A Process for Carbon Dioxide Capture Using Schiff Bases Containing a Trimethoprim Unit
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentations
2.3. Adsorption Experiment
2.4. Synthesis of Schiff Bases 1–3
3. Results and Discussion
3.1. Synthesis of Schiff Bases 1–3
3.2. SEM of 1–3
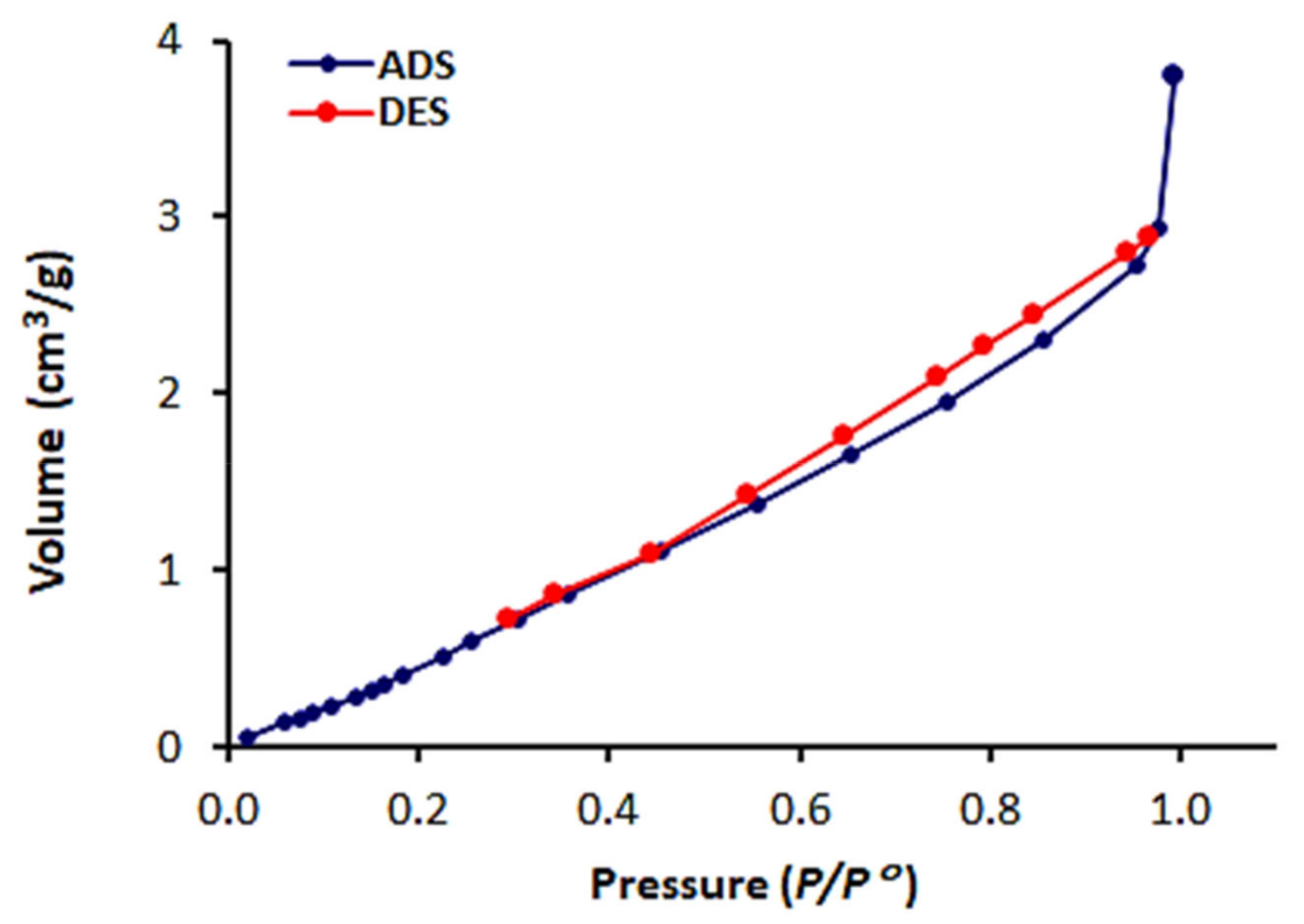
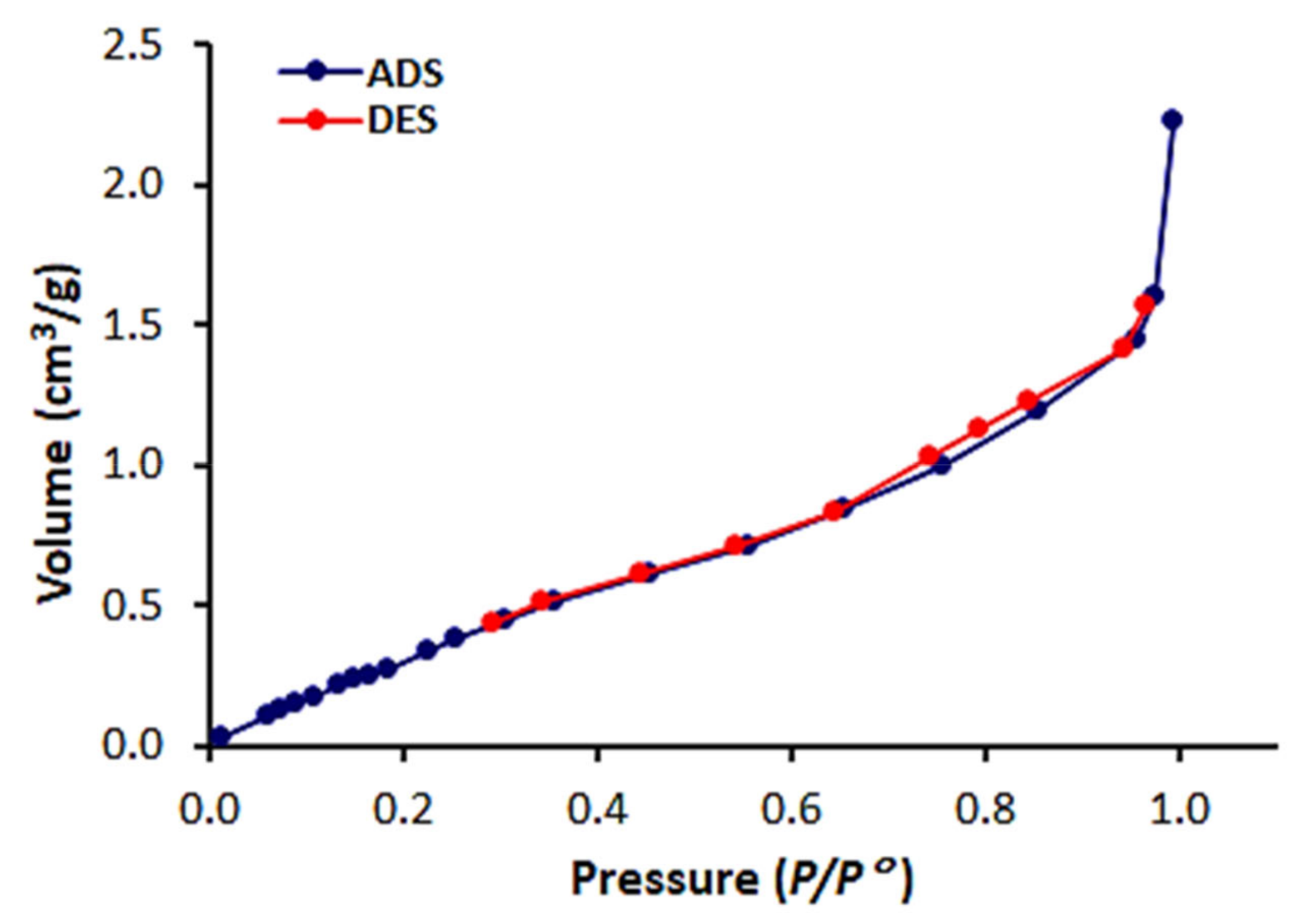
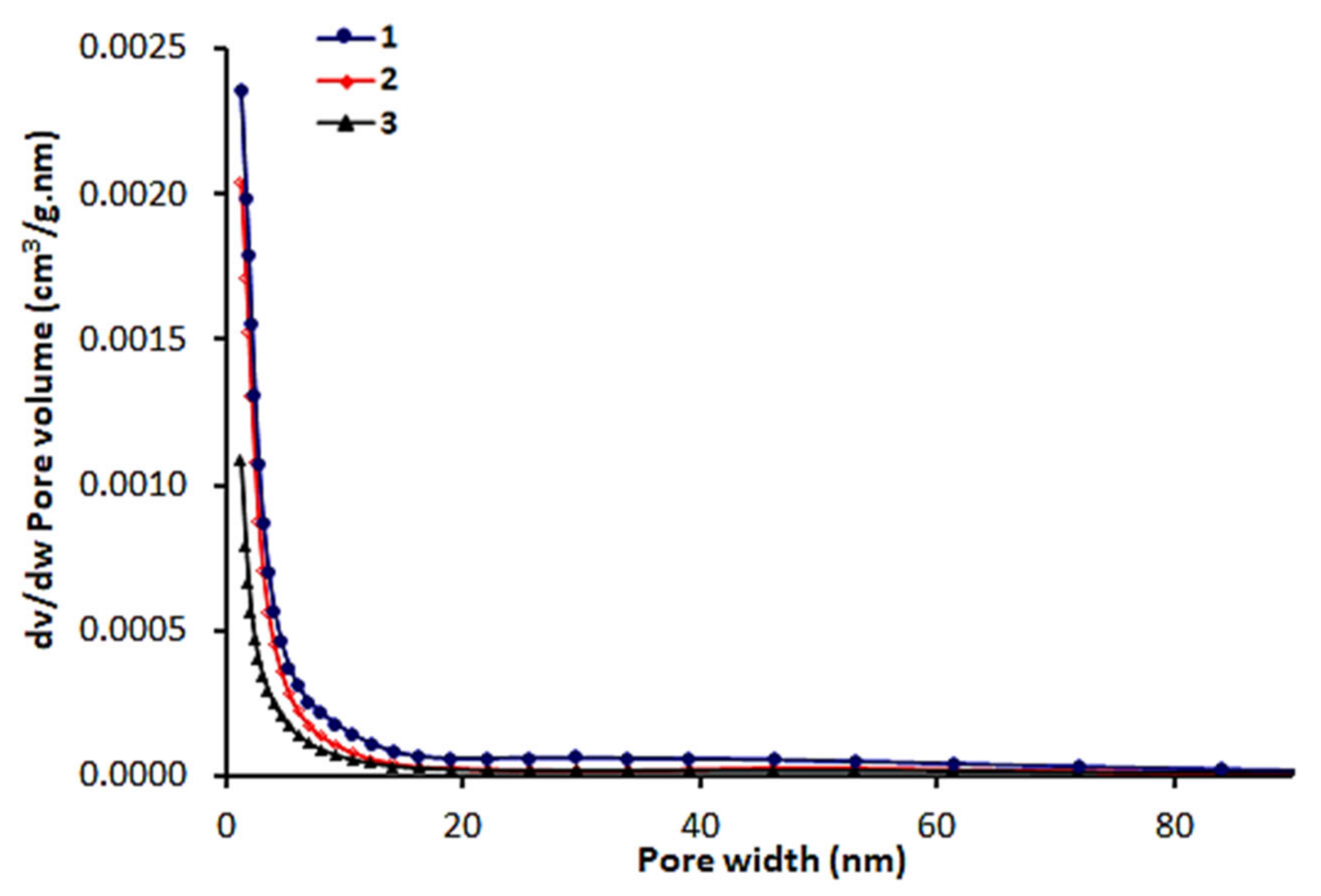
3.3. N2 Isotherms, Surface Area and Pore Size Distribution of 1–3
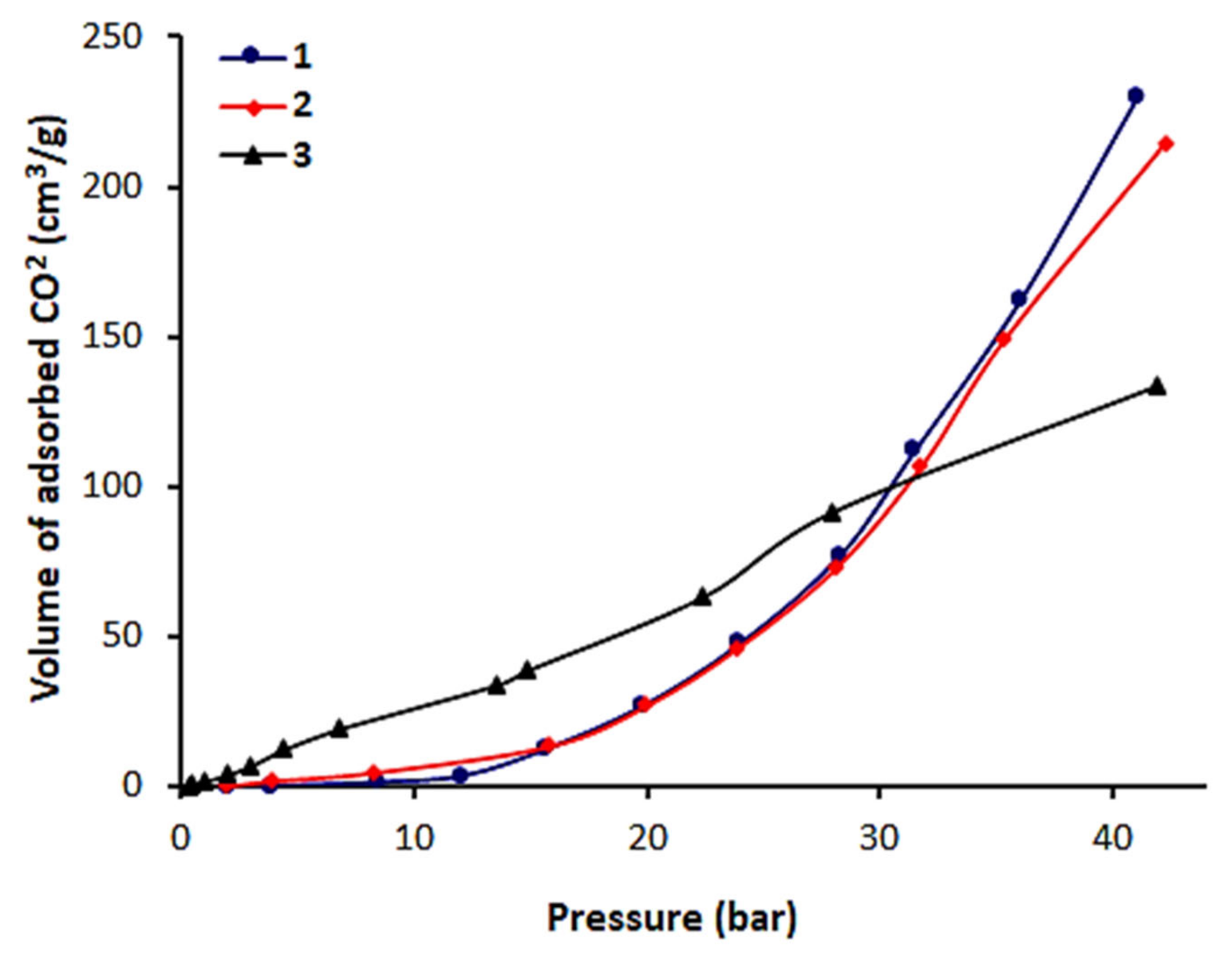
3.4. CO2 Uptake of 1–3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mardani, A.; Streimikiene, D.; Cavallaro, F.; Loganathan, N.; Khoshnoudi, M. Carbon dioxide (CO2) emissions and economic growth: A systematic review of two decades of research from 1995 to 2017. Sci. Total Environ. 2019, 649, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Yaumi, A.L.; Abu Bakar, M.Z.; Hameed, B.H. Recent advances in functionalized composite solid materials for carbon dioxide capture. Energy 2017, 124, 461–480. [Google Scholar] [CrossRef]
- Sun, H.; Xin, Q.; Ma, Z.; Lan, S. Effects of plant diversity on carbon dioxide emissions and carbon removal in laboratory-scale constructed wetland. Environ. Sci. Pollut. Res. 2019, 26, 5076–5082. [Google Scholar] [CrossRef] [PubMed]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Climate Change. Climate change 2007: Synthesis report. In Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Reisinger, A., Eds.; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2008; p. 104. [Google Scholar]
- Sanz-Pérez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct Capture of CO2 from Ambient Air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef]
- Goh, K.; Karahan, H.E.; Yang, E.; Bae, T.-H. Graphene-Based Membranes for CO2/CH4 Separation: Key Challenges and Perspectives. Appl. Sci. 2019, 9, 2784. [Google Scholar] [CrossRef]
- Jankovský, O.; Lojka, M.; Lauermannová, A.-M.; Antončík, F.; Pavlíková, M.; Pavlík, Z.; Sedmidubský, D. Carbon Dioxide Uptake by MOC-Based Materials. Appl. Sci. 2020, 10, 2254. [Google Scholar] [CrossRef]
- Osman, A.I.; Hefny, M.; Maksoud, M.I.A.A.; Elgarahy, A.M.; Rooney, D.W. Recent advances in carbon capture storage and utilisation technologies: A review. Environ. Chem. Lett. 2021. ahead of print. [Google Scholar] [CrossRef]
- Wilberforce, T.; Olabi, A.; Sayed, E.T.; Elsaid, K.; Abdelkareem, M.A. Progress in carbon capture technologies. Sci. Total Environ. 2021, 761, 143203. [Google Scholar] [CrossRef]
- Cannone, S.F.; Lanzini, A.; Santarelli, M. A Review on CO2 Capture Technologies with Focus on CO2-Enhanced Methane Recovery from Hydrates. Energies 2021, 14, 387. [Google Scholar] [CrossRef]
- Omodolor, I.S.; Otor, H.O.; Andonegui, J.A.; Allen, B.J.; Alba-Rubio, A.C. Dual-Function Materials for CO2 Capture and Conversion: A Review. Ind. Eng. Chem. Res. 2020, 59, 17612–17631. [Google Scholar] [CrossRef]
- Muslemani, H.; Liang, X.; Kaesehage, K.; Wilson, J. Business Models for Carbon Capture, Utilization and Storage Technologies in the Steel Sector: A Qualitative Multi-Method Study. Processes 2020, 8, 576. [Google Scholar] [CrossRef]
- Shukrullah, S.; Naz, M.Y.; Mohamed, N.M.; Ibrahim, K.A.; Abdel-Salam, N.M.; Ghaffar, A. CVD Synthesis, Functionalization and CO2 Adsorption Attributes of Multiwalled Carbon Nanotubes. Processes 2019, 7, 634. [Google Scholar] [CrossRef]
- Raza, A.; Gholami, R.; Rezaee, R.; Rasouli, V.; Rabiei, M. Significant aspects of carbon capture and storage—A review. Petroleum 2019, 5, 335–340. [Google Scholar] [CrossRef]
- Li, J.; Hou, Y.; Wang, P.; Yang, B. A Review of Carbon Capture and Storage Project Investment and Operational Decision-Making Based on Bibliometrics. Energies 2019, 12, 23. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon Dioxide Capture: Prospects for New Materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef]
- Shukla, S.K.; Khokarale, S.G.; Bui, T.Q.; Mikkola, J.-P.T. Ionic Liquids: Potential Materials for Carbon Dioxide Capture and Utilization. Front. Mater. 2019, 6, 42. [Google Scholar] [CrossRef]
- Mukherjee, A.; Okolie, J.A.; Abdelrasoul, A.; Niu, C.; Dalai, A.K. Review of post-combustion carbon dioxide capture technologies using activated carbon. J. Environ. Sci. 2019, 83, 46–63. [Google Scholar] [CrossRef]
- Okesola, A.A.; Oyedeji, A.A.; Abdulhamid, A.F.; Olowo, J.; Ayodele, B.E.; Alabi, T.W. Direct Air Capture: A Review of Carbon Dioxide Capture from the Air. IOP Conf. Ser. Mater. Sci. Eng. 2018, 413, 012077. [Google Scholar] [CrossRef]
- Luis, P. Use of monoethanolamine (MEA) for CO2 capture in a global scenario: Consequences and alternatives. Desalination 2016, 380, 93–99. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Electric Power Research Institute. Program on Technology Innovation: Post-Combustion CO2 Capture Technology Development; Electric Power Research Institute: Palo Alto, CA, USA, 2008. [Google Scholar]
- Figueroa, J.D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 capture technology—The U.S. Department of Energy’s Carbon Sequestration Program: A review. Int. J. Greenh. Gas Control 2008, 2, 9–20. [Google Scholar] [CrossRef]
- Asadi-Sangachini, Z.; Galangash, M.M.; Younesi, H.; Nowrouzi, M. The feasibility of cost-effective manufacturing activated carbon derived from walnut shells for large-scale CO2 capture. Environ. Sci. Pollut. Res. 2019, 26, 26542–26552. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.A.A.; Mangano, E.; Shiko, E.; Greenaway, A.G.; Gromov, A.V.; Lozinska, M.M.; Friedrich, D.; Campbell, E.E.B.; Wright, P.A.; Brandani, S. Adsorption Materials and Processes for Carbon Capture from Gas-Fired Power Plants: AMPGas. Ind. Eng. Chem. Res. 2016, 55, 3840–3851. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. A review on solid adsorbents for carbon dioxide capture. J. Ind. Eng. Chem. 2015, 23, 1–11. [Google Scholar] [CrossRef]
- Lee, S.C.; Chae, H.J.; Lee, S.J.; Choi, B.Y.; Yi, C.K.; Lee, J.B.; Ryu, C.K.; Kim, J.C. Development of Regenerable MgO-Based Sorbent Promoted with K2CO3 for CO2 Capture at Low Temperatures. Environ. Sci. Technol. 2008, 42, 2736–2741. [Google Scholar] [CrossRef]
- Wang, S.; Yan, S.; Ma, X.; Gong, J. Recent advances in capture of carbon dioxide using alkali-metal-based oxides. Energy Environ. Sci. 2011, 4, 3805–3819. [Google Scholar] [CrossRef]
- Hauchhum, L.; Mahanta, P. Carbon dioxide adsorption on zeolites and activated carbon by pressure swing adsorption in a fixed bed. Int. J. Energy Environ. Eng. 2014, 5, 349–356. [Google Scholar] [CrossRef]
- Staciwa, P.; Narkiewicz, U.; Sibera, D.; Moszyński, D.; Wróbel, R.J.; Cormia, R.D. Carbon Spheres as CO2 Sorbents. Appl. Sci. 2019, 9, 3349. [Google Scholar] [CrossRef]
- Aquino, A.S.; Vieira, M.O.; Ferreira, A.S.D.; Cabrita, E.J.; Einloft, S.; De Souza, M.O. Hybrid Ionic Liquid–Silica Xerogels Applied in CO2 Capture. Appl. Sci. 2019, 9, 2614. [Google Scholar] [CrossRef]
- Chiang, Y.-C.; Yeh, C.-Y.; Weng, C.-H. Carbon Dioxide Adsorption on Porous and Functionalized Activated Carbon Fibers. Appl. Sci. 2019, 9, 1977. [Google Scholar] [CrossRef]
- Choma, J.; Kloske, M.; Dziura, A.; Stachurska, K.; Jaroniec, M. Preparation and Studies of Adsorption Properties of Microporous Carbon Spheres. Eng. Prot. Environ. 2016, 19, 169–182. [Google Scholar] [CrossRef]
- Dawson, R.; Cooper, A.I.; Adams, D.J. Nanoporous organic polymer networks. Prog. Polym. Sci. 2012, 37, 530–563. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent Materials for Carbon Dioxide Capture from Large Anthropogenic Point Sources. ChemSusChem 2009, 2, 796–854. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, M.; Yuan, D. Carbon dioxide capture in amorphous porous organic polymers. J. Mater. Chem. A 2017, 5, 1334–1347. [Google Scholar] [CrossRef]
- Ahmed, D.S.; El-Hiti, G.A.; Yousif, E.; Ali, A.A.; Hameed, A.S. Design and synthesis of porous polymeric materials and their applications in gas capture and storage: A review. J. Polym. Res. 2018, 25, 75. [Google Scholar] [CrossRef]
- Spadaro, L.; Arena, F.; Negro, P.; Palella, A. Sunfuels from CO2 exhaust emissions: Insights into the role of photoreactor configuration by the study in laboratory and industrial environment. J. CO2 Util. 2018, 26, 445–453. [Google Scholar] [CrossRef]
- Ouyang, Y.; Yang, H.; Zhang, P.; Wang, Y.; Kaur, S.; Zhu, X.; Wang, Z.; Sun, Y.; Hong, W.; Ngeow, Y.F.; et al. Synthesis of 2,4-Diaminopyrimidine Core-Based Derivatives and Biological Evaluation of Their Anti-Tubercular Activities. Molecules 2017, 22, 1592. [Google Scholar] [CrossRef]
- Masur, H.; Brooks, J.T.; Benson, C.A.; Holmes, K.K.; Pau, A.K.; Kaplan, J.E. Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents: Updated Guidelines From the Centers for Disease Control and Prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 58, 1308–1311. [Google Scholar] [CrossRef]
- Mousa, O.G.; Yousif, E.; Ahmed, A.A.; El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, D.S. Synthesis and use of carvedilol metal complexes as carbon dioxide storage media. Appl. Petrochem. Res. 2020, 10, 157–164. [Google Scholar] [CrossRef]
- Hadi, A.G.; Jawad, K.; Yousif, E.; El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, D.S. Synthesis of Telmisartan Organotin(IV) Complexes and their use as Carbon Dioxide Capture Media. Molecules 2019, 24, 1631. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Yousif, E.; El-Hiti, G.A. Synthesis and Use of Valsartan Metal Complexes as Media for Carbon Dioxide Storage. Materials 2020, 13, 1183. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, Z.N.; Alias, M.; El-Hiti, G.A.-R.; Ahmed, D.S.; Yousif, E. Synthesis and use of new porous metal complexes containing a fusidate moiety as gas storage media. Korean J. Chem. Eng. 2021, 38, 179–186. [Google Scholar] [CrossRef]
- Omer, R.M.; Al-Tikrity, E.T.B.; El-Hiti, G.A.; Alotibi, M.F.; Ahmed, D.S.; Yousif, E. Porous Aromatic Melamine Schiff Bases as Highly Efficient Media for Carbon Dioxide Storage. Processes 2020, 8, 17. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Hameed, A.S.; Yousif, E.; Alotaibi, M.H.; Ahmed, D.S.; El-Hiti, G.A. New Porous Silicon-Containing Organic Polymers: Synthesis and Carbon Dioxide Uptake. Processes 2020, 8, 1488. [Google Scholar] [CrossRef]
- Ahmed, D.S.; El-Hiti, G.A.; Yousif, E.; Hameed, A.S.; Abdalla, M. New Eco-Friendly Phosphorus Organic Polymers as Gas Storage Media. Polymers 2017, 9, 336. [Google Scholar] [CrossRef]
- Satar, H.A.; Ahmed, A.A.; Yousif, E.; Ahmed, D.S.; Alotibi, M.F.; El-Hiti, G.A. Synthesis of Novel Heteroatom-Doped Porous-Organic Polymers as Environmentally Efficient Media for Carbon Dioxide Storage. Appl. Sci. 2019, 9, 4314. [Google Scholar] [CrossRef]
- Donald, A.M. The use of environmental scanning electron microscopy for imaging wet and insulating materials. Nat. Mater. 2003, 2, 511–516. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Sotomayor, F.J.; Cychosz, K.A.; Thommes, M. Characterization of micro/mesoporous materials by physisorption: Concepts and case studies. Acc. Mater. Surf. Res. 2018, 3, 34–50. [Google Scholar]
- Sing, K. The use of nitrogen adsorption for the characterisation of porous materials. Colloids Surfaces A Physicochem. Eng. Asp. 2001, 187–188, 3–9. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Thommes, M. Progress in the Physisorption Characterization of Nanoporous Gas Storage Materials. Engineering 2018, 4, 559–566. [Google Scholar] [CrossRef]
- Arena, F.; Trunfio, G.; Negro, J.; Spadaro, L. Optimization of the MnCeOx system for the catalytic wet oxidation of phenol with oxygen (CWAO). Appl. Catal. B Environ. 2008, 85, 40–47. [Google Scholar] [CrossRef]
- Bonenfant, D.; Kharoune, M.; Niquette, P.; Mimeault, M.; Hausler, R. Advances in principal factors influencing carbon dioxide adsorption on zeolites. Sci. Technol. Adv. Mater. 2008, 9, 013007. [Google Scholar] [CrossRef]
- Kim, K.C.; Jang, S.S. Molecular Simulation Study on Factors Affecting Carbon Dioxide Adsorption on Amorphous Silica Surfaces. J. Phys. Chem. C 2020, 124, 12580–12588. [Google Scholar] [CrossRef]






| Schiff Base | Color | Ar | Yield (%) | Melting Point (°C) | Calculated (Found; %) | ||
|---|---|---|---|---|---|---|---|
| C | H | N | |||||
| 1 | Yellow | 3-HOC6H4 | 85 | 179–181 | 63.95 (64.09) | 5.62 (5.69) | 14.20 (14.28) |
| 2 | White | 4-MeOC6H4 | 83 | 188–190 | 64.69 (64.72) | 5.92 (5.95) | 13.72 (13.77) |
| 3 | Pearl yellow | 4-Me2NC6H4 | 87 | 76–79 | 65.54 (65.55) | 6.46 (6.48) | 16.62 (16.68) |
| Schiff Base | C=C | C=N (Ar) | C=N | CH (Ar) | OH | NH2 |
|---|---|---|---|---|---|---|
| 1 | 1543 | 1589 | 1657 | 3109 | 3317 | 3447 |
| 2 | 1506 | 1591 | 1655 | 3121 | — | 3446 |
| 3 | 1506 | 1589 | 1657 | 3127 | — | 3447 |
| Schiff Base | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| 1 | 20.3 | 0.0086 | 11.4 |
| 2 | 19.8 | 0.0067 | 8.3 |
| 3 | 4.2 | 0.0036 | 6.6 |
| Schiff Base | CO2 Uptake (cm3/g) | CO2 Uptake (mmol/g) | CO2 Uptake (wt%) |
|---|---|---|---|
| 1 | 230.2 | 10.3 | 46 |
| 2 | 214.6 | 9.6 | 43 |
| 3 | 133.7 | 6.0 | 27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaseen, A.A.; Al-Tikrity, E.T.B.; El-Hiti, G.A.; Ahmed, D.S.; Baashen, M.A.; Al-Mashhadani, M.H.; Yousif, E. A Process for Carbon Dioxide Capture Using Schiff Bases Containing a Trimethoprim Unit. Processes 2021, 9, 707. https://doi.org/10.3390/pr9040707
Yaseen AA, Al-Tikrity ETB, El-Hiti GA, Ahmed DS, Baashen MA, Al-Mashhadani MH, Yousif E. A Process for Carbon Dioxide Capture Using Schiff Bases Containing a Trimethoprim Unit. Processes. 2021; 9(4):707. https://doi.org/10.3390/pr9040707
Chicago/Turabian StyleYaseen, Anaheed A., Emaad T. B. Al-Tikrity, Gamal A. El-Hiti, Dina S. Ahmed, Mohammed A. Baashen, Mohammed H. Al-Mashhadani, and Emad Yousif. 2021. "A Process for Carbon Dioxide Capture Using Schiff Bases Containing a Trimethoprim Unit" Processes 9, no. 4: 707. https://doi.org/10.3390/pr9040707
APA StyleYaseen, A. A., Al-Tikrity, E. T. B., El-Hiti, G. A., Ahmed, D. S., Baashen, M. A., Al-Mashhadani, M. H., & Yousif, E. (2021). A Process for Carbon Dioxide Capture Using Schiff Bases Containing a Trimethoprim Unit. Processes, 9(4), 707. https://doi.org/10.3390/pr9040707









