in environmental conditions, explicitly irradiation and temperature. Since microalgae mass cultures grow in dense suspensions, sufficient mixing is essential in order to expose cells to light equally [35,36]. The operation regime—suitable biomass density, culture layer (optical path), cell movement patterns (averaged light/dark cycles for cells) and gas exchange (namely CO2 supply and oxygen stripping as well as cultivation unit design and spatial setting with respect to exposure to the sun)—has to be developed to maximise/photo-optimise the use of high photon flux densities reaching the surface of cultivation systems. In practical terms, this should form part of the considerations when designing cultivation systems.
Detailed reviews of the biology, physiology and management of large-scale outdoor cultures can be found elsewhere (for review see [2,11,14,34,37]).
3.1. Light
The quantity and quality of light available are the main factors limiting the productivity of microalgae mass cultures outdoors [6,35,38]. However, the impact of irradiance on productivity is rather complex due to the interaction with other environmental variables. The average amount of photon energy received by each cell is a combination of several factors: photon flux density, cell density, culture layer, light spectrum and the rate of mixing as well as type of cultivation unit and orientation to the light and the rate of mixing [10,17,18,35,39,40]. The suitable cell irradiance for photosynthesis can be estimated from the photosynthesis vs. irradiance (P-I) curve (Figure 4).
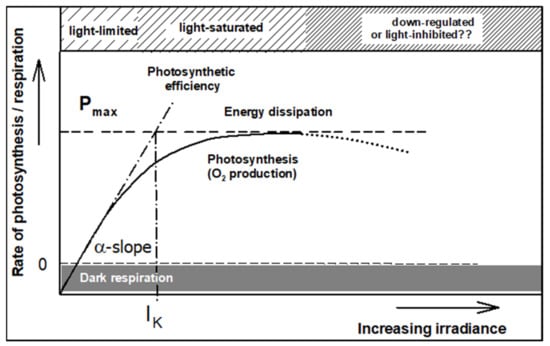
Figure 4.
An illustration of a photosynthetic light-response curve, i.e., the dependency of the rate of photosynthesis on irradiance. The α-slope shows the maximum efficiency of photosynthesis (PE). The intersect between the α-slope and maximum rate of photosynthesis Pmax indicates the light saturation (IK); surplus light energy over saturation is dissipated. At supra-optimum irradiance, the photosynthetic rate declines (dotted part of the light-response curve); this phenomenon is usually called down-regulation or photoinhibition (modified from Masojídek et al. [67]).
In any cultivation unit we have to find an optimum biomass density (g DW L−1) or optimum areal density (g DW m−2) in which productivity reaches a maximum value. If the cell density is low, the productivity is decreased as the cells are photo-stressed; on the contrary, the productivity starts to decline in denser culture because of increased cell self-shading as the light availability for individual cells is reduced. Obviously, the greater the culture depth, the lower the optimal biomass concentration. Empirically, the optimal areal density of about 60 g m−2 of dry weight was found, corresponding to a biomass concentration of about 0.6 g L−1 for a pond (10 cm culture depth), while it is about 6 g L−1 for a thin-layer cascade of 1 cm layer thickness [2,17]. For tubular PBRs, the biomass concentration depends on their diameter and usually ranges between the values indicated for ponds and cascades.
Another important experience is that microalgae photosynthetic apparatus can utilize strong light efficiently only when it is delivered intermittently in ‘pulses’. Early reports in the 1930s showed that intermittent (pulsed) light is an important condition for microalgae growth [41]. Later, the so called ‘flashing light’ effect on photosynthesis was described in Chlorella in by Kok [42] as the enhancement of photosynthesis was observed when microalgae cells were exposed to flashing lights of various frequencies and dark/light duration ratios. A crucial point is that the fast turbulence of microalgae culture induces rapid light/dark (L/D) cycling of cells [35,43]. Later, the influence of intermittent light on microalgae cultures was investigated by several research groups [8,44,45,46,47]. In the 1990s, the introduction of high-intensity LEDs as a light source for microalgae research allowed more precise measurements of the intermittent light effect in the millisecond range [48,49].
3.1.1. Photosynthetically Active Radiation
Microalgae (as higher plants) can use only the photosynthetically active radiation (PAR; 400–700 nm) for photosynthesis, with the red (600–680 nm) and blue (420–470 nm) range being the most effective. The exact spectrum requirements of microalgae cells depend on the particular pigment composition of their light-harvesting antennae [50]. Therefore, it may be possible to use only part of the available daylight solar spectrum for microalgae growth; the rest of the available spectrum might be used to generate electricity using solar panels placed above the culture [51,52,53]. If artificial lighting is used, then it becomes a major energy consumer. As LEDs have a higher efficiency, they have become frequent light sources for indoor PBRs rather than fluorescent lamps, thus reducing the power requirements for artificial light sources [54,55]. For both natural and artificial light sources, the spectral requirements of the microalgae, and not just the total light energy, is important.
3.1.2. Changing Antenna Size
One possible way to increase the productivity of microalgae cultures is to reduce the size of the light-harvesting antennae and thus lessen the photosynthetic inefficiency associated with the dissipation of excess light absorbed by surface layers of microalgae cultures [54,55]. Strains with a reduced antenna size absorb less light per cell and thus they should be able to tolerate higher irradiances and, moreover, light can penetrate to deeper layers of cells. This approach represents the synthetic variation of the natural photo-adaptive responses of high plant and microalgae in which pigments are re-organized in response to changing light conditions. This was suggested by Benemann [56,57,58,59], showing that reduced pigment content resulted in dense laboratory cultures with higher photosynthetic activity. Cells with a reduced pigment content, mutants with a smaller antenna size or transgenic microalgae with reduced antenna confirmed that this concept works at the laboratory scale size [60,61,62,63,64,65,66]. However, results in outdoor cultivation units are still ambiguous. A Chlorella mutant with truncated antennae was prepared by UV mutagenesis, which showed a 30% higher biomass productivity compared to the wild-type when grown in small-scale outdoor PBRs [60]. On the other hand, the performance of four antenna mutants of Chlamydomonas reinhardtii was compared with a wild type (WT) strain in an LED-illuminated PBR under continuous culture [61]. The highest productivities found in the mutated strains were similar to that of the wild-type. Moreover, the mutants exhibited a high sensitivity to abrupt shifts in irradiance, indicating a higher sensitivity to photodamage. Microalgae strains with a reduced antenna size may suffer lesser photoinhibition at high light intensity, particularly when cell density is low, a situation that frequently occurs at the start of the cultures outdoors. However, as the culture become denser, and thus the available light is reduced, the productivity of the reduced antenna size strain is overhauled by WTs [62]. Similar results were also obtained outdoor in thin-layer cascades comparing two Chlorella strains with different antenna sizes [63]. Thus, a strain with the reduced antenna size is not sufficient to guarantee higher biomass productivity in mass outdoor culture.
Such mutants may also result in impaired photoprotection induced by antenna alterations and/or other undesirable side effects of genetic modifications such as increased respiration that reduces the benefit of the reduced light absorption in the truncated antenna cells [60]. In the protection of the microalgae strain with truncated antenna, again reactive oxygen species (ROS) may not be as effective due to a lesser ability to dissipate excess excitation energy via a non-photochemical quenching (NPQ) process [25].
Therefore, in these studies, an important variable that needs to be considered, other than the reduced PSII antenna size, is the ratio of Pmax/Respiration, which should not be significantly affected [14]. Interestingly, a strain of Chlorella vulgaris g120 characterized by low Chl content per cell and a small antenna [64,65] showed a high respiration rate performing much better than the strain Chlamydomonas reinhardtii commonly used for photobiological hydrogen production [66].
3.1.3. Photochemical Efficiency
The P-I curve describes the relationship between irradiance intensity and the rate of photosynthesis (Figure 4). This curve is used in microalgae physiology to characterize the light-acclimation status of microalgae cells [34]. The light-saturation point IK reflects the actual light-acclimation status of the cells. The maximum photochemical efficiency (PE) is estimated on the basis of the α-slope of the P-I curve. The maximum α value is reached in the light-limited region, while the maximum rate of photochemistry, Pmax is achieved in microalgae cultures in which averaged cell irradiance is around the saturation IK (where energy losses are still low) (Figure 4; e.g., [34,67]). The α-slope, IK as well as Pmax, can change over several hours due to photoacclimation and/or unfavorable temperature and CO2 limitation. Thus, the photosynthetic rate cannot be directly used as a basis to relate light intensity to growth rate as it also includes the metabolic costs.
The term “maximum photochemical efficiency” (PE; Figure 4) has been widely discussed in the literature [68], expressing the amount of energy stored in the biomass with respect to that absorbed; in photosynthesis research, PE is understood to be the number of absorbed photons necessary to produce one oxygen molecule (the portion of light energy converted into chemical energy). Since the ratio of the incoming to absorbed quanta depends on many variables (Chl content, absorption cross-section, light spectrum, etc.), the α-slope cannot be used as a direct measure of PE and is suitable only for comparative reasons [24,69,70].
Starting from the curve plateau, when the power of absorbed light energy exceeds the capacity of the dark reaction, the energy is dissipated by photoprotective mechanisms generally as heat (Figure 4). The non-photochemical dissipation is principally antiparallel to photochemistry. Any condition that slows down the dark reactions will cause the light-generated reductants to not be used. In mass microalgae cultures it happens, for example under low temperatures or nutrient deficiency, especially under limited CO2 supply; it causes down-regulation or photoinhibition of photosynthesis. The physiological consequences of photoinhibition are a reduction in the efficiency of photochemistry and the carbon assimilation capacity (Pmax). The former is due to the damage to PSII reaction centers, which can be repaired only over several hours [71,72]. The irradiance intensity needed to overexcite the photosynthetic apparatus is different from species to species and also depends on the light acclimation status of the culture [73].
The ambient irradiance (maxima of about 2000 µmol photons m−2 s−1) available for photosynthetic antennae represents a much higher intensity than that required to saturate photosynthesis. The measurement of light intensity close to the surface in the photic layer in healthy, growing microalgae cultures showed a mean light intensity of about 200–400 µmol photons m−2 s−1, which is the usual optimum of saturating irradiance for most strains and it guarantees a high productivity [17].
From a biotechnological point of view, the irradiance saturating photosynthesis (IK in Figure 4) required in commercial microalgae PBRs is only about 10 to 20% of the maximum photosynthetically active irradiance (PAR) [46,67,74]. In other words, the light regime for an optimal growth should be adjusted to this irradiance level, otherwise as much as 90% of the photons captured by the cells is uselessly emitted as heat. The prerequisites for efficient use of high irradiance intensities in microalgae cultures have been clarified: the most important are a short light-path combined with a highly turbulent flow at high cell densities (i.e., >5 g DW L−1) [10,39,75].
Photochemical efficiency (photon to carbohydrate) in Equation (1) [2] gives only the energy demand to produce simple 3-carbon products (carbohydrates) from CO2 and water. However, cellular growth requires biomass containing not only sugars but also lipids and proteins, macromolecules that are more reduced than sugars. Thus, any increase in the protein or lipid content in a cell is inevitably linked with higher photon/carbon (P/C) values [24]. The minimum P/C values can be achieved only under low light; the photon-use efficiency drops down under saturating light intensities (Figure 4). Not all electrons from water-splitting are used for carboxylation. Alternative electron cycling is important for nitrogen and sulphur assimilation. Like in higher plants, alternative electron cycling around PSI is an important regulatory mechanism to prevent the cells from being photo-damaged by reactive oxygen species (Mehler reaction) [26].
The theoretical maximum of PE is about 10%, a value that could never be achieved in a microalgae culture as cultivation variables (such as cell irradiance, temperature, predation, technical failure, nutrition, etc.) come into play. In outdoor microalgae mass cultures, photosaturation has been believed to be the main factor suppressing the efficiency; photoinhibition, respiration and reflection are also relevant. Thus, considering the partial reactions, a more realistic figure of photosynthetic efficiency (photon energy converted into biomass energy) of about 4.5% for microalgae and C3 plants can be found (e.g., [67,76,77]).
3.2. Temperature
Besides light, temperature is the most critical variable to rule the microalgae culture. Due to seasonal and diurnal fluctuations, temperature represents one of the important biological limitations for outdoor mass production of microalgae. The temperature plays the significant role played in productivity as well as costs related to the PBR cooling. It is a weighty variable for strain selection in outdoor cultivation [14]. Harvesting at the suitable moment of the day can significantly contribute to the biomass value. Nonetheless, in order to optimize production, we have to consider the balance between the productivity of biomass and the content of a valuable compound.
Certain microalgae strains tolerate a wide range of temperatures between 15 and 40 °C (e.g., Chlorella), while Eustigmatophyceae (e.g., Nannochloropsis) usually require a much narrower range (20 °C to 28 °C). Some psychrophilic strains (e.g., Monoraphidium) grow well in mass cultures, even at lower temperatures between 5 and 15 °C. Nevertheless, the majority of freshwater microalgae have an optimum temperature range between 25 and 35 °C [3,78]. When they grow at an optimum temperature, the available light is used more effectively by microalgae and they are less prone to photoinhibition [14,79].
A narrow gap between optimal and lethal temperature can indicate that a strain is sensitive to heat stress, while a wide range may indicate that another strain is able to acclimate to high temperatures. Temperature tolerance of microalgae is large below the optimal value but usually smaller above the optimum temperature [80]. Long-term adaptation strategies to temperature increases have shown that some species are able to adapt to supra-optimal temperature.
Whereas photochemical reactions proceed at rates more or less independent of temperature, the rates of enzyme-mediated dark reactions decrease at lower temperatures [81]. Thus, the exposure of microalgae to sub-optimal temperatures may result in absorbing more light energy than can be utilized by the photosynthetic apparatus through carbon fixation and some mechanisms must be mobilized to maintain the energy balance. It was shown by Chl fluorescence measurements that low temperature conditions cause increased reduction of the PSII complex and the redox state of the quinone acceptors may act as a signal to trigger acclimation processes [82]. Possible mechanisms may include: (a) alterations in light harvesting and primary photochemistry to reduce light energy input; (b) increased rates of cyclic or pseudocyclic electron transport to spend energy (Mehler reaction); (c) higher rates of photorespiration, or (d) increased activity of the Calvin–Benson cycle. These processes are described in more detail later in this article.
Any temperature extremes strongly affect carbon fixation in the Calvin–Benson cycle as it mostly involves biochemical (enzymatic) reactions [14]. The night-time temperature is essential as it affects the respiration rate of the microalgae. A temperature decrease during the night (within the physiological range) slows down biomass loss due to lower respiration [17,27]. A decrease to sub-optimal temperatures can be used to modify lipid composition towards unsaturated fatty acids. For example, changes in biomass composition have been documented in oleaginous species such as Nannochloropsis [83,84]. Increased content of eicosapentaenoid acid (EPA) was found in the microalgae cultures grown at the sub-optimal temperature, reaching up to 27% of the total fatty acids. Supra-optimal temperature also modifies the redox status of PSII acceptors and decreases the rate of photosynthetic electron transport through both photosystems [85].
Valuable information on low temperature adaptation was gained studying Chlamydomonas strains. Contrary to the model green microalga Chlamydomonas reinhardtii, the psychrophilic Chlamydomonas nivalis can grow well at low temperatures maintaining a normal level of photosynthetic activity due to the reduced light-harvesting ability and the enhanced cyclic electron transport around PSI, which limits the photodamage to the photosynthetic apparatus [86]. Furthermore, the protective processes—increased cyclic electron transport rate, carotenoid content and antioxidant enzyme activities against reactive oxygen species (ROS)—reduce photooxidative damage to the cell.
The culture temperature balance can also be strongly affected by the culture system. For example, in closed PBRs, due to the greenhouse effect, the rise in culture temperature in the morning is much faster than that in open ponds [87]. It can easily exceed the physiological optimum and employing a cooling system may become mandatory and significantly increase biomass cost. Outdoor cultures in open units are naturally cooled by evaporation [17]. The importance of exploitation of various strains adapted to different temperatures in various periods of the year can be useful to extend the cultivation season. This strategy has been successfully used in Spirulina (now Arthrospira) cultures at Earthrise Farm in California, USA [88].
3.3. Dissolved Oxygen
The essential product of photosynthesis is oxygen. However, the photosynthetic electron transport represents the major source of ROS generating singlet oxygen, hydrogen peroxide and superoxide radical [89]. The generation of ROS is enhanced when the photosynthetic apparatus absorbs excess light or under conditions where CO2 or NADP is limited [90,91]. ROS were shown to play an important role in signal transduction [92,93]; a basal level of ROS is vital for the functions of living cells [94]. It was found that ROS triggers the accumulation of carotenoids in Dunaliella [95] and also amplifies expression of carotenoid biosynthesis-related genes in Haematococcus pluvialis under stress conditions [96].
Microalgae, especially in dense mass cultures, can generate high dissolved concentrations of O2. Though the oxygen evolution is sometimes overlooked in large-scale microalgae cultures, high concentrations of dissolved oxygen (DO) concentrations in outdoor cultures occurring especially under high irradiance at midday in outdoor cultures can result in photoinhibition and photorespiration, which initiate a reduction in photosynthetic activity and growth. In open ponds, the build-up of DO concentrations several times over air saturation are frequently observed during the day, which usually inhibit the growth of many microalgae [97]. The maintenance of DO levels below this concentration requires degassing or culture mixing. In closed tubular PBRs, the DO concentration can increase even quicker while the CO2 concentration decreases due to photosynthetic activity as the culture flows through the photostage (of the PBR; O2 has to be stripped from the medium in the degasser [98]). High DO concentrations during the day might have a significant impact due to potential inhibition of photosynthesis and growth [99]. An increase in DO concentration may cause photorespiration, which can significantly reduce productivity [100]. Photorespiration results in the light stimulated oxidation of the products of photosynthesis to CO2, which is run by the oxygenase activity of Rubisco [26,101,102]. At high O2 and low CO2 concentrations, Rubisco catalyzes the conversion of ribulose-l,5-bisphosphate to 3-phosphoglyceric acid and 2-phosphoglycolate (Section 2.2) [102,103]. At high [O2]/[CO2] ratios, the Mehler reaction can also be activated, especially at high irradiances and sub-optimal low temperatures [104].
In outdoor culture, the synergism of various environmental constraints is quite frequent. This interplay has often been studied in our experiments. The combination of a low temperature and high irradiance can frequently occur at the beginning of the cultivation season, when the ambient temperature drops greatly below the optimum, but the irradiance is high to drive massive photosynthesis. Indeed, it is common that light intensity can rise within 1 or 2 h, while the increase in temperature is a much slower process. In Monodus cultures, even a relatively short exposure to suboptimal morning temperatures induced photoinhibitory damage [105]. The culture heated in the early morning showed a significant increase in productivity of about 60% compared to the non-heated culture. The synergism of sub-optimal temperature and/or high DO concentrations (200–400% saturation) with excess irradiance can cause a decrease in photosynthetic activity and growth [63,97,105,106,107]. It happens when the rise in light intensity is much faster than the rise in temperature, with a delay of hours, or the build-up of DO concentration due to high photosynthetic activity is enormous in a culture unit. Such de-synchronization between these two important environmental variables can down-regulate photosynthesis and reduce the growth rate of outdoor cultures. In this case, photoinhibition may be induced at relatively moderate light intensity due to the exposure to suboptimal temperatures or high DO concentration.
The exposure of microalgae cultures to unfavourable conditions—combining several constraints—can induce the accumulation of some valuable products which can be used in biotechnological production. For example, Chlorophyta (e.g., Chlorella) possess the ability to synthesize larger amounts of protective or storage compounds (carotenoids, polysaccharides and lipids) under stress conditions, e.g., under high irradiance with nutrient deficiency [108]. The high content of β-carotene makes Dunaliella attractive to biotechnologists for large-scale production in high-salinity under high solar radiation (>30 °C) [109]. The accumulation of astaxanthin is induced in Haematococcus cells under stress conditions (nutrient deficiency, salinity, and high temperatures, in combination with high irradiance) [110]. Microalgae can synthesize unsaturated fatty acids under low temperatures; nutrient deprivation and high irradiance promote accumulation of PUFAs (e.g., EPA in Nannochloropsis) [111].
3.4. Culture Turbulence
Following inoculation of a culture, the cells are subjected to a transition from high light, acclimating to low irradiance as the culture density increases. This condition causes the change in cell pigmentation with a consequent reduction of light penetration reducing productivity. The increased turbulence should reduce the level of dark acclimation by preventing the increase in cell pigmentation [14]. Efficient mixing of the microalgae culture causes rapid circulation of cells between the dark and illuminated zones of the cultivation units that can increase both the photosynthetic activity and productivity. As already mentioned, the growth of a microalgae culture is also partly modulated not only by incoming light intensity, cell density, culture depth and frequency of mixing, but also by the frequency of light–dark (L/D) cycling of cells [17,40,54,58]. Thus, in ‘short’ light-path (<50 mm) cultivation systems we can induce fast L/D cycling of cells. High frequency of the L/D cycles, close to the turnover of the photosynthetic apparatus, facilitates an increase in biomass productivity. If the individual cells are exposed to intermittent irradiance due to the L/D cycles, light is diluted and it is available in smaller doses to more cells within a certain time period [37]; thus light energy can be used more efficiently when compared with the cells illuminated in poorly stirred cultures.
Light attenuation in dense microalgae cultures is partitioning the culture to several zones with two extremes: a highly illuminated external microalgae layer in which cells are exposed to light irradiance higher than that required for saturation, and a bottom one where cells are in darkness. The light is reduced exponentially through the culture according to the Lambert–Beer law. In well-mixed dense cultures in cultivation units, microalgae are exposed to a highly fluctuating light field. Although the decrease of irradiance is continuous to the culture depth, several zones can be described in a dense microalgae culture exposed outdoors to direct solar irradiance [77]: (i) The surface layers in which irradiance is excessive and down-regulation of photosynthesis appears; (ii) the light saturated zone where culture irradiance is saturating and the high photosynthetic rate (Pmax) is reached; (iii) the light limited zone with maximum light use efficiency; (iv) the dark zone where the irradiance is below the photosynthesis compensation point and only respiration takes place. The three upper zones, in which light is sufficient to activate photosynthesis, represent the photic volume (depth of light penetration). Besides, the light penetration in the culture varies with wavelength as green light, badly absorbed by Chls, penetrates better than blue or red light [74].
A question has arisen concerning the potential enhancement of productivity due to the increased mixing rate of a culture. When cultivation conditions, such as temperature, nutrition and others, are satisfied, then culture turbulence can create an optimal light/dark regime for the cells to increase the biomass culture yield [14]. Mixing rate affects productivity in different ways: (i) it prevents cell sedimentation; (ii) the cells travel through an optical gradient of culture depth with different quantity and quality of the light (iii) it increases mass transfer preventing the build-up of DO gradients in the culture as a result of photosynthesis.
However, the assessment of the effect of mixing on culture productivity is rather complex, making it difficult to separate the contribution of the different factors influenced by mixing, particularly in mass cultures where it is more difficult to separate the effect of single environmental factors. The degree of culture mixing is potentially limited by two factors: (i) the shear sensitivity of the microalgae cells and (ii) the cost of the energy provided for the mixing. Some species of microalgae with a thin cell wall, or those with flagella, are shear stress sensitive (e.g., Dunaliella) [14].
In mass microalgae cultures, high photosynthetic yields can be achieved in full sunlight once the turbulence and density of the culture are adjusted to proper light intermittence, i.e., the L/D cycling of cells is adequately short (in the order of tens to hundreds of microseconds), close to the time scale of the rate-limiting processes of photosynthesis [17,18,47,112]. While flash frequencies of 60 Hz are beyond engineering possibility, flash frequencies close to 1 Hz seems to be attainable in thin-layer cultures and small tube diameter PBRs [8,9].
3.5. Inorganic Carbon
Aquatic photosynthetic organisms fix about 50% of the 109 metric tons of carbon annually assimilated into organic matter [113]. Of this, the major part of inorganic carbon is fixed by phytoplankton in the open ocean, although locally the primary productivity in freshwater water bodies can be high on an aerial basis [1]. Whereas terrestrial plants generally utilize CO2 from the atmosphere, aquatic phototrophs acquire inorganic carbon from water where much of the available dissolved inorganic carbon (DIC) is available as bicarbonate (HCO3−) rather than as CO2. At present, commercial production of microalgae biomass is about 4 × 104 metric tons per year, i.e., about 2 × 104 metric tons of carbon is fixed annually (V. Verdelho, personal communication).
Since microalgae are generally found in aquatic habitats, inorganic carbon exists as CO2, HCO3− and CO32−. In microalgae mass cultures, the addition of an inorganic carbon, either as CO2 or HCO3−, is needed to enhance productivity [114]. Carbon dioxide serves as the main carbon source; in mass cultures it is usually added on the basis of a pH-stat system. Due to low ambient CO2 concentration (i.e., at present it is about 0.04%; v/v), it is common practice to add pure CO2 to large-scale cultures to improve biomass yields. Thus, microalgae cultures become C-limited over time if the carbon supply is not sufficient. The addition of CO2 to the culture creates a buffering system with water that can be described as follows:
pK1 pK2
CO2 + H2O/H2CO3 ↔ HCO3− + H+ ↔ CO2− + 2 H+
CO2 + H2O/H2CO3 ↔ HCO3− + H+ ↔ CO2− + 2 H+
The dissociation constants of the inorganic carbon system at 25 °C in freshwater are: pK1 = 6.35 and pK2 = 10.33 [113]. The majority of species take up both CO2 and HCO3−, where a pH value of between 7 and 8 is optimal [115]. To optimize the yield of the CO2 supply, it is recommended to operate on-demand injection of CO2 at a pH close to the limiting value for photosynthesis to maximize the utilization while minimizing losses by decarbonisation [13]. In outdoor trials, using thin-layer cascades, CO2 was supplied on the basis of pH measurements to keep the value of about 8 [97].
The CO2 compensation point is often determined using pH-drift experiments (i.e., when microalgae activity is stopped at a certain pH where there is insufficient available CO2 and HCO3− [100]). The addition of CO2 to a culture therefore not only serves as a source of carbon, but it also stabilizes the pH, and ensures that more carbon is available to the microalgae cells in the form of CO2 and HCO3−. Most microalgae can take up both CO2 and HCO3−, but there are a few species which can only uptake either CO2 or HCO3− [116]. Diffusional transport of inorganic compounds into the microalgae cells happens via molecular diffusion if culture turbulence is sufficient [100]. Since photosynthetic carbon fixation in the culture cannot be easily monitored by infrared gas analysis, special electrodes are used to measure the partial pressure of carbon dioxide (pCO2) in solutions. The principle is based on the relationship between pH, and the concentration of CO2 and bicarbonate in the solution:
KS = [HCO3] × [H+]/[CO2]
Subsequently, the Equation (7) was elaborated semi-empirically to correlate pH and CO2 partial pressure [100]. The logarithm of pCO2 in suspension decreased with the increasing pH following the formula:
log pCO2 = 11.679 − 1.46 × pH
The increase in O2 concentration due to photosynthetic activity is generally accompanied by a decline in the CO2 concentration measured as the increase in pH (CO2 uptake). A thorough analysis of CO2 and O2 gas exchange was carried out in Chlorella cultures in thin-layer cascades [100,117]. Since the ambient concentration of CO2 in the atmosphere is low (currently about 400 ppm; pCO2 ~ 0.04 kPa), mass microalgae cultures mostly depend on the addition of gaseous CO2 or solid bicarbonate to the culture. The adequate supply of inorganic carbon to phototrophic cultures is extremely important, as almost all large-scale microalgae cultures are CO2 limited. The design of the CO2 supply system is very important in order to maximize the utilization, but minimize the CO2 release to the atmosphere in order to minimize losses. Mass outdoor cultures require the CO2 partial pressure in the suspension to be at least tenfold higher than that in the atmosphere [100]. Pure COß is supplied on the basis of a pH-stat adjusting the pH value to about 8–8.5, which is sufficient for growth. If calculated according to the formula (7), there is still about 1–0.2 vol% of CO2 dissolved in the culture (5–25 times more than in the atmosphere), which is sufficient for good microalgae growth.
The effect of CO2 concentrations on microalgae growth have focused on concentrations lower than 0.5% CO2, as would be expected in the current, or in future environments with increased atmospheric CO2 concentrations [118,119]. However, if the microalgae cultures are to be used for CO2 bioremediation, it is desirable that the organisms have the ability to grow using sources with a high CO2 content (5–10% CO2). It should not be a problem for robust strains, as 5–10% v/v represents a pH of about 7.5–7.3 (see Equation (7)).
3.6. Nutrition
In microalgae cultures maintained close to the optimum biomass density, supplied nutrient amounts are essential to optimize productivity. Thus, the assay and optimisation of the nutrient requirements is essential for large-scale biomass production. Microalgae are capable of various kinds of trophy (nourishment), but basically operate two major forms of nutrition, namely autotrophy (photoautotrophy) and heterotrophy, of which the former is used mostly outdoors [120]. Phototrophic microalgae obtain the energy for reduction of CO2 through the absorption of light, while heterotrophic microalgae obtain their carbon and energy supply from organic compounds. Since nutrients represent a significant part of the cost of the microalgae production, a good knowledge of the nutrient requirements for each microalgae strain is essential in large-scale production [36].
In addition to carbon as the major biomass element, microalgae also require a supply of other macronutrients, namely nitrogen and phosphorus to maintain growth [14]. The average element composition of freshwater microalgae biomass normalized to carbon is about C:N:P = 100:13:1 [121], although the N content in particular may vary according to the environmental conditions and nutrient status (e.g., [122,123]). Nitrogen is another important macronutrient required for microalgae growth, which is usually supplied to the media as nitrate, ammonium or urea [14,124]. Phosphorus is usually added in the form of phosphate salts, although using wastewater, P may also be available in the form of polyphosphate and organic P-containing compounds [125]. Other inorganic micronutrients and trace elements found in microalgae are: K, Mg, Ca, S, Fe, Cu, Mn, Zn, Mo, Na, Co, V, Si, Se, Cr, Cd, Cl, B, I [126]. The content of these micronutrients is more variable depending on the taxonomical group, environment and cultivation conditions [127].
The most important microelements are those used in redox reactions (e.g., Fe, Mn, Cu), acid-base catalysis (e.g., Zn, Ni), transfer and storage of information (e.g., K, Ca) and in structural cross-linking (e.g., S and Si) [128]. Iron is an important element of cytochromes, ferredoxin or Fe-S proteins to maintain electron transport chains [120,129]. Fe limitation of phytoplankton has been found in the oceans [130]. Microalgae also require a range of other trace elements. For example, Mn is an important part of the oxygen-evolving complex, plastocyanin contains Cu, Se is the major biological of selenocystein or selenomethionin, while, for example, Zn, Mo, Co, V, Ni, Se and Cd are cofactors of some enzymes of the photosynthetic machinery [128]. The need to supply these trace elements depends, in part, on the composition of the water source used for culturing.
For long-term, semi-continuous or continuous cultivation, the nutrient supply has to be regularly reloaded to maintain high productivities. The biochemical composition of microalgae biomass can be manipulated by the concentration and form the supplied nutrients.
3.7. Cell Density and Culture Depth
Since light represents the most important substrate controlling productivity, cell density of the culture has a significant impact on the light availability for the cells. In order to achieve the best growth, light intensity has to be balanced with the cell density and culture layer (i.e., the unit depth). In dense cultures, the irradiance decreases rapidly from the surface, deeper into the culture; thus, cells in deeper layers may actually stay in the dark [18,77]. For given culture conditions, a certain value of cell density will exist, at which the individual cells will be exposed to the optimal irradiance regime. At this optimal cell density (OCD; g DW L−1), the highest photosynthetic efficiency should be reached, and found as the highest yield of biomass production per illuminated area [35]. Thus, OCD is defined as the cell concentration which results in the highest production of biomass and/or desired compound. In other words, the OCD facilitates the most efficient use of the irradiance received by the culture in a cultivation unit. Studies on L/D cycles have indicated that the cell turbulence is a relevant variable for the enhancement of photosynthetic productivity. In short light-path systems (<10 mm), e.g., flat-panel PBRs or thin-layer cascades, the time period of the L/D cycles can be hundreds of milliseconds [39].
A textbook example of this can be seen in low-depth units such as sloping cascades where dense, high-productivity cultures can be maintained due to high turbulence of thin-layer culture, first proposed by Šetlík and co-workers [131]. The latest generation of these units has a culture depth of about 6 mm with a culture area inclined at 0.5% [18]. A hydrodynamic model showed that fast flow (about 0.5 m s−1) causes turbulence, which induces rapid L/D cycling of cells (with a frequency of 0.47 s−1) [17]. In these trials, the optimum areal density (for maximal productivity) was 40–80 g DW m−2 and the culture grew well up to a biomass concentration of 300 g DW m−2, i.e., about 50 g DW L−1 [17]. Such high biomass densities and a daylight productivity up to 55 g DW m−2 day−1 are most likely caused by the short L/D cell cycling frequency of about 2 Hz (0.5 s−1), which these cultures experience [18].
Principally, thin-layer cultivation systems ensure high areal or volumetric productivity as they have a high exposed surface-to-volume ratio (S/V ratio). The other aspect is that the overall photic volume should comprise ~5 to 10% of the optical path [74,77] as the light penetration depth depends on cell density. Generally, a thinner culture layer means a smaller areal volume, which induces a higher volumetric productivity [132].
3.8. Cultivation Unit Design and Culture Productivity
The geometry of the culture system used for microalgae growth represents a crucial technical parameter affecting the productivity. Indeed, microalgae are currently produced in a wide variety of culturing units including open raceway ponds with a typical culture depth between 10 and 30 cm mixed by paddle wheels, tubular PBRs made of transparent tubes with an internal diameter typically between 4 and 10 cm, vertical flat-panel PBRs with an optical path ranging between 1–20 cm, or thin-layer cascades in which the culture depth is lower than 1 cm [18,133]. Table 1 shows the advantages and disadvantages of open and closed systems, which have been extensively discussed in recent reviews [13,134,135].

Table 1.
Comparison of advantages and disadvantages of basic types of cultivation units which are used for microalgae production (modified from [136]).
Ponds and cascades are open cultivation units in which the culture is in direct contact with the environment, while PBRs are closed systems in which the contact with the environment is limited. Open systems are strongly dependent on the weather/climate, have increased risk of microbial contamination, high CO2 losses and require higher areas compared to closed systems. Yet the cost of construction is about one order of magnitude lower than that of closed systems [137,138]. Raceway systems are used commercially worldwide including the United States, Thailand, China, Chile, and Israel to produce microalgae for relatively high-value applications related to human consumption [13]. The open raceway pond is currently the most frequently used and cheapest cultivation system for commercial production of microalgae. Cost investment for these open systems range from 130,000 to 370,000 Euro ha−1 at 100-ha scale [137,139] while the investment costs of tubular PBRs of about 500,000 Euro ha−1 were reported [139].
In general, the decision of using a certain type of cultivation unit strongly depends on a cultivated strain and desired product. Typically, closed systems are used for microalgae strains susceptible to contamination by other strains, such as Haematococcus pluvialis, or strains requiring a strict control of temperature (such as marine Phaeodactylum tricornutum) used for the production of lipids and aquaculture purposes. Open systems are usually used for the production of fast-growing microalgae or in selective condition (e.g., Chlorella or Spirulina). A higher productivity is expected in thin-layer cascades and closed systems characterized by shorter light paths. An important parameter that has to be considered while comparing the performance of different culture systems is the light conversion efficiency (LCE) into biomass. Higher LCE means a reduced biomass production cost. A comparison of four outdoor pilot-scale PBRs under the same climatic conditions using the same strain was carried out by de Vree et al. [140] The LCE values ranged between 2.2–4.2% in vertical systems (vertical flat-panel and vertical tubular PBRs) while it was found between 1.5 and 1.8% in horizontal tubular PBRs. The lowest values, less than 1%, were obtained in raceway ponds. These differences can be explained by the way the light approaches the surface of a cultivation unit, and by temperature profile. Vertical PBRs take advantage of a high ratio between the illuminated area of the PBR and the ground area occupied [141]. This type of PBR intercepts sunlight at large angles and thus “dilutes” light compared to horizontal PBRs, thus being expected to be more efficient than horizontal ones in terms of solar-energy utilization [140,142]. However, it must be pointed out that, notwithstanding these advancements in the PBR design, the theoretical LCE (10%) is still far from being obtained (see Section 3.1 Light).
The supply of pure carbon dioxide can constitute up to 30% of the overall microalgae production cost [36]. When flue gases are used instead of pure CO2 to decrease the production costs, the sustainability of the entire process is increased [13]. Thus, the usage of alternative nutrient sources is an important step towards a more ecological and economical microalgae cultivation. By the utilization of wastewater as a nutrient source, the production costs can be reduced to under 5 Euro kg−1 [143].
3.9. Altering Cultivation Conditions to Modulate Biomass Composition
Only about 10 microalgae strains are grown commercially in mass cultures to produce biomass containing certain bioactive compounds for specific applications. Microalgae strains are chosen as they can be cultured on a large scale and their biomass composition can be ‘manipulated’ by cultivation conditions for production of desired compounds. However, only a limited number of cultivation variables can be manipulated practically and cost-effectively, in large-scale cultures [14].
The three classes of cellular macromolecules—proteins, carbohydrates and lipids—contain up to 90% of the cellular carbon. Using Fourier Transform Infrared Spectroscopy (FTIR), carbon allocation analysis can be performed, which allows the quantification of the relative amounts of major biomass components [144]. The relative amounts of these three groups varies among species and also depends on growth conditions.
The organic compounds of the cell can be generally classified as either primary or secondary metabolites. Primary metabolites are directly participating in cell growth, normally performing an intrinsic physiological function. Secondary metabolites are not essential for the normal functioning of the cell, but may have an auxiliary (protecting) role in cells under particular conditions. They are usually related to primary metabolites as, for example, some carotenoids may be the same but serve another physiological function. Most of the valuable microalgae compounds, such as β-carotene, astaxanthin or triacylglycerols, are the secondary metabolites produced by microalgae under nutrient limitation. A common feature of these compounds is that they are accumulated by the cells under growth-limiting conditions (often called ‘stressed’ cultures, usually using the synergism effect of nutrient limitation, extreme temperature and high irradiance). Various cultivation strategies have been employed, or proposed, to produce these desired metabolites in cultures. A crucial point is how to distinguish the proper moment to harvest the biomass enriched in these compounds using fast-response monitoring techniques. Several attempts have been made to correlate changes in photosynthetic activity and metabolite production [40,145]. In freshwater, Chlorella cultures treated under nutrient deficiency (distilled H2O) physiological stress became evident when Chl fluorescence variables rETR, FV/FM and NPQ were substantially modified, which indicated the onset of neutral lipid synthesis [145]. Similar data were found when laboratory cultures of the microalga Chlorella fusca were subjected to nitrogen and sulphur limitation inducing lipid and/or starch accumulation [146]. The nutrient deficiency became evident when photosynthetic activity was substantially reduced, which was seen as a decline of rETR counteracted by a rise of NPQ. These changes indicated the point of lipid accumulation (while the starch content was decreased). A substantial development of NPQ indicated that the culture still possessed an effective photoprotective ability to dissipate excess light energy under mild early nutrient deficiency stress [147].
3.10. Microalgae Biomass with Added Value
In the past six decades, there have been numerous attempts by researchers and companies to commercialize microalgae biomass production, primarily as food supplements due to its potential to enhance nutritional value, as probiotics (life-enhancing agents) or so-called functional foods (which, in addition to the basic nutritional function, support health). Microalgae biomass is also widely used as a feed supplement for farm and domestic animals (poultry, ruminants, pigs, ornamental fish and birds) and aquaculture (mollusc, crustacean and fish larvae in hatcheries) to improve the quality of products, vitality, health resistance and color (e.g., [3]).
The recent annual market is 30–40 thousand tons of microalgae biomass (dry weight), but only some technologies are established and sustainable, others are still under development for commercial use (Table 2).

Table 2.
The current industrial-scale biotechnology applications of the most exploited microalgae (modified from [3]).
4. Genetic Improvement of Photosynthetic Reactions
Enhanced knowledge about the genes and proteins involved in the photosynthetic machinery and acclimation of microalgae would improve our understanding of their acclimation pathways, and enable the genetic improvement of stress-tolerant strains [89]. Genetic manipulation approaches can generate strains with desirable commercial traits, by either expressing new biosynthetic pathways or enhancing the yield of a product of interest already present in a given strain. Bioengineering attempts to reduce the light absorption as a larger population of cells can contribute to overall productivity, was showed in the model microalga Chlamydomonas reinhardtii as mutants with truncated light-harvesting antenna were obtained by random mutagenesis [54,57]. The mutant tla1 showed a significant reduction of Chl content per cell and a smaller functional antenna size than that of WT. Mutants with a reduced antenna size were also obtained in Nannochloropsis gaditana and Chlorella sorokiniana by random mutagenesis and phenotypic selection; the strains showed higher photosynthetic efficiency than WT and improved photoresistance in both lab-scale and pilot-scale PBRs [60,148]. Nevertheless, it is not yet clear if such engineered strains with both modulated antenna size to improve light penetration and enhanced resistance to excess light, can be optimized for mass culture towards higher biomass productivity and stress resistance [149]. An alternative approach represents bioprospecting, particularly in extreme environments, which might provide such strains with improved resistance, which exhibits high productivity and the robustness of growth (for example Chlorella ohadii, a strain isolated found in the Sinai desert [150]).
The rate-limiting step of the Calvin–Benson cycle is the fixation of inorganic carbon catalysed by Rubisco, as the complex has a low turnover rate and a low substrate specificity. Moreover, it also shows affinity for O2, which leads to futile reactions (in the Section 2.2 of this article). The construction of microalgae strains with improved carboxylation activity would be crucial for higher efficiency of solar energy conversion [25]. The combination of mutations of different isoforms might be a way to obtain Rubisco with the improved Vmax of carboxylation catalysis [151]. Rubisco-improved variants were obtained by site-directed mutagenesis, targeting either the gene (Rubisco large subunit) or the subunit that interacts with Rubisco activase [152,153]. Besides Rubisco, other enzymes of the Calvin–Benson cycle and accessory proteins have been targeted, e.g., sedoheptulose 1,7-bisphosphatase from Chlamydomonas reinhardtii has been successfully over-expressed in Dunaliella bardawii, resulting in a significant enhancement of photosynthetic efficiency [154]. A strong rise in the photosynthetic productivity of Synechocystis was obtained by over-expressing Rubisco, sedoheptulose1,7-biphosphatase, fructose-bisphosphate aldolase and trans-ketolase [155].
The triose phosphate produced by photosynthesis represents the main metabolic pathway of the microalgae cell; therefore, the improvement potentially results in increased production of lipids, proteins and other high value compounds. The major research target is the engineering of strains for a significant increase of total lipid accumulation, and/or the optimization of fatty acid chain-length profiles by targeting single or multiple genes involved in the lipid biosynthesis or by down-regulating competing pathways [156,157]. Saturated and mono-unsaturated C14–C20 fatty acids from microalgae are exploited for renewable liquid biofuel production, while the engineering biosynthesis of polyunsaturated fatty acids (PUFAs), important components of the human diet, might become a viable option in the market of high-value food additives [158].
Regarding the endogenous isoprenoids, carotenoids represent commercially successful products from microalgae: β-carotene from Dunaliella salina and astaxanthin from Haematococcus pluvialis are high-price products mainly used in aquaculture, and as food coloring agents and nutraceuticals (Table 2) [159]. In Haematococcus pluvialis, the endogenous phytoene desaturase was modified by site-directed mutagenesis, enhancing both the resistance to the herbicide norflurazon and the astaxanthin productivity [160]. Significant enhancements in carotenoid productivity were obtained by modification of the astaxanthin synthesis pathway—either over-expressing the β-carotene ketolase, or by expressing the endogenous phytoene desaturase in the chloroplast [161,162].
5. Photosynthesis Monitoring
Successful cultivation requires constant monitoring to maintain a favorable cultivation regime in microalgae cultures, namely physico-chemical variables, such as pH, temperature, DO concentration, and nutrient levels, but most importantly monitoring photosynthetic performance. Any irregularity in photosynthesis can signal changes which may indicate early symptoms of stress; thus, counteraction may be taken to prevent culture failure. The classical method for culture control is direct sensual (appearance, color, smell) or microscopic examination in order to follow physiological and structural changes and contamination of the culture. The presence of ‘invaders’ (other microalgae strains, protozoa, bacteria or fungi) usually indicates that the culture has not been in favorable conditions for growth. Such contaminations are frequently one of the more serious problems in large-scale microalgae cultivations.
Finding fast and robust techniques to evaluate variations in microalgae activity has been one of the major tasks for the effective monitoring of pilot and large-scale cultivation units. Various biophysical and biochemical monitoring methods have been used to adjust growth conditions for the production of biomass, or certain valuable compounds. The primary symptoms of adverse growth conditions can be detected as an inhibition of photosynthetic activity of a microalgae culture which subsequently slows down its growth and productivity. Changes in dissolved O2 concentrations, or CO2 consumption (if operated at regulated pH by its addition) and Chl fluorescence have been used for monitoring microalgae culture, as they provide primary information on culture photosynthetic performance and consequently are reflected in culture growth [14,70].
One direct approach to monitoring microalgae cultures is to follow the photosynthetic activity, such as oxygen production or Chl fluorescence measurement on-line/in-situ tracking of the actual situation. The other method is to assess the culture state ex-situ using microalgae samples withdrawn from a cultivation unit. Some variables, such as photosynthetic oxygen production, or PSII photochemical yield and electron transport rate (measured by oximeters and fluorimeters), have been used to correlate photosynthetic activity and growth. At present, techniques and protocols have been elaborated for microalgae culture monitoring to optimize their growth in various cultivation systems [70,78,106,163,164,165,166].
5.1. Measurement of Photosynthetic Carbon Fixation
As photosynthetic carbon assimilation in microalgae culture cannot be easily followed by infrared gas analysis, some special electrodes have been used to measure the partial pressure of carbon dioxide (pCO2) in solutions. The principle is based on the correlation between pH, and the concentration of CO2 and bicarbonate in the solution (KS = [HCO3−] × [H+]/[CO2]).
The technique of 14C radiolabelling has been used to study the carbon assimilation rate in photosynthesis. The culture of microalgae is exposed to 14C labelled compounds for a certain time and then the amount of 14C incorporated in to the biomass is assayed using a scintillation counter. This technique is often employed in phytoplankton studies [167], but it is not used much in microalgae mass cultivations.
5.2. Measurements of Photosynthetic Oxygen Evolution
For regular measurements of photosynthetic oxygen production and DO concentration in microalgae cultures, polarographic oxygen sensors (based on the Clark-type electrode) are usually used [168]. Optical oxygen sensors have also been developed for DO measurement in gaseous and liquid phases (e.g., by Mettler Toledo) that are based on fluorescence quenching, utilizing the interaction between a fluorescing chromophore and oxygen molecules. Although not widely used, these sensors have a sensitivity comparable to Clark-type electrodes and can be used in-situ. These sensors have shown a few advantages compared to polarographic sensors, namely they have no need for polarization, no consumption of oxygen, stability against electrical and thermal disturbances, and high storage and mechanical stability.
Photosynthetic oxygen production can also be measured by mass spectrometry using nuclear isotopes (18O) and measuring their distribution [169]. Using 16O2 and 18O2 isotopes, the net photosynthesis and respiration can be distinguished.
Recently, a photo-respirometry technique for quantifying the rates of the main metabolic processes in microalgae-bacteria consortia has been developed and tested [170]. When studying microalgae-bacteria consortia, for example whether wastewater used as a nutrient source, it is beneficial to distinguish between the contribution to oxygen balance by both groups. Distinguishing between heterotrophic and nitrifying activity in microalgae cultures can be done using the oxygen production/consumption rates. The proposed protocol is based on the application of dark and light periods to the microalgae-bacteria consortia using different substrates when measuring the rate of oxygen production. The methodology allows us to distinguish between the metabolisms of the three main populations: the microalgae, the heterotrophic bacteria and the nitrifying bacteria. Firstly, samples of the microalgae cultures were taken and subjected to nutrient starvation to remove the organic matter and the ammonium present in the medium. Subsequently, the samples are placed inside the jacketed flask and the variation in dissolved oxygen over time was measured during four light–dark periods of 4 min when the variation in dissolved oxygen over time was measured and registered. This allowed us to distinguish between microalgae and heterotrophic and nitrifying activity using the oxygen production/consumption rates.
5.3. Chlorophyll Fluorescence Monitoring
Since the 1990s, traditional measuring methods used in microalgae biotechnology were supplemented by biophysical techniques, namely Chl fluorescence measurements, which give fast and more complex information about the physiology and ‘health’ of the mass cultures (Figure 5) [106,171,172,173,174]. This approach was qualitatively different from physiological methods, upgrading it to a molecular level.

Figure 5.
In-situ Chl fluorescence measurements in microalgae cultures using various PAM fluorimeters. (A) Tubular indoor PBR with Haematococcus, Institute of Physical Biology, University of South Bohemia, Nové Hrady, Czech Republic; (B) tubular outdoor PBR with Arthrospira, Institute of Ecosystem Studies, CNR, Sesto Fiorentino, Italy; (C) outdoor thin-layer cascade with Chlorella, and (D) outdoor thin-layer raceway pond with Monoraphidium, both in Centre Algatech, Institute of Microbiology, Třeboň, Czech Republic. The fiber optics of the fluorimeter were placed on the wall of a PBR tube (panels (A,B), or were submerged directly in the culture using solar irradiance as actinic light (panel (C)). Sensors of dissolved oxygen concentration, temperature, irradiance and Chl fluorescence were submerged in the culture side by side to measure data in parallel (D).
Chl fluorescence measurements have become one of the most useful and frequently used approaches to monitor microalgae mass cultures for their fast response and sensitivity as well as a wide choice of fluorimeters that are easily used in-situ with provision for numerous variables [70,105,106,166,171,173,175,176,177,178]. These measurements directly indicate the functioning of photobiochemical processes in the photosynthetic apparatus, namely in the PSII complex, which is a key regulatory point of light-driven processes. At present, it is relatively easy to evaluate data, but care must always be taken to select sensible variables and elucidation of their changes. This is particularly important when working with outdoor mass cultures, where growth variables, such as irradiance, temperature, and other adverse factors can occur side by side. In this case, Chl fluorescence represents a good tool which allows rapid monitoring of physiological status of microalgae cultures. Nevertheless, Chl fluorescence techniques used to measure photosynthetic performance under certain conditions need to be compared with some other measurements: for example, oxygen production as a complementary technique that can even distinguish photosynthetic and respiratory rates [70,168]. Both Chl fluorescence techniques and oxygen production assays have often been combined to optimize growth regimes as well as to assess the influence of adverse environmental conditions—temperature extremes, high dissolved oxygen concentration and high irradiance—and their synergism on microalgae growth [17,70,145,166,173,179]. Chl fluorescence compared with measurements of O2 production and/or CO2 uptake provides analogous information, but the former techniques are considerably quicker, more sensitive and moreover show energy distribution photochemical and non-photochemical (heat dissipation) processes (for a review see [70,166,178]).
Basically, two Chl fluorescence techniques have been utilized for photosynthesis monitoring in microalgae mass cultures at present: rapid fluorescence induction kinetics and the saturating pulse analysis of fluorescence quenching, i.e., pulse-amplitude modulation (PAM) techniques (for recent reviews see [180,181,182,183,184,185]). While the fast fluorescence induction kinetics provides information on the redox status of the photosynthetic electron transport chain, the PAM-technique presents information on the absorbed energy distribution between the energy distribution between the photochemical and non-photochemical processes in photosynthesis. The fast Chl fluorescence induction kinetics (also called the Kautsky curve) of all oxygenic photosynthetic organisms is characterized by a polyphasic rise measured in the time range between 50 µs to about 1 s when the signal rises rapidly from the origin (F0 = O) to the highest peak (P = FM) via two infections, J and I [186,187]. The current understanding of the OJIP transient rise is that it reflects the filling-up (i.e., reduction) of the electron acceptor pool of PSII [181]. After exposure to light the rapid O-J rise (2–5 ms) in fluorescence represents the photochemical phase, and reflects the accumulation of the reduced ‘primary’ acceptor of PSII, QA− (gradual closing of open the reaction centre of PSII). The second inflection I occurs some 30–50 ms after illumination and it is thought to reflect different redox states, e.g., QA QB2− or QA− QB2− [188,189]. The dip after the J inflection reflects the movement of electrons from one quencher to the next (e.g., QA → QB). An important variable that is frequently used in graphical presentations of fluorescence induction data is the variable fluorescence at time t, Vt = (Ft−0)/(FM − F0) (Table 3 (reviewed in [190]). The rise of fluorescence from J to the peak P (200–500 ms) reflects the thermal phase influenced by the two-step reduction of QB (QB → QB− → QB2−) and the heterogeneity in the reduction of the PQ pool. The peak P shows that the PQ pool becomes fully reduced (equivalent to maximum fluorescence yield FM; fully closed reaction centre). Fast fluorescence induction curves can be normalized on both F0 and FM to better illustrate the reduction status of the J (Vj) and I (Vi) transients [183]. Due to the complex nature of the numerous interactions influencing the Chl fluorescence induction kinetics, however, it has been generally agreed which particular phase reflects the individual photosynthetic processes [181,187,189,190].

Table 3.
Selected variables calculated from the time-course of fast fluorescence induction curves. F0, FJ, FI and FP—fluorescence yields measured at O-step (0.05 ms), J-step (~2 ms), I-step (~20 ms) and P-step (200–400 ms) of fluorescence induction curve (modified and compiled from [166,189,195]).
The PAM technique has been a considerable step forward as it has made it easy to estimate photosynthetic activity in-situ in microalgae cultures under actinic (ambient) irradiance [191]. Chl fluorescence measurements illustrate that alterations in some fluorescence variables correlate well with changes in cultivation conditions, physiological status and growth of a microalgae cultures in a selected cultivation system [17,70,78,106,173,176,184,185]. Several basic variables can be estimated in microalgae cultures by using the PAM technique (Table 4). The maximum quantum yield FV/FM, calculated as (FM − F0)/FM, is considered a measure of the maximum photochemical PSII efficiency, an estimate of the physiological status. The variables F0, FV and FM reflect the minimum, variable and maximum fluorescence yields, respectively, measured in the ‘relaxed’, dark-adapted culture. It is important to mention that measurement of FV/FM in cyanobacteria is quite complex and strongly depends on the culture status and conditions. In these microorganisms, respiratory pathways interconnect with electron flow in the photosynthetic membrane and both electron pathways share the plastoquinone pool [192]. Due to higher ratios of PSI/PSII reaction centers and increased fluorescence from phycobilisomes in cyanobacteria, the F0 (constant fluorescence yield in the dark) values are increased [193]. Cyanobacteria have higher levels of F0 and lower levels of FM (maximum fluorescence yield) when using standard fluorescence measuring protocols for green microalgae and plants. When the quantum yield of PSII FV/FM is calculated as FV/FM = (FM − F0)/FM, then the values give lower quantum yields in cyanobacteria when compared to green microalgae. The effective, or actual quantum yield YII or ΔF′/FM′ = (FM′ − F′)/FM′) reflects the acclimation of photosystem II (PSII) when the culture is exposed to light as it depends on the actual redox state of the PSII electron carriers. This variable can be used as a measure of photosynthetic performance (and growth) [179,194]. The variables FM′ and F′ show the maximum and steady state fluorescence yield, respectively measured in the light-adapted sample.

Table 4.
Basic variables calculated from saturating pulse analysis of Chl fluorescence quenching when measuring [3,147,166,182]: F0, FV, FM—minimum, variable and maximum fluorescence yield in the dark-adapted state; F′, FV′, FM′—steady-state, variable and maximum fluorescence in the light-adapted state; EPAR—photosynthetically active irradiance intensity; a* is the Chl-specific absorption cross-sections and fPSII is the fraction of absorbed quanta by PSII [196].
In outdoor trials, PSII photochemical yields (FV/FM or ∆F′/FM′) should decrease by 20–30% at midday as compared with morning values; this is typical for well-performing microalgae cultures [17,70,184]. A lower or higher decline may indicate that the culture is either low-light acclimated or photo-stressed. The decrease in temperature below 20 °C during the night time is beneficial as it can minimize the biomass loss due to respiration to below 10%. It is important from a biotechnological point of view when culturing outdoor mass cultures that everything is optimized for varying climatic conditions.
Chl fluorescence measurements in microalgae cultures in-situ show that diurnal variations of the relative PSII electron transport can be correlated with simultaneous changes in the daily productivities of the cultures grown under various conditions [17,173,176]. The relative electron transport rate through PSII, rETR (Table 2; calculated as YII × irradiance in the culture) recorded in-situ have also shown to be one of simple and reliable variables for estimation of the photosynthetic performance of outdoor microalgae mass cultures [17,70,173]. The non-photochemical quenching NPQ calculated according to the so-called Stern–Volmer formalism [=(FM − FM′)/FM′] is inversely related to the actual photochemical yield (YII or ∆F′/FM′). An increased NPQ (indicating dissipation of absorbed energy, mostly as heat) is considered as a protection mechanism of PSII reaction centres against damage by excess irradiance [197]. Semi-empirically, NPQ has been solved into two components—Y(NPQ) and Y(NO), which quantify regulated (∆pH- and xanthophyll-regulated quenching in the light-harvesting antennae) and non-regulated (due to energy trapped in closed PSII reaction centres) thermal dissipation processes, respectively [147]. If summarized YII + Y(NPQ) + Y(NO) should equal 1.
NPQ arises from a number of processes in the thylakoid membranes, and several major components of NPQ can be identified based on the kinetic curves of the relaxation of PSII fluorescence [198]. The fastest component of NPQ, immediately triggered upon exposure to light, is the energy-dependent quenching (qE). State transitions represent changes in the relative antenna sizes of photosystems [47]; however, although this fluorescence decline (called qT) has been included in NPQ, an additional quenching component, that rises and relaxes at a longer time scale called qZ, is found in some eukaryotic microalgae and plants [199,200]. The response is dependent on a low lumenal pH and requires Chl-xanthophyll-binding proteins. Under high-light conditions, lumen acidification triggers the so-called xanthophyll cycle, which involves the xanthophylls violaxanthin and zeaxanthin, and consists of a light-dependent, rapid and reversible de-epoxidation of violaxanthin to zeaxanthin [199,201]. The NPQ mechanism is highly relevant for the maintenance of the photosynthetic efficiency, which contributes to acclimation to the different light environments. The relative contribution of each of the NPQ components changes between organisms and irradiances: qE activates based on sudden increases in light intensity, while ST responds to changes in the light spectrum under low light conditions [25].
Simple and fast procedures (manuals) for microalgae biotechnologists have been worked out based on Chl fluorescence measurements for monitoring photosynthetic performance (and physiological changes) of microalgae cultures in order to optimize growth [70,106,166]. Nevertheless, though the interpretation of fluorescence data has been well elaborated, the elucidation of fluorescence signals in various microalgae groups may not be as straightforward [70,166,181,182,195,202,203]. Recently, a quick, one-day test based on photosynthetic measurements was elaborated for preliminary optimization of growth regime in microalgae cultures [78]. Three techniques—measurements of oxygen production, saturating pulse analysis of fluorescence quenching, and fast fluorescence induction kinetics—are used to estimate the preliminary growth regime, which should correspond to a high growth rate.
In order to control an outdoor microalgae culture, on-line monitoring is desirable to follow the culture health and detect any deviation from the usual behaviour. It is important to find suitable monitoring techniques and variables (markers) which correlate with growth in order to optimize biomass productivity. In one series of trials, the Chlorella R-117 culture was grown in an outdoor thin-layer cascade at an optimal cell density (about 6 g DW L−1) exposed to about 1800 μmol photons m−2 s−1 at 25 to 30 °C, which are rather favorable conditions for this culture [70]. Photosynthetic activity in-situ was monitored by following oxygen production (by the Clark-type electrode) and the actual PSII photochemical yield (by a PAM fluorimeter). If the culture was exposed to a CO2 shortage, a continuous pH increase was accompanied by an immediate decrease in the actual photochemical yield ∆F′/FM′ that indicated an inhibition of electron transport through the PSII complex. The course of ∆F′/FM′ was matched by a simultaneous decrease in the gradient of dissolved oxygen concentration (ΔDO concentration; measured as the difference of dissolved oxygen concentration between the upper and lower ends of the cultivation surface). At pH 8.4, the CO2 partial pressure is about 0.2 kPa, which is insufficient for the fast-growing Chlorella mass cultures [100] and thus photosynthetic oxygen production and the photochemical yield considerably dropped (ΔDO decrease from 13 to 2 mg L−1 and ∆F′/FM′ from 0.55 to 0.33). Then, after 30 min, the supply of CO2 was restored, the immediate recovery of photosynthetic activity was observed within minutes as a simultaneous increase of ∆F′/FM′ and ΔDO (Figure 12 in Malapascua et al. [70]). This experiment clearly illustrated that measurement of photochemical yield by Chl fluorescence and oxygen production can alternate/substitute for the monitoring of photosynthetic activity of the outdoor mass culture.
In our recent trials, culture variables—ambient irradiance and temperature—were monitored in parallel with photosynthetic activity in the culture of the psychrophilic microalga Monoraphidium (Chlorophyceae) grown in an outdoor thin-layer raceway pond (Figure 6). The culture was inoculated with a starting biomass density of 0.4 g DW L−1, suitable for a thin-layer raceway pond with a culture depth between 1.5 and 2.5 cm. It grew relatively well as the specific growth rate µ was about 0.23 day−1. In these experiments, photosynthetic activity was measured as O2 production (estimated as DO saturation) and rETR in-situ in the culture using a submerged oxygen sensor, fiber optics and a fluorimeter light sensor (rETR calculated as irradiance intensity multiplied by ∆F′/FM′ in the photic zone). On days 1–3, maximum ambient irradiance in the greenhouse was between 144–380 µmol photons m−2 s−1 depending on if we worked under overcast or clear skies in November–December (Figure 7A). The greenhouse was heated to keep the culture temperature between 10–20 °C (Figure 7C). The culture revealed midday maxima of 110–120% O2 saturation and rETR between 4–25, corresponding to the irradiance inside the culture of about 10–30 µmol photons m−2 s−1 (Figure 7G). At night the temperature was between 12–15 °C, which resulted in the value of O2 saturation between 75 and 80% due to respiration. Starting on day 5, the ambient irradiance maxima at midday reached between 570–640 µmol photons m−2 s−1. The increased ambient light caused an increase in the inside-culture irradiance between 100–180 µmol photons m−2 s−1 at midday, which logically induced an increase in the photosynthetic activity corresponding to the rETR value between 50–77 and 120–145% of O2 saturation in the culture (Figure 7B,F,G). One important point to note was the correlation between the increased culture temperature during the night and the corresponding rate of respiration. On days 7–8, when the weather was fair with high sunshine, the heating during the night was switched off (t = 7–10 °C) and weak illumination (about 20 µmol photons m−2 s−1) was used. The O2 saturation was similar to that found in the air, showing that respiration was suppressed. In these trials, a very good correlation was found between the course and range of individual variables, i.e., ambient irradiance, irradiance inside the culture (in the photic layer), temperature, dissolved O2 concentration (oxygen production and respiration), actual photochemical yield ΔF′/FM′, rETR and NPQ measured by Chl fluorescence in the photic layer (i.e., depth of light penetration) in the microalgae culture volume. This is an important finding to select and validate suitable monitoring techniques and important variables—markers for management of large-scale outdoor units.

Figure 6.
Thin-layer raceway pond used for the cultivation of Monoraphidium. The unit of 5 m2 with a working volume of 100 litres was placed in a polycarbonate greenhouse to protect against un-favourable weather conditions and cross-contamination (Centre Algatech Třeboň). Temperature control was maintained by heaters and fans to keep a suitable cultivation regime. The culture layer thickness in the unit was between 15–25 mm and flow velocity about 0.2 m s−1 managed by a paddle wheel [15].
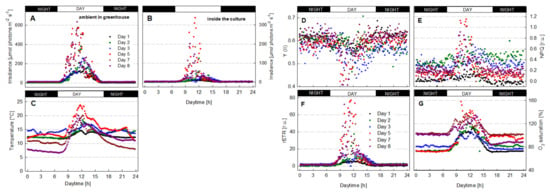
Figure 7.
Diurnal changes of growth conditions and photosynthetic activity in the Monoraphidium culture (class Chlorophyceae) grown during an 8-day trial in an outdoor thin-layer raceway pond (November–December 2020). (A) Ambient irradiance intensity in the greenhouse; (B) irradiance intensity in the culture measured in the middle of the photic zone (the depth of light penetration); (C) culture temperature; (D) actual photochemical yield ΔF′/FM′ (E) non-photochemical quenching NPQ; (F) electron transport rate ETR rETR = ∆F′/FM′ × PAR estimated in the middle of the photic zone; (G) dissolved oxygen concentration in the culture; (E) relative electron transport rate.
6. Conclusions
In microalgae cultivation, we make an effort to manipulate growth conditions in order to suit culture requirements. In this article, major variables affecting photosynthesis of microalgae mass cultures have been reviewed. There are a number of impacts which influence microalgae growth in large-scale facilities. These are irradiance, temperature, dissolved oxygen concentration, CO2 supply, nutrition, culture turbulence, contamination and construction of cultivation unit. Effective cultivation must involve continuous monitoring of photosynthesis and physiology performance to maintain suitable cultivation conditions. Any ‘abnormality’ indicates changes in the culture that may show early symptoms of stress; thus, counteraction may be taken to prevent culture stress, or even death.
Methods and protocols have been developed to monitor outdoor mass cultures of selected strains so as to optimize their growth in specific cultivation systems. Finding fast and robust techniques to evaluate variations in microalgae activity has been one of the main tasks for the successful management of large-scale cultivation units. Changes in oxygen production, CO2 consumption and/or Chl fluorescence have been mostly used, which give primary and prompt information on culture photosynthetic activity and consequently reflect the growth. Contamination of the culture may be an overlooked problem in mass culture, particularly for species growing in non-selective media. Therefore, it is important to develop rapid and reliable techniques for monitoring alien species, particularly insidious are Chytrid fungi, which can have devastating effects on culture growth and thus the necessity to replace the culture and a rise in production costs. One direct approach to culture monitoring is to follow photosynthesis variation in-situ during the diurnal cycle and thus obtain the information about the actual situation in the culture. The other possibility is to measure ex-situ using microalgae samples withdrawn from a cultivation unit. For some variables, like photosynthetic oxygen production, the photochemical quantum yield of PSII and the electron transport rate (measured using oximeters and fluorimeters) have been found to directly reflect photosynthetic activity and growth.
Author Contributions
Conceptualization, J.M., G.T.; methodology, J.M., K.R., G.T., A.M.S.B., G.E.L.; investigation, K.R., A.M.S.B., J.M., G.T., G.E.L.; data curation, K.R., A.M.S.B.; writing—original draft preparation, J.M.; writing—review and editing, K.R., G.T., G.E.L.; visualization, K.R., J.M.; supervision, J.M.; funding acquisition, J.M., K.R., G.E.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the EU Horizon 2020 programme (project SABANA, grant no. 727874) and in part by European Regional Development Fund—InterReg V-A Austria–Czech Republic (project Algae4Fish, grant no. ATCZ221) and by International mobility MSCA-IF III, (project no. CZ.02.2.6910.0/0.0/19_074/0014484 awarded to G.E.L.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors thank Soňa Pekařová and Michal Bureš and João Câmara Manoel for technical assistance, Tomáš Grivalský for helpful discussion, Eduard Pareis and Richard Lhotský for administrative support and Miroslav Kajan for consulting.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Beardall, J.; Raven, J.A. Carbon acquisition by microalgae. In The Physiology of Microalgae; Borowitzka, M., Beardall, J., Raven, J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 89–99. [Google Scholar]
- Richmond, A. Biological principles of mass cultivation of photoautotrophic microalgae. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Richmond, A., Hu, Q., Eds.; Wiley-Blackwell: Chichester, UK, 2013; pp. 171–204. [Google Scholar]
- Masojídek, J.; Torzillo, G. Mass Cultivation of Freshwater Microalgae. In Earth Systems and Environmental Sciences, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; p. 13. [Google Scholar]
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Algal biotechnology products and processes—Matching science and economics. J. Appl. Phycol. 1992, 4, 267–279. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Commercial production of microalgae: Ponds, tanks, tubes and fermenters. J. Biotechnol. 1999, 70, 313–321. [Google Scholar] [CrossRef]
- Richmond, A. Microalgal biotechnology at the turn of the millennium: A personal view. J. Appl. Phycol. 2000, 12, 441–451. [Google Scholar] [CrossRef]
- Grobbelaar, J.U. Upper limits of photosynthetic productivity and problems of scaling. J. Appl. Phycol. 2009, 21, 519–522. [Google Scholar] [CrossRef]
- Grobbelaar, J.U. Microalgal biomass production: Challenges and realities. Photosynth. Res. 2010, 106, 135–144. [Google Scholar] [CrossRef]
- Grobbelaar, J.U. Microalgae mass culture: The constraints of scaling-up. J. Appl. Phycol. 2012, 24, 315–318. [Google Scholar] [CrossRef]
- Posten, C.; Walter, C. Microalgal Biotechnology: Potential and Production; Walter de Gruyter: Boston, MA, USA, 2012; ISBN 9783110225020. [Google Scholar]
- Tredici, M. Mass production of microalgae: Photobioreactors. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell Science: Oxford, UK, 2004; pp. 178–214. ISBN 9780470995280. [Google Scholar]
- Acién, F.G.; Molina, E.; Reis, A.; Torzillo, G.; Zittelli, G.C.; Sepúlveda, C.; Masojídek, J. Photobioreactors for the production of microalgae. In Microalgae-Based Biofuels and Bioproducts. From Feedstock Cultivation to End-Products; Woodhead Publishing: Cambridge, UK, 2017; pp. 1–44. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Algal physiology and large-scale outdoor cultures of microalgae. In The Physiology of Microalgae; Borowitzka, M.A., Beardall, J., Raven, J.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 601–652. [Google Scholar]
- Grivalský, T.; Ranglová, K.; da Câmara Manoel, J.A.; Lakatos, G.E.; Lhotský, R.; Masojídek, J. Development of thin-layer cascades for microalgae cultivation: Milestones (review). Folia Microbiol. 2019, 64, 603–614. [Google Scholar] [CrossRef]
- Grobbelaar, J.U.; Nedbal, L.; Tichý, L.; Šetlík, L. Variation in some photosynthetic characteristics of microalgae cultured in outdoor thin-layered sloping reactors. J. Appl. Phycol. 1995, 7, 175–184. [Google Scholar] [CrossRef]
- Masojídek, J.; Kopecký, J.; Giannelli, L.; Torzillo, G. Productivity correlated to photobiochemical performance of Chlorella mass cultures grown outdoors in thin-layer cascades. J. Ind. Microbiol. Biotechnol. 2011, 38, 307–317. [Google Scholar] [CrossRef]
- Masojídek, J.; Sergejevová, M.; Malapascua, J.R.; Kopecký, J. Thin-layer systems for mass cultivation of microalgae: Flat panels and sloping cascades. In Algal Biorefineries; Springer International Publishing: Cham, Switzerland, 2015; pp. 237–261. [Google Scholar]
- Hall, D.; Rao, K. Photosynthesis, 6th ed.; Cambridge University Press: Cambridge, UK, 1999; ISBN 0-521-64497-6. [Google Scholar]
- Hill, R.; Bendall, F. Function of the two cytochrome components in chloroplasts: A working hypothesis. Nature 1960, 186, 136–137. [Google Scholar] [CrossRef]
- Masojídek, J.; Koblížek, M.; Torzillo, G. Photosynthesis in Microalgae. In Handbook of Microalgal Mass Cultures: Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell Science Ltd.: Oxford, UK, 2004; pp. 20–39. [Google Scholar]
- Mitchell, P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol. Rev. 1966, 41, 445–501. [Google Scholar] [CrossRef]
- Bolton, J.R.; Hall, D.O. The maximum efficiency of photosynthesis. Photochem. Photobiol. 1991, 53, 545–548. [Google Scholar] [CrossRef]
- Wilhelm, C.; Jakob, T. Balancing the conversion efficiency from photon to biomass. In Microalgal Biotechnology: Potential and Production; Posten, C., Walter, C., Eds.; Walter de Gruyter: Boston, MA, USA, 2012; pp. 39–53. [Google Scholar]
- Vecchi, V.; Barera, S.; Bassi, R.; Dall’Osto, L. Potential and Challenges of Improving Photosynthesis in Algae. Plants 2020, 9, 1–25. [Google Scholar]
- Raven, J.A.; Beardall, J.; Quigg, A. Light-driven oxygen consumption in the water-water cycles and photorespiration, and light stimulated mitochondrial respiration. In Photosynthesis in Algae: Biochemical and Physiological Mechanisms; Larkum, A., Grossman, A., Raven, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 161–178. [Google Scholar]
- Torzillo, G.; Sacchi, A.; Materassi, R.; Richmond, A. Effect of temperature on yield and night biomass loss in Spirulina platensis grown outdoors in tubular photobioreactors. J. Appl. Phycol. 1991, 3, 103–109. [Google Scholar] [CrossRef]
- Miyake, C.; Asada, K. The water-water cycle in algae. In Photosynthesis in Algae; Larkum, A.W.D., Douglas, S., Raven, J.A., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 184–204. [Google Scholar]
- Grobbelaar, J.U.; Soeder, C.J. Respiration losses in planktonic green algae cultivated in raceway ponds. J. Plankton Res. 1985, 7, 497–506. [Google Scholar]
- Beardall, J.; Burger-Wiersma, T.; Rijkeboer, M.; Sukenik, A.; Lemoalle, J.; Dubinsky, Z.; Fontvielle, D. Studies on enhanced post-illumination respiration in microalgae. J. Plankton Res. 1994, 16, 1401–1410. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Dubinsky, Z.; Wyman, K. Growth-irradiance relationships in phytoplankton. Limnol. Oceanogr. 1985, 30, 311–321. [Google Scholar] [CrossRef]
- Falkowski, P.; Raven, J. Aquatic Photosynthesis; Blackwell Scientific: Oxford, UK, 1997; 375p. [Google Scholar]
- Borowitzka, M.A.; Vonshak, A. Scaling up microalgal cultures to commercial scale. Eur. J. Phycol. 2017, 52, 407–418. [Google Scholar] [CrossRef]
- Torzillo, G.; Vonshak, A. Environmental Stress Physiology with Reference to Mass Cultures. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Richmond, A., Hu, Q., Eds.; Wiley-Blackwell: Chichester, UK, 2013; pp. 90–113. [Google Scholar]
- Hu, Q.; Richmond, A. Productivity and photosynthetic efficiency of Spirulina platensis as affected by light intensity, cell density and rate of mixing in a flat plate photobioreactor. J. Appl. Phycol. 1996, 8, 139–145. [Google Scholar]
- Acién, F.G.; Fernández, J.M.; Magán, J.J.; Molina, E. Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol. Adv. 2012, 30, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fernandez, C.; Muñoz, R. Microalgae-Based Biofuels and Bioproducts, 1st ed.; Woodhead Publishing, Elsevier Ltd.: Duxford, UK, 2017; ISBN 9780081010235. [Google Scholar]
- Burlew, J. Algal Culture: From Laboratory to Pilot Plant; Publication n.o. 600; Carnegie Institution of Washington: Washington, DC, USA, 1953. [Google Scholar]
- Richmond, A. Growth characteristics of ultrahigh-density microalgal cultures. Biotechnol. Bioprocess Eng. 2003, 8, 349–353. [Google Scholar] [CrossRef]
- Jerez, C.; Navarro, E.; Malpartida, I.; Rico, R.; Masojídek, J.; Abdala, R.; Figueroa, F. Hydrodynamics and photosynthesis performance of Chlorella fusca (Chlorophyta) grown in a thin-layer cascade (TLC) system. Aquat. Biol. 2014, 22, 111–122. [Google Scholar] [CrossRef]
- Emerson, R.; Arnold, W. A separation of the reactions in photosynthesis by means of intermittent light. J. Gen. Physiol. 1932, 15, 391–420. [Google Scholar] [CrossRef] [PubMed]
- Kok, B. Experiments on photosynthesis by Chlorella in flashing light. In Algal Culture from Laboratory to Pilot Plant; Burlew, J., Ed.; Carnegie Institution of Washington: Washington, DC, USA, 1953; pp. 63–75. [Google Scholar]
- Grobbelaar, J.U. Do light/dark cycles of medium frequency enhance phytoplankton productivity? J. Appl. Phycol. 1989, 1, 333–340. [Google Scholar] [CrossRef]
- Laws, E.A.; Terry, K.L.; Wickman, J.; Chalup, M.S. A simple algal production system designed to utilize the flashing light effect. Biotechnol. Bioeng. 1983, 25, 2319–2335. [Google Scholar] [CrossRef]
- Tennessen, D.J.; Bula, R.J.; Sharkey, T.D. Efficiency of photosynthesis in continuous and pulsed light emitting diode irradiation. Photosynth. Res. 1995, 44, 261–269. [Google Scholar] [CrossRef]
- Gordon, J.M.; Polle, J.E.W. Ultrahigh bioproductivity from algae. Appl. Microbiol. Biotechnol. 2007, 76, 969–975. [Google Scholar] [CrossRef]
- Zarmi, Y.; Bel, G.; Aflalo, C. Theoretical analysis of culture growth in flat-plate bioreactors: The essential role of timescales. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Richmond, A., Hu, Q., Eds.; Wiley-Blackwell: Chichester, UK, 2013; pp. 205–224. [Google Scholar]
- Matthijs, H.C.P.; Balke, H.; van Hes, U.M.; Kroon, B.M.A.; Mur, L.R.; Binot, R.A. Application of light-emitting diodes in bioreactors: Flashing light effects and energy economy in algal culture (Chlorella pyrenoidosa). Biotechnol. Bioeng. 1996, 50, 98–107. [Google Scholar] [CrossRef]
- Nedbal, L.; Tichý, V.; Xiong, F.; Grobbelaar, J.U. Microscopic green algae and cyanobacteria in high-frequency intermittent light. J. Appl. Phycol. 1996, 8, 325–333. [Google Scholar] [CrossRef]
- Larkum, A.W.D. Light-harvesting in cyanobacteria and eukaryotic algae: An overview. In Biochemical and Physiological Mechanisms; Larkum, A., Grossman, A., Raven, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 207–260. [Google Scholar]
- Moheimani, N.R.; Parlevliet, D. Sustainable solar energy conversion to chemical and electrical energy. Renew. Sustain. Energy Rev. 2013, 27, 494–504. [Google Scholar] [CrossRef]
- Schulze, P.S.C.; Barreira, L.A.; Pereira, H.G.C.; Perales, J.A.; Varela, J.C.S. Light emitting diodes (LEDs) applied to microalgal production. Trends Biotechnol. 2014, 32, 422–430. [Google Scholar] [CrossRef]
- Sergejevová, M.; Malapascua, J.R.; Kopecký, J.M.J. Photobioreactors with internal illumination. In Algal Biorefineries: Volume 2: Products and Refinery Design; Prokop, A., Bajpai, R.K., Zappi, M.E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 237–262. ISBN 9783319202006. [Google Scholar]
- Polle, J.; Kanakagiri, S.; Jin, E.; Masuda, T.; Melis, A. Truncated chlorophyll antenna size of the photosystems—a practical method to improve microalgal productivity and hydrogen production in mass culture. Int. J. Hydrogen Energy 2002, 27, 1257–1264. [Google Scholar] [CrossRef]
- Raven, J.A.; Ralph, P.J. Enhanced biofuel production using optimality, pathway modification and waste minimization. J. Appl. Phycol. 2015, 27, 1–31. [Google Scholar] [CrossRef]
- Benemann, J. The future of microalgal biotechnology. In Algal and Cyanobacteria Biotechnology; Cresswell, R., Rees, T., Shah, N., Eds.; Longman Scientific and Technical: New York, NY, USA, 1989; pp. 317–337. [Google Scholar]
- Polle, J.; Kanakagiri, S.-D.; Melis, A. tla1, a DNA insertional transformant of the green alga Chlamydomonas reinhardtii with a truncated light-harvesting chlorophyll antenna size. Planta 2003, 217, 49–59. [Google Scholar]
- Mitra, M.; Kirst, H.; Dewez, D.; Melis, A. Modulation of the light-harvesting chlorophyll antenna size in Chlamydomonas reinhardtii by TLA1 gene over-expression and RNA interference. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3430–3443. [Google Scholar] [CrossRef]
- Perrine, Z.; Negi, S.; Sayre, R.T. Optimization of photosynthetic light energy utilization by microalgae. Algal Res. 2012, 1, 134–142. [Google Scholar] [CrossRef]
- Cazzaniga, S.; Dall’Osto, L.; Szaub, J.; Scibilia, L.; Ballottari, M.; Purton, S.; Bassi, R. Domestication of the green alga Chlorella sorokiniana: Reduction of antenna size improves light-use efficiency in a photobioreactor. Biotechnol. Biofuels 2014, 7, 157. [Google Scholar] [CrossRef]
- De Mooij, T.; Janssen, M.; Cerezo-chinarro, O.; Mussgnug, J.H. Antenna size reduction as a strategy to increase biomass productivity: A great potential not yet realized. J. Appl. Phycol. 2015, 27, 1063–1077. [Google Scholar] [CrossRef]
- Chow, Y.; Thung, L. Quantifying the competitive advantage of light green algal cells in batch culturetle. J. Appl. Phycol. 2015, 27, 1805–1812. [Google Scholar]
- Masojídek, J.; Ranglová, K.; Torzillo, G.; Celis Pla, P.; Rearte, T.A.; Silva Benavides, A.M.; Neori, A.; Gómez, C.; Caporgno, M.P.; Alvárez Gómez, F.; et al. Changes in photosynthesis, growth and biomass composition in outdoor Chlorella g120 culture during the metabolic shift from heterotrophic to phototrophic cultivation regime. Algal Res. 2021, 56, 102303. [Google Scholar] [CrossRef]
- Babaei, A.; Ranglová, K.; Malapascua, J.R.; Torzillo, G.; Shayegan, J.; Silva Benavides, A.M.; Masojídek, J. Photobiochemical changes in Chlorella g120 culture during trophic conversion (metabolic pathway shift) from heterotrophic to phototrophic growth regime. J. Appl. Phycol. 2020. [Google Scholar] [CrossRef]
- Mylenko, M.; Vu, D.L.; Kuta, J.; Ranglová, K.; Kubáč, D.; Lakatos, G.; Grivalský, T.; Caporgno, M.; Câmara Manoel, J.; Kopecký, J.; et al. Selenium incorporation to amino acids in Chlorella cultures grown in phototrophic and heterotrophic regime. J. Agric. Food Chem. 2019, 68, 1654–1665. [Google Scholar]
- Touloupakis, E.; Faraloni, C.; Silva Benavides, A.M.; Masojidek, J.; Torzillo, G. Sustained photobiological hydrogen production by Chlorella vulgaris without nutient starvation. Int. J. Hydrogen Energy 2021, 46, 3684–3694. [Google Scholar]
- Masojídek, J.; Torzillo, G.; Koblížek, M. Photosynthesis in microalgae mass cultures. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Richmond, A., Hu, Q., Eds.; Wiley-Blackwell: Chichester, UK, 2013; pp. 21–36. ISBN 9781118567166. [Google Scholar]
- Pirt, S.J.; Lee, Y.-K.; Richmond, A.; Pirt, M.W. The photosynthetic efficiency of Chlorella biomass growth with reference to solar energy utilisation. J. Chem. Technol. Biotechnol. 1980, 30, 25–34. [Google Scholar] [CrossRef]
- Blache, U.; Jakob, T.; Su, W.; Wilhelm, C. The impact of cell-specific absorption properties on the correlation of electron transport rates measured by chlorophyll fluorescence and photosynthetic oxygen production in planktonic algae. Plant Physiol. Biochem. 2011, 49, 801–808. [Google Scholar] [CrossRef]
- Malapascua, J.R.F.; Jerez, C.G.; Sergejevová, M.; Figueroa, F.L.; Masojídek, J. Photosynthesis monitoring to optimize growth of microalgal mass cultures: Application of chlorophyll fluorescence techniques. Aquat. Biol. 2014, 22, 123–140. [Google Scholar] [CrossRef]
- Nixon, P.J.; Michoux, F.; Yu, J.; Boehm, M.; Komenda, J. Recent advances in understanding the assembly and repair of photosystem II. Ann. Bot. 2010, 106, 1–16. [Google Scholar] [CrossRef]
- Campbell, D.A.; Tyystjärvi, E. Parameterization of photosystem II photoinactivation and repair. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 258–265. [Google Scholar] [CrossRef]
- Raven, J.A. The cost of photoinhibition. Physiol. Plant. 2011, 142, 87–104. [Google Scholar] [CrossRef]
- Richmond, A. Biological principles of mass cultivation. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell Science: Oxford, UK, 2004; pp. 125–177. ISBN 9780470995280. [Google Scholar]
- Qiang, H.; Zarmi, Y.; Richmond, A. Combined effects of light intensity, light-path and culture density on output rate of Spirulina platensis (cyanobacteria). Eur. J. Phycol. 1998, 33, 165–171. [Google Scholar] [CrossRef]
- Walker, D.A. Biofuels, facts, fantasy, and feasibility. J. Appl. Phycol. 2009, 21, 509–517. [Google Scholar] [CrossRef]
- Tredici, M.R. Photobiology of microalgae mass cultures: Understanding the tools for the next green revolution. Biofuels 2010, 1, 143–162. [Google Scholar] [CrossRef]
- Ranglová, K.; Lakatos, G.E.; Manoel, J.A.C.; Grivalský, T.; Masojídek, J. Rapid screening test to estimate temperature optima for microalgae growth using photosynthesis activity measurements. Folia Microbiol. 2019. [Google Scholar] [CrossRef]
- Torzillo, G.; Vonshak, A. Effect of light and temperature on the photosynthetic activity of the cyanobacterium Spirulina platensis. Biomass Bioenergy 1994, 6, 399–403. [Google Scholar] [CrossRef]
- Ras, M.; Steyer, J.-P.; Bernard, O. Temperature effect on microalgae: A crucial factor for outdoor production. Rev. Environ. Sci. Bio Technol. 2013, 12, 153–164. [Google Scholar] [CrossRef]
- Raven, J.A.; Geider, R.J. Temperature and algal growth. New Phytol. 1988, 110, 441–461. [Google Scholar] [CrossRef]
- Maxwell, D.P.; Falk, S.; Trick, C.G.; Huner, N.P.A. Growth at low-temperature mimics high-light acclimation in Chlorella-vulgaris. Plant Physiol. 1994, 105, 535–543. [Google Scholar] [CrossRef]
- Carneiro, M.; Cicchi, B.; Maia, I.B.; Pereira, H.; Zittelli, G.C.; Varela, J.; Malcata, F.X.; Torzillo, G. Effect of temperature on growth, photosynthesis and biochemical composition of Nannochloropsis oceanica, grown outdoors in tubular photobioreactors. Algal Res. 2020, 49, 101923. [Google Scholar] [CrossRef]
- Chini Zittelli, G.; Lavista, F.; Bastianini, A.; Rodolfi, L.; Vincenzini, M.; Tredici, M.R. Production of eicosapentaenoic acid by Nannochloropsis sp. cultures in outdoor tubular photobioreactors. J. Biotechnol. 1999, 70, 299–312. [Google Scholar] [CrossRef]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B Biol. 2014, 137, 116–126. [Google Scholar] [CrossRef]
- Zheng, Y.; Xue, C.; Chen, H.; He, C.; Wang, Q. Low-Temperature Adaptation of the Snow Alga Chlamydomonas nivalis Is Associated With the Photosynthetic System Regulatory Process. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Torzillo, G.; Pushparaj, B.; Bocci, F.; Balloni, W.; Materassi, R.; Florenzano, G. Production of Spirulina biomass in closed photobioreactors. Biomass 1986, 11, 61–74. [Google Scholar] [CrossRef]
- Belay, A. Mass culture of Spirulina outdoors—The Earthrise Farm experience. In Spirulina platensis (Arthrospira): Physiology, Cell-biology and Biotechnology; Vonshak, A., Ed.; Springer Science+Business Media: Berlin/Heidelberg, Germany, 1997; pp. 131–158. [Google Scholar]
- Barati, B.; Gan, S.-Y.; Lim, P.-E.; Beardall, J.; Phang, S.-M. Green algal molecular responses to temperature stress. Acta Physiol. Plant. 2019, 41, 26. [Google Scholar] [CrossRef]
- Asada, K. Mechanisms for scavenging reactive molecules generated in chloroplasts under light stress. In Photoinhibition of Photosynthesis: From Molecular Mechanisms to the Field; Baker, N.B., Bowyer, J.R., Eds.; BIOS Scientific Publishing Limited: Oxford, UK, 1994; pp. 129–142. [Google Scholar]
- Nishiyama, Y.; Murata, N. Revised scheme for the mechanism of photoinhibition and its application to enhance the abiotic stress tolerance of the photosynthetic machinery. Appl. Microbiol. Biotechnol. 2014, 98, 8777–8796. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; van Gollery, M.B.F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Dietz, K.-J.; Turkan, I.; Krieger-Liszkay, A. Redox- and Reactive Oxygen Species-Dependent Signaling into and out of the Photosynthesizing Chloroplast. Plant Physiol. 2016, 171, 1541–1550. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Ye, Z.-W.; Jiang, J.-G.; Wu, G.-H. Biosynthesis and regulation of carotenoids in Dunaliella: Progresses and prospects. Biotechnol. Adv. 2008, 26, 352–360. [Google Scholar] [CrossRef]
- Vidhyavathi, R.; Venkatachalam, L.; Sarada, R.; Ravishankar, G.A. Regulation of carotenoid biosynthetic genes expression and carotenoid accumulation in the green alga Haematococcus pluvialis under nutrient stress conditions. J. Exp. Bot. 2008, 59, 1409–1418. [Google Scholar] [CrossRef]
- Rearte, T.A.; Celis-Plá, P.S.M.; Neori, A.; Masojídek, J.; Torzillo, G.; Gómez-Serrano, C.; Silva Benavides, A.M.; Álvarez-Gómez, F.; Abdala-Díaz, R.T.; Ranglová, K.; et al. Photosynthetic performance of Chlorella vulgaris R117 mass culture is moderated by diurnal oxygen gradients in an outdoor thin layer cascade. Algal Res. 2021, 54, 102176. [Google Scholar] [CrossRef]
- Rubio, C.; Fernández, A.; Pérez, S.; Camacho, G.; Grima, M.; Rubio, F.C.; Acie, F.G.; Grima, E.M. Prediction of dissolved oxygen and carbon dioxide concentration profiles in tubular photobioreactors for microalgal culture. Biotechnol. Bioeng. 1999, 62, 71–86. [Google Scholar] [CrossRef]
- Hidasi, N.; Belay, A. Diurnal variation of various culture and biochemical parameters of Arthrospira platensis in large-scale outdoor raceway ponds. Algal Res. 2018, 29, 121–129. [Google Scholar] [CrossRef]
- Lívanský, K. Dependence of the apparent CO2 mass transfer coefficient KLa on the nutrient solution pH in outdoor algal culture units. Arch. Hydrobiol. Suppl. Algol. Stud. 1993, 71, 111–119. [Google Scholar]
- Beardall, J.; Raven, J. Structural and biochemical features of carbon acquisition in algae. In Photosynthesis in algae: Biochemical and physiological mechanisms; Larkum, A., Grossman, A., Raven, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 141–160. [Google Scholar]
- Beardall, J.; Quigg, A.; Raven, J. Oxygen consumption: Photorespiration and chlororespiration. In Photosynthesis in Algae; Larkum, A., Douglas, S., Raven, J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 157–181. [Google Scholar]
- Hough, R.A.; Wetzel, R.G. Photorespiration and CO2 compensation point in Najas flexilis. Limnol. Oceanogr. 1978, 23, 719–724. [Google Scholar] [CrossRef]
- Badger, M.R.; von Caemmerer, S.; Ruuska, S.; Nakano, H. Electron flow to oxygen in higher plants and algae: Rates and control of direct photoreduction (Mehler reaction) and rubisco oxygenase. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2000, 355, 1433–1446. [Google Scholar] [CrossRef]
- Vonshak, A.; Torzillo, G.; Masojidek, J.; Boussiba, S. Sub-optimal morning temperature induces photoinhibition in dense outdoor cultures of the alga Monodus subterraneus (Eustigmatophyta). Plant. Cell Environ. 2001, 24, 1113–1118. [Google Scholar] [CrossRef]
- Torzillo, G.; Accolla, P.; Pinzani, E.; Masojidek, J. In situ monitoring of chlorophyll fluorescence to assess the synergistic effect of low temperature and high irradiance stresses in Spirulina cultures grown outdoors in photobioreactors. J. Appl. Phycol. 1996, 8, 283–291. [Google Scholar] [CrossRef]
- Torzillo, G.; Chini Zittelli, G.; Silva Benavides, A.M.; Ranglova, K.; Masojidek, J. Culturing of microalgae for food applications. In Cultured Microalgae in the Food Industry; Lafarga, T., Acien-Fernandez, F.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Iwamoto, H. Chlorella. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell Science Ltd.: Oxford UK, 2004; pp. 255–263. [Google Scholar]
- Ben-Amotz, A. Industrial Production of Microalgal Cell-mass and Secondary Products – Major Industrial Species: Dunaliella. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell Science Ltd.: Oxford UK, 2004; pp. 273–280. [Google Scholar]
- Cysewski, G.R.; Todd Lorenz, R. Industrial Production of Microalgal Cell-mass and Secondary Products—Species of High Potential: Haematococcus. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell Science Ltd.: Oxford, UK, 2004; pp. 281–288. [Google Scholar]
- Chini Zittelli, G.; Rodolfi, L.; Tredici, M.R. Industrial Production of Microalgal Cell-mass and Secondary Products – Species of High Potential: Mass Cultivation of Nannochloropsis in Closed Systems. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell Science Ltd.: Oxford, UK, 2004; pp. 298–303. [Google Scholar]
- Janssen, M.; de Bresser, L.; Baijens, T.; Tramper, J.; Mur, L.; Snel, J.; Wijffels, R. Scale-up aspects of photobioreactors: Effects of mixing-induced light/dark cycles. J. Appl. Phycol. 2000, 12, 225–237. [Google Scholar]
- Falkowski, P.; Raven, J. Aquatic Photosynthesis, 2nd ed.; Blackwell Science: Malden, MA, USA, 2007; 488p. [Google Scholar]
- Sutherland, D.L.; Howard-Williams, C.; Turnbull, M.H.; Broady, P.A.; Craggs, R.J. The effects of CO2 addition along a pH gradient on wastewater microalgal photo-physiology, biomass production and nutrient removal. Water Res. 2015, 70, 9–26. [Google Scholar] [CrossRef]
- Moheimani, N.R.; Borowitzka, M.A. Increased CO2 and the effect of pH on growth and calcification of Pleurochrysis carterae and Emiliania huxleyi (Haptophyta) in semicontinuous cultures. Appl. Microbiol. Biotechnol. 2011, 90, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Colman, B.; Huertas, I.E.; Bhatti, S.; Dason, J.S. The diversity of inorganic carbon acquisition mechanisms in eukaryotic microalgae. Funct. Plant Biol. 2002, 29, 261. [Google Scholar] [CrossRef] [PubMed]
- Lívanský, K.; Doucha, J. CO2 and O2 gas exchange in outdoor thin-layer high density microalgal cultures. J. Appl. Phycol. 1996, 8, 353–358. [Google Scholar] [CrossRef]
- Raven, J.A.; Giordano, M.; Beardall, J.; Maberly, S.C. Algal and aquatic plant carbon concentrating mechanisms in relation to environmental change. Photosynth. Res. 2011, 109, 281–296. [Google Scholar] [CrossRef]
- Raven, J.A.; Giordano, M.; Beardall, J.; Maberly, S.C. Algal evolution in relation to atmospheric CO2: Carboxylases, carbon-concentrating mechanisms and carbon oxidation cycles. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 493–507. [Google Scholar] [CrossRef]
- Grobbelaar, J.U. Inorganic Algal Nutrition. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Richmond, A., Hu, Q., Eds.; Wiley-Blackwell: Chichester, UK, 2013; pp. 123–133. ISBN 9781118567166. [Google Scholar]
- Oswald, W. Large-scale algal culture systems (engineering aspects). In Microalgal Biotechnology; Borowitzka, M., Borowitzka, L., Eds.; Cambridge University Press: Cambridge, UK, 1988; pp. 357–395. [Google Scholar]
- Beardall, J.; Young, E.; Roberts, S. Approaches for determining phytoplankton nutrient limitation. Aquat. Sci. 2001, 63, 44–69. [Google Scholar] [CrossRef]
- Geider, R.; La Roche, J. Redfield revisited: Variability of C:N:P in marine microalgae and its biochemical basis. Eur. J. Phycol. 2002, 37, 1–17. [Google Scholar] [CrossRef]
- Raven, J.A.; Giordano, M. Combined nitrogen. In The Physiology of Microalgae; Borowitzka, M., Beardall, J., Raven, J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 143–154. [Google Scholar]
- Cembella, A.D.; Antia, N.J.; Harrison, P.J. The utilization of inorganic and organic phosphorous compounds as nutrients by eukaryotic microalgae: A multidisciplinary perspective: Part, I. CRC Crit. Rev. Microbiol. 1982, 10, 317–391. [Google Scholar] [CrossRef]
- O’Kelley, J.C. Inorganic nutrients. In Algal Physiology and Biochemistry; Steward, W., Ed.; Blackwell Scientific Publications: London, UK, 1974; pp. 610–635. [Google Scholar]
- Finkel, Z.V.; Beardall, J.; Flynn, K.J.; Quigg, A.; Rees, T.A.V.; Raven, J.A. Phytoplankton in a changing world: Cell size and elemental stoichiometry. J. Plankton Res. 2010, 32, 119–137. [Google Scholar] [CrossRef]
- Quigg, A. Micronutrients. In The Physiology of Microalgae; Borowitzka, M., Beardall, J., Raven, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 211–231. [Google Scholar]
- Marchetti, A.; Maldonado, M.T. Iron. In The Physiology of Microalgae; Borowitzka, M., Beadrdall, J., Raven, J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 233–279. [Google Scholar]
- Behrenfeld, M.J.; Westberry, T.K.; Boss, E.S.; O’Malley, R.T.; Siegel, D.A.; Wiggert, J.D.; Franz, B.A.; McClain, C.R.; Feldman, G.C.; Doney, S.C.; et al. Satellite-detected fluorescence reveals global physiology of ocean phytoplankton. Biogeosciences 2009, 6, 779–794. [Google Scholar] [CrossRef]
- Šetlík, I.; Šust, V.; Málek, I. Dual purpose open circulation units for large scale culture of algae in temperate zones. I. Basic design considerations and scheme of a pilot plant. Arch. Hydrobiol. Suppl. Algol. Stud. 1970, 1, 111–164. [Google Scholar]
- Richmond, A.; Cheng-Wu, Z. Optimization of a flat plate glass reactor for mass production of Nannochloropsis sp. outdoors. J. Biotechnol. 2001, 85, 259–269. [Google Scholar] [CrossRef]
- Zittelli, G.C.; Rodolfi, L.; Bassi, N.; Biondi, N.; Tredici, M.R. Photobioreactors for Microalgal Biofuel Production. In Algae for Biofuels and Energy; Borowitzka, M.A., Moheimani, N.R., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 115–131. ISBN 978-94-007-5479-9. [Google Scholar]
- Assunção, J.; Malcata, F.X. Enclosed ”non-conventional” photobioreactors for microalga production: A review. Algal Res. 2020, 52, 102107. [Google Scholar]
- Zittelli, G.C.; Biondi, N.; Rodolfi, L.; Tredici, M.R. Photobioreactors for Mass Production of Microalgae. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Richmond, A., Hu, Q., Eds.; Wiley-Blackwell: Chichester, UK, 2013; pp. 225–266. [Google Scholar]
- Narala, R.R.; Garg, S.; Sharma, K.K.; Thomas-Hall, S.R.; Deme, M.; Li, Y.; Schenk, P.M. Comparison of Microalgae Cultivation in Photobioreactor, Open Raceway Pond, and a Two-Stage Hybrid System. Front. Energy Res. 2016, 4, 1–10. [Google Scholar] [CrossRef]
- Chisti, Y. Raceways-based production of algal crude oil. In Microalgal Biotechnology: Potential and Production; Clemens, P., Christian, W., Eds.; Walter de Gruyter: Boston, MA, USA, 2012; pp. 113–146. [Google Scholar]
- Chisti, Y. Constraints to commercialization of algal fuels. J. Biotechnol. 2013, 167, 201–214. [Google Scholar] [CrossRef]
- Norsker, N.-H.; Barbosa, M.J.; Vermuë, M.H.; Wijffels, R.H. Microalgal production—A close look at the economics. Biotechnol. Adv. 2011, 29, 24–27. [Google Scholar] [CrossRef]
- De Vree, J.H.; Bosma, R.; Janssen, M.; Barbosa, M.J.; Wijffels, R.H. Biotechnology for biofuels: Comparison of four outdoor pilot—scale photobioreactors. Biotechnol. Biofuels 2015, 1–12. [Google Scholar] [CrossRef]
- Posten, C. Design principles of photo-bioreactors for cultivation of microalgae. Eng. Life Sci. 2009, 9, 165–177. [Google Scholar] [CrossRef]
- Cuaresma, M.; Janssen, M.; End, E.J.; Vílchez, C.; Wijffels, R.H. Luminostat operation: A tool to maximize microalgae photosynthetic efficiency in photobioreactors during the daily light cycle? Bioresour. Technol. 2011, 102. [Google Scholar] [CrossRef]
- Del Mar Morales-Amaral, M.; Gómez-Serrano, C.; Acién, F.G.; Fernández-Sevilla, J.M.; Molina-Grima, E. Production of microalgae using centrate from anaerobic digestion as the nutrient source. Algal Res. 2015, 9, 297–305. [Google Scholar] [CrossRef]
- Wagner, H.; Liu, Z.; Langner, U.; Stehfest, K.; Wilhelm, C. The use of FTIR spectroscopy to assess quantitative changes in the biochemical composition of microalgae. J. Biophotonics 2010, 3, 557–566. [Google Scholar] [CrossRef]
- White, S.; Anandraj, A.; Bux, F. PAM fluorometry as a tool to assess microalgal nutrient stress and monitor cellular neutral lipids. Bioresour. Technol. 2011, 102, 1675–1682. [Google Scholar] [CrossRef]
- Jerez, C.G.; Malapascua, J.R.; Sergejevová, M.; Figueroa, F.L.; Masojídek, J. Effect of nutrient starvation under high irradiance on lipid and starch accumulation in Chlorella fusca (Chlorophyta). Mar. Biotechnol. 2016, 18, 24–36. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Perin, G.; Cimetta, E.; Monetti, F.; Morosinotto, T.; Bezzo, F. Novel micro-photobioreactor design and monitoring method for assessing microalgae response to light intensity. Algal Res. 2016, 19, 69–76. [Google Scholar] [CrossRef]
- Dall’Osto, L.; Cazzaniga, S.; Guardini, Z.; Barera, S.; Bemedett, M.; Mannino, G.; Maffei, M.E.; Bassi, R. Combined resistance to oxidative stress and reduced antenna size enhance light-to-biomass conversion efficiency in Chlorella vulgaris cultures. Biotechnol. Biofuels 2019, 12, 1–17. [Google Scholar]
- Treves, H.; Raanan, H.; Finkel, O.M.; Berkowicz, S.M.; Keren, N.; Shotland, Y.; Kaplan, A. A newly isolated Chlorella sp. from desert sand crusts exhibits a unique resistance to excess light intensity. FEMS Microbiol. Ecol. 2013, 86, 373–380. [Google Scholar]
- Karkehabadi, S.; Peddi, S.R.; Anwaruzzaman, M.; Taylor, T.C.; Cederlund, A.; Genkov, T.; Andersson, I.; Spreitzer, R.J. Chimeric Small Subunits Influence Catalysis without Causing Global Conformational Changes in the Crystal Structure of Ribulose-1,5-bisphosphate Carboxylase/Oxygenase. Biochemistry 2005, 44, 9851–9861. [Google Scholar] [CrossRef]
- Larson, E.M.; O’Brien, C.M.; Zhu, G.; Spreitzer, R.J.; Portis, A.R. Specificity for Activase Is Changed by a Pro-89 to Arg Substitution in the Large Subunit of Ribulose-1,5-bisphosphate Carboxylase/Oxygenase. J. Biol. Chem. 1997, 272, 17033–17037. [Google Scholar] [CrossRef]
- Ott, C.M.; Smith, B.D.; Portis, A.R.; Spreitzer, R.J. Activase Region on Chloroplast Ribulose-1,5-bisphosphate Carboxylase/Oxygenase. J. Biol. Chem. 2000, 275, 26241–26244. [Google Scholar] [CrossRef]
- Fang, L.; Lin, H.X.; Low, C.S.; Wu, M.H.; Chow, Y.; Lee, Y.K. Expression of the Chlamydomonas reinhardtii Sedoheptulose-1,7-bisphosphatase in Dunaliella bardawil leads to enhanced photosynthesis and increased glycerol production. Plant Biotechnol. J. 2012, 10, 1129–1135. [Google Scholar] [CrossRef]
- Liang, F.; Englund, E.; Lindberg, P.; Lindblad, P. Engineered cyanobacteria with enhanced growth show increased ethanol production and higher biofuel to biomass ratio. Metab. Eng. 2018, 46, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, N.M.D.; Parisien, A.; Wang, B.; Lan, C.Q. Enhancement of lipid production using biochemical, genetic and transcription factor engineering approaches. J. Biotechnol. 2009, 141. [Google Scholar] [CrossRef]
- Kao, P.-H.; Ng, I.-S. CRISPRi mediated phosphoenolpyruvate carboxylase regulation to enhance the production of lipid in Chlamydomonas reinhardtii. Bioresour. Technol. 2017, 245, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Leu, S.; Boussiba, S. Advances in the Production of High-Value Products by Microalgae. Ind. Biotechnol. 2014, 10, 169–183. [Google Scholar] [CrossRef]
- Jin, E.S.; Polle, J.E.W.; Lee, H.K.; Hyun, S.M.; Chang, M. Xanthophylls in microalgae: From biosynthesis to biotechnological mass production and application. J. Microbiol. Biotechnol. 2003, 13, 165–174. [Google Scholar]
- Steinbrenner, J.; Sandmann, G. Transformation of the Green Alga Haematococcus pluvialis with a Phytoene Desaturase for Accelerated Astaxanthin Biosynthesis. Appl. Environ. Microbiol. 2006, 72, 7477–7484. [Google Scholar] [CrossRef]
- Kathiresan, S.; Chandrashekar, A.; Ravishankar, G.A.; Sarada, R. Regulation of astaxanthin and its intermediates through cloning and genetic transformation of β-carotene ketolase in Haematococcus pluvialis. J. Biotechnol. 2015, 196–197, 33–41. [Google Scholar] [CrossRef]
- Galarza, J.I.; Gimpel, J.A.; Rojas, V.; Arredondo-Vega, B.O.; Henríquez, V. Over-accumulation of astaxanthin in Haematococcus pluvialis through chloroplast genetic engineering. Algal Res. 2018, 31, 291–297. [Google Scholar] [CrossRef]
- Kromkamp, J.C.; Forster, R.M. The use of variable fluorescence measurements in aquatic ecosystems: Differences between multiple and single turnover measuring protocols and suggested terminology. Eur. J. Phycol. 2003, 38, 103–112. [Google Scholar] [CrossRef]
- Ralph, P.J.; Gademann, R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Enríquez, S.; Borowitzka, M.A. The use of the fluorescence signal in studies of seagrasses and macroalgae. In Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications; Springer: Dordrecht, The Netherlands, 2010; pp. 187–208. [Google Scholar]
- Torzillo, G.; Faraloni, C.; Silva, A.M.; Kopecký, J.; Pilný, J.; Masojídek, J. Photoacclimation of Phaeodactylum tricornutum (Bacillariophyceae) cultures grown in outdoors photobioreactors and open ponds. Eur. J. Phycol. 2012, 47, 169–181. [Google Scholar]
- Oh, H.M.; Maeng, J.; Rhee, G.-Y. Nitrogen and carbon fixation by Anabaena sp. isolated from a rice paddy and grown under P and light limitations. J. Appl. Phycol. 1991, 3, 335–343. [Google Scholar] [CrossRef]
- Walker, D.A. Polarographic measurement of oxygen. In Photosynthesis and Production in a Changing Environment; Hall, O., Scurlock, J., Bolha-Nordenkampf, H., Leegood, R., Long, S., Eds.; Chapman & Hall: London, UK, 1993; pp. 168–180. [Google Scholar]
- Van Gorkom, H.J.; Gast, P. Measurement of photosynthetic oxygen evolution. In Biophysical Techniques in Phytosynthesis; Amesz, J., Hoff, A., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; pp. 391–405. [Google Scholar]
- Sánchez Zurano, A.; Gómez Serrano, C.; Acién-Fernández, F.G.; Fernández-Sevilla, J.M.; Molina-Grima, E. Modeling of photosynthesis and respiration rate for microalgae–bacteria consortia. Biotechnol. Bioeng. 2021, 118, 952–962. [Google Scholar] [CrossRef]
- Knoppová, J.; Pokorný, J.; Masojídek, J. Chlorophyll fluorescence quenching caused by inorganic carbon depletion in the green alga Scenedesmus quadricauda. Photosynthetica 1993, 28, 281–288. [Google Scholar]
- Vonshak, A.; Torzillo, G.; Tomaseli, L. Use of chlorophyll fluorescence to estimate the effect of photoinhibition in outdoor cultures of Spirulina platensis. J. Appl. Phycol. 1994, 6, 31–34. [Google Scholar] [CrossRef]
- Torzillo, G.; Bernardini, P.; Masojídek, J. On-line monitoring of fluorescence to access the extent of photoinhibition of photosynthesis induced by high oxygen concentration and low temperature and its effect on the productivity of outdoor cultures of Spirulina platensis (Cyanobacteria). J. Phycol. 1998, 34, 504–510. [Google Scholar] [CrossRef]
- Masojídek, J.; Trivedi, S.; Halshaw, L.; Alexiou, A.; Hall, D.O. The synergistic effect of drought and light stresses in sorghum and pearl millet. Plant Physiol. 1991, 96, 198–207. [Google Scholar] [CrossRef]
- Masojídek, J.; Torzillo, G.; Koblížek, M.; Kopecký, J.; Bernardini, P.; Sacchi, A.; Komenda, J. Photoadaptation of two members of the Chlorophyta (Scenedesmus and Chlorella) in laboratory and outdoor cultures: Changes in chlorophyll fluorescence quenching and the xanthophyll cycle. Planta 1999, 209, 126–135. [Google Scholar] [CrossRef]
- Masojídek, J.; Torzillo, G.; Kopecký, J.; Koblížek, M.; Nidiaci, L.; Komenda, J.; Lukavská, A.; Sacchi, A. Changes in chlorophyll fluorescence quenching and pigment composition in the green alga Chlorococcum sp. grown under nitrogen deficiency and salinity stress. J. Appl. Phycol. 2000, 12, 417–426. [Google Scholar] [CrossRef]
- Masojidek, J. Photosystem II Electron Transport Rates and Oxygen Production in Natural Waterblooms of Freshwater Cyanobacteria During a Diel Cycle. J. Plankton Res. 2001, 23, 57–66. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis In Vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, F.L.; Jerez, C.G.; Korbee, N. Use of in vivo chlorophyll fluorescence to estimate photosynthetic activity and biomass productivity in microalgae grown in different culture systems. Lat. Am. J. Aquat. Res. 2013, 41, 801–819. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.; Srivastava, A.; Tsimili-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing, Photosynthesis Mechanisms, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor & Francis: London, UK, 2004; pp. 445–483. [Google Scholar]
- Schreiber, U. Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: An overview. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Springe: Dordrecht, The Netherlands, 2004; pp. 279–319. [Google Scholar]
- Masojídek, J.; Vonshak, A.; Torzillo, G. Chlorophyll Fluorescence Applications In Microalgal Mass Cultures. In Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications Developments in Applied Phycology; Suggett, D.J., Prášil, O., Borowitzka, M.A., Eds.; Springer Science+Business Media B.V.: Berlin/Heidelberg, Germany, 2011; pp. 277–292. ISBN 978-90-481-9268-7. [Google Scholar]
- Masojídek, J.; Papáček, Š.; Sergejevová, M.; Jirka, V.; Červený, J.; Kunc, J.; Korečko, J.; Verbovikova, O.; Kopecký, J.; Štys, D.; et al. A closed solar photobioreactor for cultivation of microalgae under supra-high irradiance: Basic design and performance. J. Appl. Phycol. 2003, 15, 239–248. [Google Scholar] [CrossRef]
- Masojídek, J.; Sergejevová, M.; Rottnerová, K.; Jirka, V.; Korečko, J.; Kopecký, J.; Zaťková, I.; Torzillo, G.; Štys, D. A two-stage solar photobioreactor for cultivation of microalgae based on solar concentrators. J. Appl. Phycol. 2009, 21, 55–63. [Google Scholar] [CrossRef]
- Strasser, B.J.; Strasser, R.J. Measuring fast fluorescence transients to address environmental questions: The JIP test. In Photosynthesis from Light to Biosphere; Mathis, P., Ed.; Kluwer: Dordrecht, The Netherlands, 1995; Volume 5, pp. 977–980. ISBN 978-0-7923-3862-8. [Google Scholar]
- Srivastava, A.; Strasser, R.J. Govindjee Polyphasic rise of chlorophyll a fluorescence in herbicide-resistant D1 mutants of Chlamydomonas reinardtii. Photosynth. Res. 1995, 43, 131–141. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. ISBN 978-1-4020-3218-9. [Google Scholar]
- Goltsev, V.N.; Kalaji, H.M.; Paunov, M.; Bąba, W.; Horaczek, T.; Mojski, J.; Kociel, H.; Allakhverdiev, S.I. Variable chlorophyll fluorescence and its use for assessing physiological condition of plant photosynthetic apparatus. Russ. J. Plant Physiol. 2016, 63, 869–893. [Google Scholar] [CrossRef]
- Stirbet, A.G. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B 2011, 104, 236–254. [Google Scholar]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef]
- Matthijs, H.C.P.; Ludérus, E.M.E.; Löffler, H.J.M.; Scholts, M.J.C.; Kraayenhof, R. Energy metabolism in the cyanobacterium Plectonema boryanum. Participation of the thylakoid photosynthetic electron transfer chain in the dark respiration of NADPH and NADH. Biochim. Biophys. Acta Bioenerg. 1984, 766, 29–37. [Google Scholar] [CrossRef]
- Schuurmans, R.M.; van Alphen, P.; Schuurmans, J.M.; Matthijs, H.C.P.; Hellingwerf, K.J. Comparison of the photosynthetic yield of cyanobacteria and green algae: Different methods give different answers. PLoS ONE 2015, 10, e0139061. [Google Scholar] [CrossRef]
- Kromkamp, J.C.; Beardall, J.; Sukenik, A.; Kopecky, J.; Masojidek, J.; van Bergeijk, S.; Gabai, S.; Shaham, E.; Yamshon, A. Short-term variations in photosynthetic parameters of Nannochloropsis cultures grown in two types of outdoor mass cultivation systems. Aquat. Microb. Ecol. 2009, 56, 309–322. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A. Govindjee Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Johnsen, G.; Sakshaug, E. Biooptical characteristics of PSII and PSI in 33 species (13 pigment groups) of marine phytoplankton, and the relevance for pulse-amplitude-modulated and fast-repetition-rate fluorometry. J. Phycol. 2007, 43, 1236–1251. [Google Scholar] [CrossRef]
- Bilger, W.; Björkman, O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef]
- Narayan, A.; Misra, M.; Singh, R. Chlorophyll Fluorescence in Plant Biology. Biophysics 2012, 7, 171–192. [Google Scholar] [CrossRef]
- Masojidek, J.; Kopecky, J.; Koblizek, M.; Torzillo, G. The xanthophyll cycle in green algae (Chlorophyta): Its rle in the photosynthetic apparatus. Plant Biol. 2004, 6, 342–349. [Google Scholar]
- Quaas, T.; Berteotti, S.; Ballottari, M.; Flieger, K.; Bassi, R.; Wilhelm, C.; Goss, R. Non-photochemical quenching and xanthophyll cycle activities in six green algal species suggest mechanistic di erences in the process of excess energy dissipation. J. Plant Physiol. 2015, 172, 92–103. [Google Scholar]
- Demmig, B.; Winter, K.; Kruger, A.; Czygan, F.C. Photoinhibition and zeaxanthin formation in intact leaves—A possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol 1987, 84, 218–224. [Google Scholar]
- Schreiber, U.; Endo, T.; Mi, H.; Asada, K. Quenching analysis of chlorophyll fluorescence by the saturation mulse Method: Particular aspects relating to the study of eukaryotic algae and Cyanobacteria. Plant Cell Physiol. 1995, 36, 873–882. [Google Scholar] [CrossRef]
- Campbell, D.; Hurry, V.; AK, C.; Gustafsson, P.; Oquist, G. Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiol. Mol. Biol. Rev. 1998, 62, 667–683. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).