Recent Advances in the Synthesis of Nanocellulose Functionalized–Hybrid Membranes and Application in Water Quality Improvement
Abstract
1. Introduction
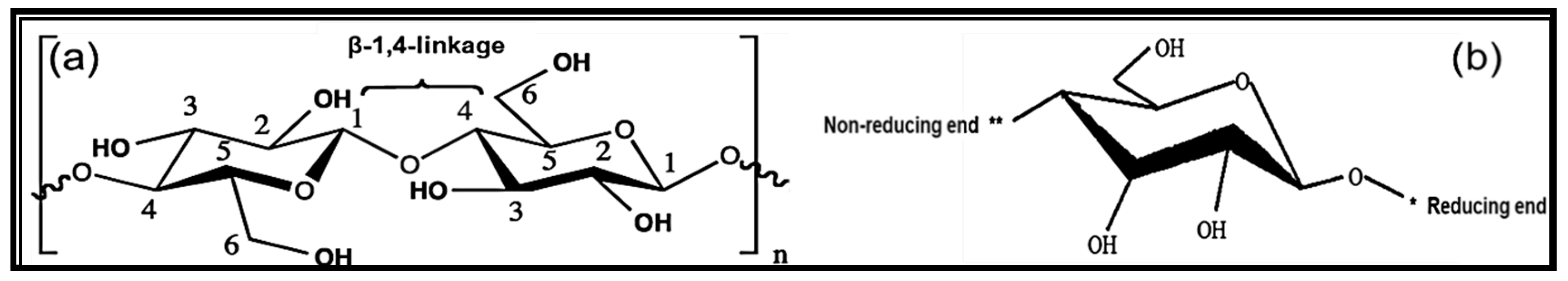
2. Nanocelluloses
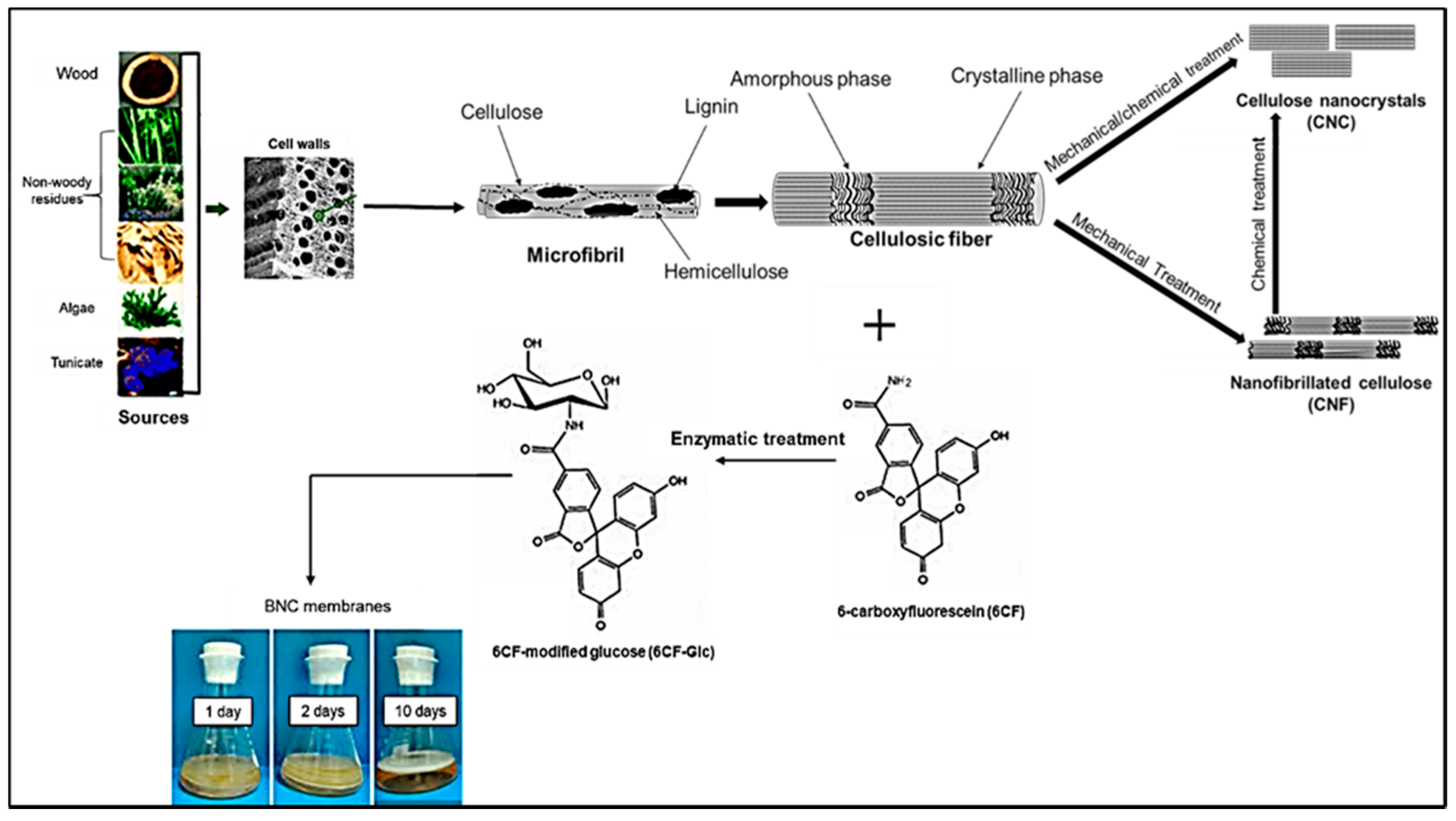
2.1. Sources and Categorization
2.2. Nanocelluloses Properties Relevant to Membrane Filtration
2.2.1. Enhanced Mechanical Properties
2.2.2. Enhanced Surface Chemistry
2.2.3. Biodegradability, Biocompatibility, and Toxicity
2.2.4. Dimensions and Aspect Ratios
3. Production Techniques of NCs
3.1. Pre-Treatment Technologies for the Production of NCs
3.2. Mechanical Isolation of NCs
3.3. Chemical Isolation of NCs
3.4. Biological Isolation of NCs
4. Surface Functionalization of NCs for Membranes Performance Improvement
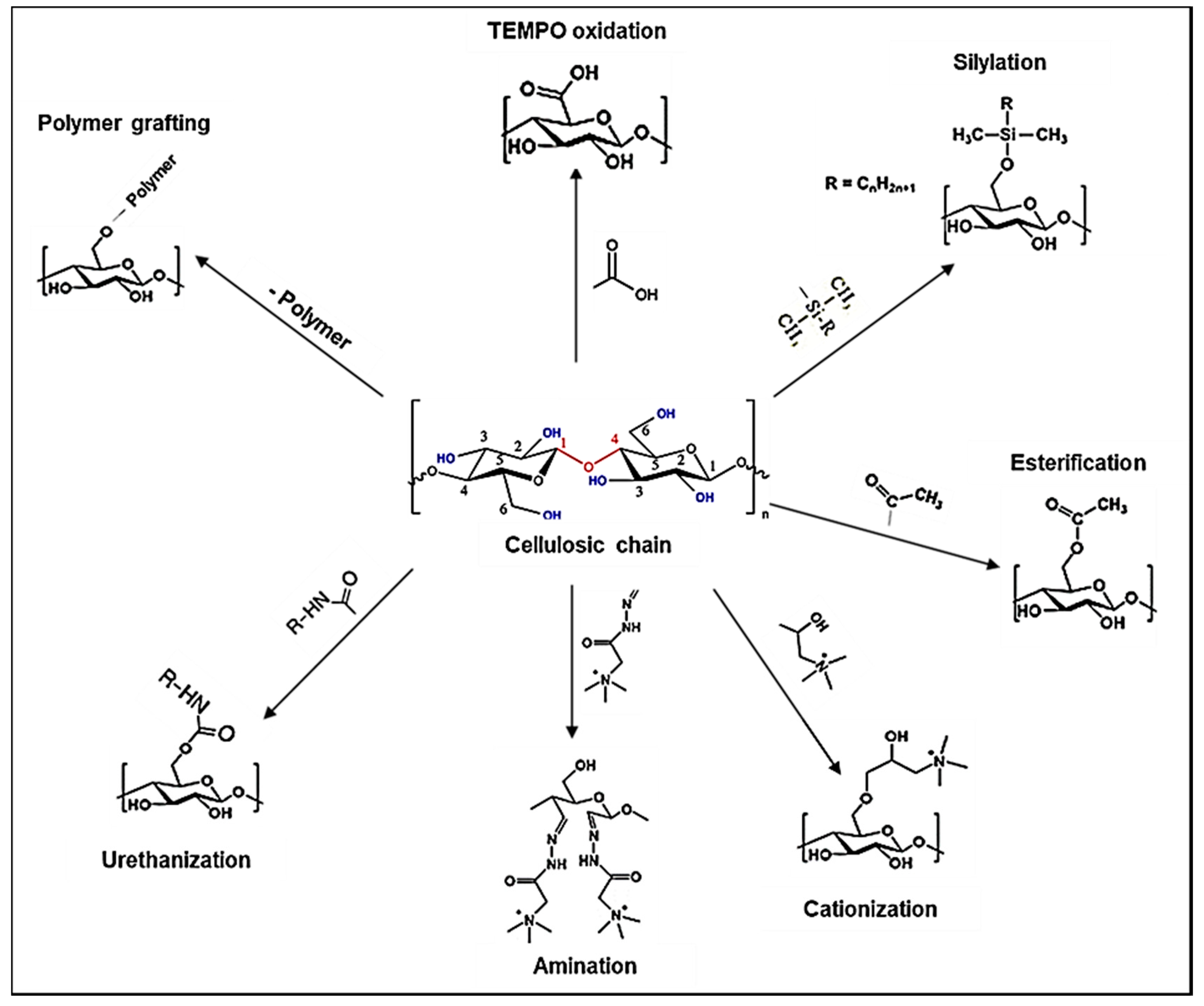
4.1. Non-Covalent Surface Functionalization of NCs
4.2. Chemical Surface Functionalization of NCs
4.2.1. Chemical Functionalization by Oxidation
4.2.2. Chemical Functionalization by Cationization
4.2.3. Chemical Functionalization by Esterification
4.2.4. Chemical Functionalization by Silane Coupling Reactions
4.2.5. Chemical Functionalization by Amidation
4.2.6. Chemical Functionalization by Urethanization
4.3. Polymer Grafting on NCs
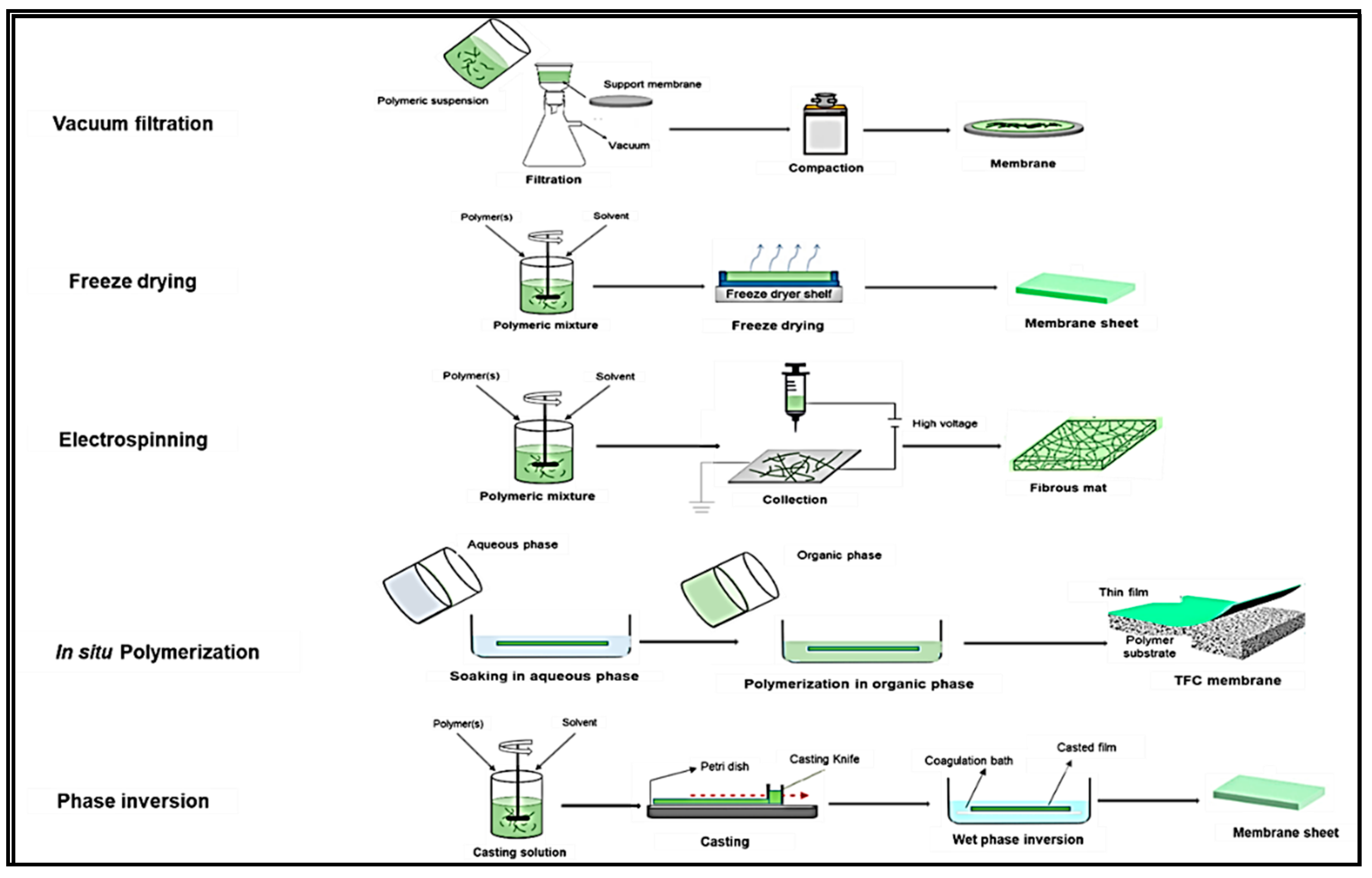
5. The NCs-Based Composite Membranes: Processing and Applications in Water Quality Improvement
5.1. The Phase Inversion Technique
5.2. The Vacuum Filtration Technique
5.3. The Electrospinning Technique
5.4. The Interfacial Polymerization Technique
5.5. The Freeze-Drying Technique
6. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Z.; Wu, A.; Ciacchi, L.C.; Wei, G. Recent Advances in Nanoporous Membranes for Water Purification. Nanomaterials 2018, 8, 65. [Google Scholar] [CrossRef]
- Dongre, R.S.; Sadasivuni, K.K.; Deshmukh, K.; Mehta, A.; Basu, S.; Meshram, J.S.; Al-Maadeed, M.A.A.; Karim, A. Natural polymer based composite membranes for water purification: A review. Polym. Technol. Mater. 2019, 58, 1295–1310. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Maifadi, S.; Mamba, B.B.; Verliefde, A.R.; Mhlanga, S.D. Spectroscopic Determination of Water Salinity in Brackish Surface Water in Nandoni Dam, at Vhembe District, Limpopo Province, South Africa. Water 2018, 10, 990. [Google Scholar] [CrossRef]
- Rafieian, F.; Jonoobi, M.; Yu, Q. A novel nanocomposite membrane containing modified cellulose nanocrystals for copper ion removal and dye adsorption from water. Cellulose 2019, 26, 3359–3373. [Google Scholar] [CrossRef]
- Sumisha, A.; Arthanareeswaran, G.; Thuyavan, Y.L.; Ismail, A.; Chakraborty, S. Treatment of laundry wastewater using polyethersulfone/polyvinylpyrollidone ultrafiltration membranes. Ecotoxicol. Environ. Saf. 2015, 121, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Nthunya, L.N.; Khumalo, N.P.; Verliefde, A.R.; Mamba, B.B.; Mhlanga, S.D. Quantitative analysis of phenols and PAHs in the Nandoni Dam in Limpopo Province, South Africa: A preliminary study for dam water quality management. Phys. Chem. Earth Parts A/B/C 2019, 112, 228–236. [Google Scholar] [CrossRef]
- Vasudevan, S.; Oturan, M.A. Electrochemistry: As cause and cure in water pollution—an overview. Environ. Chem. Lett. 2014, 12, 97–108. [Google Scholar] [CrossRef]
- Siraj, K.T.; Rao, P.V.V.P. Review on current world water resources scenario and water treatment technologies and tech-niques. Int. J. Appl. Res. Stud. 2016, 2, 262–266. [Google Scholar]
- Mofokeng, M.; Nthunya, L.N.; Gutierrez, L.; Matabola, P.; Mishra, S.; Nxumalo, E.N. Perflurooctyltriethoxy silane and carbon nanotubes-modified PVDF superoleophilic nanofibre membrane for oil-in-water adsorption and recovery. J. Environ. Chem. Eng. 2020, 8, 104497. [Google Scholar] [CrossRef]
- Makgabutlane, B.; Nthunya, L.N.; Maubane-Nkadimeng, M.S.; Mhlanga, S.D. Green synthesis of carbon nanotubes to address the water-energy-food nexus: A critical review. J. Environ. Chem. Eng. 2021, 9, 104736. [Google Scholar] [CrossRef]
- Liu, Z.; Mi, Z.; Jin, S.; Wang, C.; Wang, D.; Zhao, X.; Zhou, H.; Chen, C. The influence of sulfonated hyperbranched polyethersulfone-modified halloysite nanotubes on the compatibility and water separation performance of polyethersulfone hybrid ultrafiltration membranes. J. Membr. Sci. 2018, 557, 13–23. [Google Scholar] [CrossRef]
- Farahani, M.H.D.A.; Rabiee, H.; Vatanpour, V. Comparing the effect of incorporation of various nanoparticulate on the performance and antifouling properties of polyethersulfone nanocomposite membranes. J. Water Process. Eng. 2019, 27, 47–57. [Google Scholar] [CrossRef]
- Voisin, H.; Bergström, L.; Liu, P.; Mathew, A.P. Nanocellulose-Based Materials for Water Purification. Nanomaterials 2017, 7, 57. [Google Scholar] [CrossRef]
- Nasrollahi, N.; Aber, S.; Vatanpour, V.; Mahmoodi, N.M. The effect of amine functionalization of CuO and ZnO nanoparticles used as additives on the morphology and the permeation properties of polyethersulfone ultrafiltration nanocomposite membranes. Compos. Part B Eng. 2018, 154, 388–409. [Google Scholar] [CrossRef]
- Kim, D.; Vovusha, H.; Schwingenschlögl, U.; Nunes, S.P. Polyethersulfone flat sheet and hollow fiber membranes from solutions in ionic liquids. J. Membr. Sci. 2017, 539, 161–171. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Hashaikeh, R.; Hilal, N. Fouling control in reverse osmosis membranes through modification with conductive carbon nanostructures. Desalination 2019, 470, 114118. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Gutierrez, L.; Derese, S.; Mamba, B.B.; Verliefde, A.R.; Mhlanga, S.D. Adsorption of phenolic compounds by polyacrylonitrile nanofibre membranes: A pretreatment for the removal of hydrophobic bearing compounds from water. J. Environ. Chem. Eng. 2019, 7, 103254. [Google Scholar] [CrossRef]
- Ruiz-García, A.; Ruiz-Saavedra, E. 80,000h operational experience and performance analysis of a brackish water reverse osmosis desalination plant. Assessment of membrane replacement cost. Desalination 2015, 375, 81–88. [Google Scholar] [CrossRef]
- Nassrullah, H.; Anis, S.F.; Hashaikeh, R.; Hilal, N. Energy for desalination: A state-of-the-art review. Desalination 2020, 491, 114569. [Google Scholar] [CrossRef]
- Ruiz-García, A.; Ruiz-Saavedra, E.; Báez, S.O.P. Evaluation of the first seven years operating data of a RO brackish water desalination plant in Las Palmas, Canary Islands, Spain. Desalination Water Treat. 2014, 54, 3193–3199. [Google Scholar] [CrossRef]
- Shak, K.P.Y.; Pang, Y.L.; Mah, S.K. Nanocellulose: Recent advances and its prospects in environmental remediation. Beilstein J. Nanotechnol. 2018, 9, 2479–2498. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, A.; Teramoto, Y. Recent Advances in Nanocellulose Composites with Polymers: A Guide for Choosing Partners and How to Incorporate Them. Polymers 2018, 10, 517. [Google Scholar] [CrossRef]
- Karim, Z.; Claudpierre, S.; Grahn, M.; Oksman, K.; Mathew, A.P. Nanocellulose based functional membranes for water cleaning: Tailoring of mechanical properties, porosity and metal ion capture. J. Membr. Sci. 2016, 514, 418–428. [Google Scholar] [CrossRef]
- Carpenter, A.W.; De Lannoy, C.-F.; Wiesner, M.R. Cellulose Nanomaterials in Water Treatment Technologies. Environ. Sci. Technol. 2015, 49, 5277–5287. [Google Scholar] [CrossRef] [PubMed]
- Ashori, A.; Rafieyan, F.; Kian, F.; Jonoobi, M.; Tavabe, K.R. Effect of cellulose nanocrystals on performance of polyethersulfone nanocomposite membranes using electrospinning technique. Polym. Compos. 2019, 40, E835–E841. [Google Scholar] [CrossRef]
- Han, A.; Zhang, H.; Yuan, R.; Ji, H.; Du, P. Crystalline Copper Phosphide Nanosheets as an Efficient Janus Catalyst for Overall Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 2240–2248. [Google Scholar] [CrossRef]
- Bai, L.; Bossa, N.; Qu, F.; Winglee, J.; Li, G.; Sun, K.; Liang, H.; Wiesner, M.R. Comparison of Hydrophilicity and Mechanical Properties of Nanocomposite Membranes with Cellulose Nanocrystals and Carbon Nanotubes. Environ. Sci. Technol. 2017, 51, 253–262. [Google Scholar] [CrossRef]
- Mautner, A. Nanocellulose water treatment membranes and filters: A review. Polym. Int. 2020, 69, 741–751. [Google Scholar] [CrossRef]
- Malakhov, A.O.; Anokhina, T.S.; Petrova, D.A.; Vinokurov, V.A.; Volkov, A.V. Nanocellulose as a Component of Ultrafiltration Membranes. Pet. Chem. 2018, 58, 923–933. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nano-composites—A review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef]
- Liang, H.; Hu, X. A quick review of the applications of nano crystalline cellulose in wastewater treatment. J. Bioresour. Bioprod. 2016, 1, 199–204. [Google Scholar]
- Xie, H.; Du, H.; Yang, X.; Si, C. Recent Strategies in Preparation of Cellulose Nanocrystals and Cellulose Nanofibrils Derived from Raw Cellulose Materials. Int. J. Polym. Sci. 2018, 2018, 1–25. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial Cellulose as a Raw Material for Food and Food Packaging Applications. Front. Sustain. Food Syst. 2019, 3. [Google Scholar] [CrossRef]
- Kalita, E.; Nath, B.; Agan, F.; More, V.; Deb, P. Isolation and characterization of crystalline, autofluorescent, cellulose nanocrystals from saw dust wastes. Ind. Crop. Prod. 2015, 65, 550–555. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Chirayil, C.J.; Mathew, L.; Thomas, S. Review of recent research in nano cellulose preparation from different lignocellulosic fibers. Rev. Adv. Mater. Sci. 2014, 20–28. [Google Scholar]
- Dresselhaus, M.S.; Dresselhaus, G.; Charlier, J.-C.; Hernández, E. Electronic, thermal and mechanical properties of carbon nanotubes. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2004, 362, 2065–2098. [Google Scholar] [CrossRef]
- Saito, T.; Kuramae, R.; Wohlert, J.; Berglund, L.A.; Isogai, A. An Ultrastrong Nanofibrillar Biomaterial: The Strength of Single Cellulose Nanofibrils Revealed via Sonication-Induced Fragmentation. Biomacromolecules 2013, 14, 248–253. [Google Scholar] [CrossRef]
- Vikman, M.; Vartiainen, J.; Tsitko, I.; Korhonen, P. Biodegradability and Compostability of Nanofibrillar Cellulose-Based Products. J. Polym. Environ. 2015, 23, 206–215. [Google Scholar] [CrossRef]
- Murray, A.R.; Kisin, E.; Leonard, S.S.; Young, S.H.; Kommineni, C.; Kagan, V.E.; Castranova, V.; Shvedova, A.A. Oxidative stress and inflammatory response in dermal toxicity of single-walled carbon nanotubes. Toxicology 2009, 257, 161–171. [Google Scholar] [CrossRef]
- Aschberger, K.; Johnston, H.J.; Stone, V.; Aitken, R.J.; Hankin, S.M.; Peters, S.A.K.; Tran, C.L.; Christensen, F.M. Review of carbon nanotubes toxicity and exposure—Appraisal of human health risk assessment based on open literature. Crit. Rev. Toxicol. 2010, 40, 759–790. [Google Scholar] [CrossRef]
- Shatkin, J.A.; Wegner, T.H.; Bilek, E.; Cowie, J. Market projections of cellulose nanomaterial-enabled products? Part 1: Applications. Tappi J. 2014, 13, 9–16. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Tammelin, T.; Schulfter, K.; Kiiskinen, H.; Samela, J.; Bismarck, A. High Performance Cellulose Nanocomposites: Comparing the Reinforcing Ability of Bacterial Cellulose and Nanofibrillated Cellulose. ACS Appl. Mater. Interfaces 2012, 4, 4078–4086. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.; Azadmanjiri, J.; Nikzad, M.; Sbarski, I.; Wang, J.; Yu, A. Cellulose Nanocrystals: Production, Functionalization and Advanced Applications. Rev. Adv. Mater. Sci. 2019, 58, 1–16. [Google Scholar] [CrossRef]
- Börjesson, M.; Westman, M.B.A.G. Cellulose: Fundamental Aspects and Current Trends; IntechOpen: London, UK, 2015. [Google Scholar]
- Portela, R.; Leal, C.R.; Almeida, P.L.; Sobral, R.G. Bacterial cellulose: A versatile biopolymer for wound dressing applications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Wang, X.; Zhou, Y.; Zhang, L. Preparation and characterization of poly(vinylidene fluoride) composite membranes blended with nano-crystalline cellulose. Prog. Nat. Sci. 2012, 22, 250–257. [Google Scholar] [CrossRef]
- Ma, H.; Burger, C.; Hsiao, B.S.; Chu, B. Nanofibrous Microfiltration Membrane Based on Cellulose Nanowhiskers. Biomacromolecules 2012, 13, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhang, D.; Shao, Z.; Han, B.; Lv, Y.; Gao, K.; Peng, X. Superior effect of TEMPO-oxidized cellulose nanofibrils (TOCNs) on the performance of cellulose triacetate (CTA) ultrafiltration membrane. Desalination 2014, 332, 117–125. [Google Scholar] [CrossRef]
- Karim, Z.; Mathew, A.P.; Kokol, V.; Wei, J.; Grahn, M. High-flux affinity membranes based on cellulose nanocomposites for removal of heavy metal ions from industrial effluents. RSC Adv. 2016, 6, 20644–20653. [Google Scholar] [CrossRef]
- Qiu, K.; Netravali, A. In Situ Produced Bacterial Cellulose Nanofiber-Based Hybrids for Nanocomposites. Fibers 2017, 5, 31. [Google Scholar] [CrossRef]
- Jahan, Z.; Niazi, M.B.K.; Gregersen, Ø.W. Mechanical, thermal and swelling properties of cellulose nanocrystals/PVA nanocomposites membranes. J. Ind. Eng. Chem. 2018, 57, 113–124. [Google Scholar] [CrossRef]
- Qu, P.; Tang, H.; Gao, Y.; Zhang, L.P.; Wang, S. Polyethersulfone composite membrane blended With cellulose fibrils. Bio-Resources 2010. [Google Scholar] [CrossRef]
- Lalia, B.S.; Guillen, E.; Arafat, H.A.; Hashaikeh, R. Nanocrystalline cellulose reinforced PVDF-HFP membranes for membrane distillation application. Desalination 2014, 332, 134–141. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Y.-Q.; Xing, Y.-Y.; Yang, J.-F.; Wang, S.-F. Properties of novel polyvinyl alcohol/cellulose nanocrystals/silver nanoparticles blend membranes. Carbohydr. Polym. 2013, 98, 1573–1577. [Google Scholar] [CrossRef]
- Peresin, M.S.; Habibi, Y.; Zoppe, J.O.; Pawlak, J.J.; Rojas, O.J. Nanofiber Composites of Polyvinyl Alcohol and Cellulose Nanocrystals: Manufacture and Characterization. Biomacromolecules 2010, 11, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Barud, H.S.; Souza, J.L.; Santos, D.B.; Crespi, M.S.; Ribeiro, C.A.; Messaddeq, Y.; Ribeiro, S.J. Bacterial cellulose/poly(3-hydroxybutyrate) composite membranes. Carbohydr. Polym. 2011, 83, 1279–1284. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, S.; Hu, W.; Yin, N.; Zhang, W.; Xiang, C.; Wang, H. Flexible luminescent CdSe/bacterial cellulose nanocomoposite membranes. Carbohydr. Polym. 2012, 88, 173–178. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Sunasee, R.; Hemraz, U.D. Synthetic Strategies for the Fabrication of Cationic Surface-Modified Cellulose Nanocrystals. Fibers 2018, 6, 15. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Jonoobi, M.; Ashori, A.; Siracusa, V. Characterization and properties of polyethersulfone/modified cellulose nanocrystals nanocomposite membranes. Polym. Test. 2019, 76, 333–339. [Google Scholar] [CrossRef]
- Bai, L.; Liu, Y.; Ding, A.; Ren, N.; Li, G.; Liang, H. Fabrication and characterization of thin-film composite (TFC) nanofiltration membranes incorporated with cellulose nanocrystals (CNCs) for enhanced desalination performance and dye removal. Chem. Eng. J. 2019, 358, 1519–1528. [Google Scholar] [CrossRef]
- Park, C.H.; Jeon, S.; Park, S.-H.; Shin, M.G.; Park, M.S.; Lee, S.-Y.; Lee, J.-H. Cellulose nanocrystal-assembled reverse osmosis membranes with high rejection performance and excellent antifouling. J. Mater. Chem. A 2019, 7, 3992–4001. [Google Scholar] [CrossRef]
- Xu, C.; Chen, W.; Gao, H.; Xie, X.; Chen, Y. Cellulose nanocrystal/silver (CNC/Ag) thin-film nanocomposite nanofiltration membranes with multifunctional properties. Environ. Sci. Nano 2020, 7, 803–816. [Google Scholar] [CrossRef]
- Bai, L.; Wu, H.; Ding, J.; Ding, A.; Zhang, X.; Ren, N.; Li, G.; Liang, H. Cellulose nanocrystal-blended polyethersulfone membranes for enhanced removal of natural organic matter and alleviation of membrane fouling. Chem. Eng. J. 2020, 382, 122919. [Google Scholar] [CrossRef]
- Wang, R.; Guan, S.; Sato, A.; Wang, X.; Wang, Z.; Yang, R.; Hsiao, B.S.; Chu, B. Nanofibrous microfiltration membranes capable of removing bacteria, viruses and heavy metal ions. J. Membr. Sci. 2013, 446, 376–382. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Ferraz, N.; Carlsson, D.O.; Hong, J.; Larsson, R.; Fellström, B.; Nyholm, L.; Strømme, M.; Mihranyan, A. Haemocompatibility and ion exchange capability of nanocellulose polypyrrole membranes intended for blood purification. J. R. Soc. Interface 2012, 9, 1943–1955. [Google Scholar] [CrossRef]
- Razaq, A.; Nyström, G.; Strømme, M.; Mihranyan, A.; Nyholm, L. High-Capacity Conductive Nanocellulose Paper Sheets for Electrochemically Controlled Extraction of DNA Oligomers. PLoS ONE 2011, 6, e29243. [Google Scholar] [CrossRef]
- Pereira, M.M.; Raposo, N.R.B.; Brayner, R.; Teixeira, E.M.; Oliveira, V.; Quintão, C.C.R.; Camargo, L.S.A.; Mattoso, L.H.C.; Brandão, H.M. Cytotoxicity and expression of genes involved in the cellular stress response and apoptosis in mammalian fibroblast exposed to cotton cellulose nanofibers. Nanotechnology 2013, 24, 075103. [Google Scholar] [CrossRef]
- Jeong, S.I.; Lee, S.E.; Yang, H.; Jin, Y.-H.; Park, C.-S.; Park, Y.S. Toxicologic evaluation of bacterial synthesized cellulose in endothelial cells and animals. Mol. Cell. Toxicol. 2010, 6, 370–377. [Google Scholar] [CrossRef]
- Kim, G.-D.; Yang, H.; Park, H.R.; Park, C.-S.; Park, Y.S.; Lee, S.E. Evaluation of immunoreactivity of in vitro and in vivo models against bacterial synthesized cellulose to be used as a prosthetic biomaterial. BioChip J. 2013, 7, 201–209. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods—A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.A.; Bhat, A.; Yusra, A.I. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crop. Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Picheth, G.F.; Pirich, C.L.; Sierakowski, M.R.; Woehl, M.A.; Sakakibara, C.N.; de Souza, C.F.; Martin, A.A.; da Silva, R.; de Freitas, R.A. Bacterial cellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2017, 104, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Kalia, S.; Boufi, S.; Celli, A.; Kango, S. Nanofibrillated cellulose: Surface modification and potential applications. Colloid Polym. Sci. 2014, 292, 5–31. [Google Scholar] [CrossRef]
- Du, H.; Liu, C.; Zhang, Y.; Yu, G.; Si, C.; Li, B. Preparation and characterization of functional cellulose nanofibrils via formic acid hydrolysis pretreatment and the followed high-pressure homogenization. Ind. Crop. Prod. 2016, 94, 736–745. [Google Scholar] [CrossRef]
- Fernández-Bolaños, J.; Felizón, B.; Heredia, A.; Rodrȷguez, R.; Guillén, R.; Jiménez, A. Steam-explosion of olive stones: Hemicellulose solubilization and enhancement of enzymatic hydrolysis of cellulose. Bioresour. Technol. 2001, 79, 53–61. [Google Scholar] [CrossRef]
- Hassan, M.L.; Hassan, E.A.; Oksman, K.N. Effect of pretreatment of bagasse fibers on the properties of chitosan/microfibrillated cellulose nanocomposites. J. Mater. Sci. 2011, 46, 1732–1740. [Google Scholar] [CrossRef]
- Rol, F.; Karakashov, B.; Nechyporchuk, O.; Terrien, M.; Meyer, V.; Dufresne, A.; Belgacem, M.N.; Bras, J. Pilot-Scale Twin Screw Extrusion and Chemical Pretreatment as an Energy-Efficient Method for the Production of Nanofibrillated Cellulose at High Solid Content. ACS Sustain. Chem. Eng. 2017, 5, 6524–6531. [Google Scholar] [CrossRef]
- Missoum, K.; Belgacem, M.N.; Bras, J. Nanofibrillated Cellulose Surface Modification: A Review. Materials 2013, 6, 1745–1766. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.N.M.; Annamalai, P.K.; Morrow, I.C.; Martin, D. Production of cellulose nanocrystals via a scalable mechanical method. RSC Adv. 2015, 5, 57133–57140. [Google Scholar] [CrossRef]
- Li, W.; Yue, J.; Liu, S. Preparation of nanocrystalline cellulose via ultrasound and its reinforcement capability for poly(vinyl alcohol) composites. Ultrason. Sonochemistry 2012, 19, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-W.; Han, S.-Y.; Namgung, H.-W.; Seo, P.-N.; Lee, S.-Y.; Lee, S.-H. Preparation and Characterization of Cellulose Nanofibrils with Varying Chemical Compositions. BioResources 2017, 12, 5031–5044. [Google Scholar] [CrossRef]
- Trache, D.; Hussin, M.H.; Haafiz, M.K.M.; Thakur, V.K. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale 2017, 9, 1763–1786. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Yu, G.; Yu, Q.; Li, B.; Mu, X. A novel approach for the preparation of nanocrystalline cellulose by using phosphotungstic acid. Carbohydr. Polym. 2014, 110, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.A.; Laine, J.; Rojas, O.J. Microemulsion Systems for Fiber Deconstruction into Cellulose Nanofibrils. ACS Appl. Mater. Interfaces 2014, 6, 22622–22627. [Google Scholar] [CrossRef]
- Du, H.; Liu, C.; Mu, X.; Gong, W.; Lv, D.; Hong, Y.; Si, C.; Li, B. Preparation and characterization of thermally stable cellulose nanocrystals via a sustainable approach of FeCl3-catalyzed formic acid hydrolysis. Cellulose 2016, 23, 2389–2407. [Google Scholar] [CrossRef]
- Chen, S.-Q.; Lopez-Sanchez, P.; Wang, D.; Mikkelsen, D.; Gidley, M.J. Mechanical properties of bacterial cellulose synthesised by diverse strains of the genus Komagataeibacter. Food Hydrocoll. 2018, 81, 87–95. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Rojas, O.J.; Lucia, L.A. Green Modification of Surface Characteristics of Cellulosic Materials at the Molecular or Nano Scale: A Review. BioResources 2015, 10, 6095–6206. [Google Scholar] [CrossRef]
- Eyley, S.; Thielemans, W. Surface modification of cellulose nanocrystals. Nanoscale 2014, 6, 7764–7779. [Google Scholar] [CrossRef]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T. Removal of heavy metals and pollutants by membrane adsorption techniques. Appl. Water Sci. 2018, 8, 19. [Google Scholar] [CrossRef]
- Hatton, F.L.; Malmström, E.; Carlmark, A. Tailor-made copolymers for the adsorption to cellulosic surfaces. Eur. Polym. J. 2015, 65, 325–339. [Google Scholar] [CrossRef]
- Kim, J.; Montero, G.; Habibi, Y.; Hinestroza, J.P.; Genzer, J.; Argyropoulos, D.S.; Rojas, O.J. Dispersion of cellulose crystallites by nonionic surfactants in a hydrophobic polymer matrix. Polym. Eng. Sci. 2009, 49, 2054–2061. [Google Scholar] [CrossRef]
- Mautner, A.; Sehaqui, H.; Maples, H.A.; Zimmermann, T.; De Larraya, U.P.; Mathew, A.P.; Lai, C.Y.; Li, K.; Bismarck, A. Nitrate removal from water using a nanopaper ion-exchanger. Environ. Sci. Water Res. Technol. 2015, 2, 117–124. [Google Scholar] [CrossRef]
- Zhang, W.; Zhong, L.; Wang, T.; Jiang, Z.; Gao, X.; Zhang, L. Surface modification of cellulose nanofibers and their effects on the morphology and properties of polysulfone membranes. IOP Conf. Ser. Mater. Sci. Eng. 2018, 397, 012016. [Google Scholar] [CrossRef]
- Mautner, A.; Lee, K.-Y.; Tammelin, T.; Mathew, A.P.; Nedoma, A.J.; Li, K.; Bismarck, A. Cellulose nanopapers as tight aqueous ultra-filtration membranes. React. Funct. Polym. 2015, 86, 209–214. [Google Scholar] [CrossRef]
- Ma, H.; Burger, C.; Hsiao, B.S.; Chu, B. Ultra-fine cellulose nanofibers: New nano-scale materials for water purification. J. Mater. Chem. 2011, 21, 7507–7510. [Google Scholar] [CrossRef]
- Mautner, A.; Lee, K.-Y.; Lahtinen, P.; Hakalahti, M.; Tammelin, T.; Li, K.; Bismarck, A. Nanopapers for organic solvent nanofiltration. Chem. Commun. 2014, 50, 5778–5781. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Burger, C.; Hsiao, B.S.; Chu, B. Fabrication and characterization of cellulose nanofiber based thin-film nanofibrous composite membranes. J. Membr. Sci. 2014, 454, 272–282. [Google Scholar] [CrossRef]
- Li, N.; Zheng, J.; Hadi, P.; Yang, M.; Huang, X.; Ma, H.; Walker, H.W.; Hsiao, B.S. Synthesis and Characterization of a High Flux Nanocellulose–Cellulose Acetate Nanocomposite Membrane. Membranes 2019, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Hakalahti, M.; Mautner, A.; Johansson, L.-S.; Hänninen, T.; Setälä, H.; Kontturi, E.; Bismarck, A.; Tammelin, T. Direct Interfacial Modification of Nanocellulose Films for Thermoresponsive Membrane Templates. ACS Appl. Mater. Interfaces 2016, 8, 2923–2927. [Google Scholar] [CrossRef]
- Maatar, W.; Boufi, S. Microporous cationic nanofibrillar cellulose aerogel as promising adsorbent of acid dyes. Cellulose 2017, 24, 1001–1015. [Google Scholar] [CrossRef]
- Niide, T.; Shiraki, H.; Oshima, T.; Baba, Y.; Kamiya, N.; Goto, M. Quaternary Ammonium Bacterial Cellulose for Adsorption of Proteins. Solvent Extr. Res. Dev. Jpn. 2010, 17, 73–81. [Google Scholar] [CrossRef][Green Version]
- Gopakumar, D.A.; Pasquini, D.; Henrique, M.A.; De Morais, L.C.; Grohens, Y.; Thomas, S. Meldrum’s Acid Modified Cellulose Nanofiber-Based Polyvinylidene Fluoride Microfiltration Membrane for Dye Water Treatment and Nanoparticle Removal. ACS Sustain. Chem. Eng. 2017, 5, 2026–2033. [Google Scholar] [CrossRef]
- Mautner, A.; Maples, H.A.; Kobkeatthawin, T.; Kokol, V.; Karim, Z.; Li, K.; Bismarck, A. Phosphorylated nanocellulose papers for copper adsorption from aqueous solutions. Int. J. Environ. Sci. Technol. 2016, 13, 1861–1872. [Google Scholar] [CrossRef]
- Cruz-Tato, P.; Ortiz-Quiles, E.O.; Vega-Figueroa, K.; Santiago-Martoral, L.; Flynn, M.; Díaz-Vázquez, L.M.; Nicolau, E. Metalized Nanocellulose Composites as a Feasible Material for Membrane Supports: Design and Applications for Water Treatment. Environ. Sci. Technol. 2017, 51, 4585–4595. [Google Scholar] [CrossRef]
- Wang, J.; Lu, X.; Ng, P.F.; Lee, K.I.; Fei, B.; Xin, J.H.; Wu, J.-Y. Polyethylenimine coated bacterial cellulose nanofiber membrane and application as adsorbent and catalyst. J. Colloid Interface Sci. 2015, 440, 32–38. [Google Scholar] [CrossRef]
- Chitpong, N.; Husson, S.M. Polyacid functionalized cellulose nanofiber membranes for removal of heavy metals from impaired waters. J. Membr. Sci. 2017, 523, 418–429. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Gutierrez, L.; Nxumalo, E.N.; Mhlanga, S.D. Water as the Pore Former in the Synthesis of Hydrophobic PVDF Flat Sheet Membranes for Use in Membrane Distillation. Hydro Sci. Mar. Eng. 2019, 1, 1–9. [Google Scholar] [CrossRef]
- Masheane, M.L.; Nthunya, L.N.; Malinga, S.P.; Nxumalo, E.N.; Mamba, B.B.; Mhlanga, S.D. Synthesis of Fe-Ag/f-MWCNT/PES nanostructured-hybrid membranes for removal of Cr(VI) from water. Sep. Purif. Technol. 2017, 184, 79–87. [Google Scholar] [CrossRef]
- Khumalo, N.; Nthunya, L.; De Canck, E.; Derese, S.; Verliefde, A.; Kuvarega, A.; Mamba, B.; Mhlanga, S.; Dlamini, D. Congo red dye removal by direct membrane distillation using PVDF/PTFE membrane. Sep. Purif. Technol. 2019, 211, 578–586. [Google Scholar] [CrossRef]
- Gebreslase, G.A. Review on Membranes for the Filtration of Aqueous Based Solution: Oil in Water Emulsion. J. Membr. Sci. Technol. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Dai, Z.; Ottesen, V.; Deng, J.; Helberg, R.M.L.; Deng, L. A Brief Review of Nanocellulose Based Hybrid Membranes for CO2 Separation. Fibers 2019, 7, 40. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Masheane, M.L.; Malinga, S.P.; Nxumalo, E.N.; Barnard, T.G.; Kao, M.; Tetana, Z.N.; Mhlanga, S.D. Greener Approach To Prepare Electrospun Antibacterial β-Cyclodextrin/Cellulose Acetate Nanofibers for Removal of Bacteria from Water. ACS Sustain. Chem. Eng. 2016, 5, 153–160. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Derese, S.; Gutierrez, L.; Verliefde, A.R.; Mamba, B.B.; Barnard, T.G.; Mhlanga, S.D. Green synthesis of silver nanoparticles using one-pot and microwave-assisted methods and their subsequent embedment on PVDF nanofibre membranes for growth inhibition of mesophilic and thermophilic bacteria. New J. Chem. 2019, 43, 4168–4180. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Masheane, M.L.; Malinga, S.P.; Nxumalo, E.N.; Mamba, B.B.; Mhlanga, S.D. Thermally and mechanically stable β-cyclodextrin/cellulose acetate nanofibers synthesized using an environmentally benign procedure. Int. J. Smart Nano Mater. 2017, 8, 1–19. [Google Scholar] [CrossRef]
- Xiang Chunhui, F.M.W. Nanocomposite Fibers Electrospun from Biodegradable Polymer. In Proceedings of the 235th ACS National Meeting, New Orleans, LA, USA, 17 March 2008. [Google Scholar]
- Nthunya, L.N.; Gutierrez, L.; Nxumalo, E.N.; Verliefde, A.R.; Mhlanga, S.D.; Onyango, M.S. f-MWCNTs/AgNPs-coated superhydrophobic PVDF nanofibre membrane for organic, colloidal, and biofouling mitigation in direct contact membrane distillation. J. Environ. Chem. Eng. 2020, 8, 103654. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Gutierrez, L.; Derese, S.; Nxumalo, E.N.; Verliefde, A.R.; Mamba, B.B.; Mhlanga, S.D. A review of nanoparticle-enhanced membrane distillation membranes: Membrane synthesis and applications in water treatment. J. Chem. Technol. Biotechnol. 2019, 94, 2757–2771. [Google Scholar] [CrossRef]
- Karim, Z.; Mathew, A.P.; Grahn, M.; Mouzon, J.; Oksman, K. Nanoporous membranes with cellulose nanocrystals as functional entity in chitosan: Removal of dyes from water. Carbohydr. Polym. 2014, 112, 668–676. [Google Scholar] [CrossRef]
- Zhang, Q.G.; Deng, C.; Soyekwo, F.; Liu, Q.L.; Zhu, A.M. Sub-10 nm Wide Cellulose Nanofibers for Ultrathin Nanoporous Membranes with High Organic Permeation. Adv. Funct. Mater. 2015, 26, 792–800. [Google Scholar] [CrossRef]
- Li, S.; Gao, Y.; Bai, H.; Zhang, L.; Qu, P.; Bai, L. Preparation and characteristics of polysulfone dialysis composite mem-branes modified with nanocrystalline cellulose. BioResources 2011, 6, 1670–1680. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, X.; Liu, Y.; Zhang, L. Enhancing the Compatibility, Hydrophilicity and Mechanical Properties of Polysulfone Ultrafiltration Membranes with Lignocellulose Nanofibrils. Polymers 2016, 8, 349. [Google Scholar] [CrossRef]
- Zhang, D.; Karkooti, A.; Liu, L.; Sadrzadeh, M.; Thundat, T.; Liu, Y.; Narain, R. Fabrication of antifouling and antibacterial polyethersulfone (PES)/cellulose nanocrystals (CNC) nanocomposite membranes. J. Membr. Sci. 2018, 549, 350–356. [Google Scholar] [CrossRef]
- Zhong, L.; Ding, Z.; Li, B.; Zhang, L. Preparation and Characterization of Polysulfone/Sulfonated Polysulfone/Cellulose Nanofibers Ternary Blend Membranes. BioResources 2015, 10, 2936–2948. [Google Scholar] [CrossRef][Green Version]
- Lv, J.; Zhang, G.; Zhang, H.; Zhao, C.; Yang, F. Improvement of antifouling performances for modified PVDF ultrafiltration membrane with hydrophilic cellulose nanocrystal. Appl. Surf. Sci. 2018, 440, 1091–1100. [Google Scholar] [CrossRef]
- Chen, F.; Sun, Z. Preparation of homogeneous grafting cellulose and partial substitution for polyethersulfone membrane material. Carbohydr. Polym. 2013, 95, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Metreveli, G.; Wågberg, L.; Emmoth, E.; Belák, S.; Strømme, M.; Mihranyan, A. A Size-Exclusion Nanocellulose Filter Paper for Virus Removal. Adv. Healthc. Mater. 2014, 3, 1546–1550. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Abou-Zeid, R.; Hassan, E.; Berglund, L.; Aitomäki, Y.; Oksman, K. Membranes Based on Cellulose Nanofibers and Activated Carbon for Removal of Escherichia coli Bacteria from Water. Polymers 2017, 9, 335. [Google Scholar] [CrossRef] [PubMed]
- Goetz, L.A.; Naseri, N.; Nair, S.S.; Karim, Z.; Mathew, A.P. All cellulose electrospun water purification membranes nanotextured using cellulose nanocrystals. Cellulose 2018, 25, 3011–3023. [Google Scholar] [CrossRef]
- Asempour, F.; Emadzadeh, D.; Matsuura, T.; Kruczek, B. Synthesis and characterization of novel Cellulose Nanocrystals-based Thin Film Nanocomposite membranes for reverse osmosis applications. Desalination 2018, 439, 179–187. [Google Scholar] [CrossRef]
- Wang, X.; Ma, H.; Chu, B.; Hsiao, B.S. Thin-film nanofibrous composite reverse osmosis membranes for desalination. Desalination 2017, 420, 91–98. [Google Scholar] [CrossRef]
- Wang, X.; Yeh, T.-M.; Wang, Z.; Yang, R.; Wang, R.; Ma, H.; Hsiao, B.S.; Chu, B. Nanofiltration membranes prepared by interfacial polymerization on thin-film nanofibrous composite scaffold. Polymer 2014, 55, 1358–1366. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, H.; Hsiao, B.S.; Chu, B. Nanofibrous ultrafiltration membranes containing cross-linked poly(ethylene glycol) and cellulose nanofiber composite barrier layer. Polymer 2014, 55, 366–372. [Google Scholar] [CrossRef]

| NCs | CNTs | Ref. | |||
|---|---|---|---|---|---|
| CNCs | CNFs | MWCNTs | SWCNTs | ||
| Sources | Cotton, hemp, wood, algae, sugar beet, straw, potatoes. | Fossil fuel | [35] | ||
| Length (nm) | 100–250 | >1000 | >1000 | >1000 | [35,36] |
| Diameter (nm) | 5–70 | 5–100 | 2–100 | 0.4–2 | [35,36] |
| Tensile strength (GPa) | 2–6 | 2–4 | 11–63 | 13–52 | [37,38] |
| Young’s Modulus (GPa) | 50–143 | 15–150 | 0.27–0.95 | 0.32–1.47 | [37,38] |
| Ecotoxicity | Low toxicity Inflammatory cytokines in few cases | Low toxicity Pulmonary inflammation reported in few cases | Highest risk recorded with inhalation and dermal exposure Inflammation and oxidative stress observed | [39,40] | |
| Main Applications | Packaging, cement, paper, automotive, food, water treatment, biomedical and medical | Medical, packaging, paint Water treatment | Solar systems Micro-electronics, water treatment | [41,42] | |
| Disposal | Biodegradable | Non-biodegradable | [39,43] | ||
| Material and Technology | Change in Stress (%) | Change (%) in Young’s Modulus | Ref. |
|---|---|---|---|
| UF (PES + 1 wt% CNFs) | 42.2 | 10.2 | [53] |
| UF(PES + 4 wt% CNFs) | 7.8 | −5.8 | [53] |
| NF (CNFs + PHB) | 10.1 | 24 | [57] |
| UF (PVDF + 1 wt% CNCs) | 34.85 | - | [47] |
| NF (CNFs + CdSe) | −11.1 | −52.2 | [58] |
| NF (PVA + 5 wt % CNCs) | 31.9 | - | [55] |
| MD (PVDF-HFP + 2 wt % CNCs) | 36.5 | 45.8 | [54] |
| UF (CA + CNFs) | 31.5 | −30.3 | [49] |
| UF (PVA + CNCs + Ag NPs) | 42.37 | - | [55] |
| UF (PVA + 1 wt % CNCs) | 69.89 | - | [55] |
| Modification Technique | Modifying Agent | Membrane Technology | Application | PWF | Ref. |
|---|---|---|---|---|---|
| TEMPO-oxidation | TEMPO | NF | Organic solvent rejection | - | [104] |
| TEMPO-oxidation | TEMPO | UF | Polybead microsphere rejection (>99.9%) | 5-fold higher compared to a commercial UF membrane | [105] |
| TEMPO-oxidation | TEMPO | UF | BSA rejection (85%) | 100 L·m−2·h−1 | [106] |
| TEMPO-oxidation | TEMPO | NF | Micro-pollutants and divalent ions rejection | 180 L·m−2·h−1·MPa−1 | [107] |
| Cationization | EPTMAC | - | Dyes adsorption | - | [108] |
| Cationization | EPTMAC | - | Proteins adsorption | - | [109] |
| Esterification | Meldrum’s acid | MF | Fe2O3 and dyes rejection | - | [110] |
| Esterification | H3PO4 | UF | Copper II (Cu2+) ions adsorption (190 mg·m−2) | 2 L·m−2·h−1·MPa−1 | [111] |
| Silylation | APTES | MF | Licorice wastewater treatment | - | [62] |
| Silylation | MPS | UF | BSA rejection (>98%) | >90 L·m−2·h−1 | [101] |
| Silylation | APTES | UF | Cu2+ removal (90%) and dye removal (99%) | 28.42 L·m−2·h−1 | [4] |
| Silylation | APTES | Forward Osmosis (FO) | Total organic carbon rejection (90.3%) | 10.24 L·m−2·h−1·bar−1 | [112] |
| Amidation | PEI/GDE | - | Cu2+ and Pb2+ ions adsorption (>90%) | - | [113] |
| Polymer grafting | Poly(acrylic acid) | UF | Cadmium ions adsorption | - | [114] |
| Polymer grafting | Poly(N-isopropylacrylamide) | NF | Micro-pollutants and divalent ions rejection | 45 L·m−2·h−1·MPa−1 | [107] |
| Fabrication Technique | Polymers | Application | PWF | Contact Angle | Process Efficiency | Ref. |
|---|---|---|---|---|---|---|
| Phase inversion | PES + CNFs | UF for BSA rejection | 813.3 L·m−2·h−1 | 45.8° | 95% removal | [53] |
| PVDF + CNCs | UF for BSA rejection | 230.8 L·m−2·h−1 | - | 92.5% removal | [47] | |
| PS + CNCs | UF for BSA rejection | - | <50° | >96% removal | [128] | |
| PES + Lignin CNFs | UF for BSA rejection | 692.3 L·m−2·h−1 | <50° | >95% removal | [129] | |
| PS + Lignin CNFs | UF for BSA rejection | 723 L·m−2·h−1 | 33.9° | 97.1% removal | [129] | |
| PES + CNCs | UF for BSA rejection | 195 L·m−2·h−1 | 43° | 97% removal | [130] | |
| PSF + SPSF + CNFs | UF for BSA rejection | 137.6 L·m−2·h−1 | 59.5° | 95.8% removal | [131] | |
| CTA + CNFs | UF for proteins rejection | 224.68 L·m−2·h−1 | 47.10° | >95% removal | [49] | |
| PVDF + CNCs | UF for proteins rejection | 206.9 L·m−2·h−1 | 73.95° | 88.2% removal | [132] | |
| Vacuum Filtration | CNFs + CNCs | NF for Ag+, Cu2+ and Fe2+/Fe3+ rejection | 6.0 L·m−2·h−1 | - | >99.9% removal | [23] |
| BNCs | UF for Ca2+ and SO42− rejection. | 25 L·m−2·h−1 | - | - | [102] | |
| CNFs | UF for Ca2+ and SO42− rejection. | 10 L·m−2·h−1 | - | - | [102] | |
| CNCs | UF for Ca2+ and SO42− rejection. | 2.0 L·m−2·h−1 | - | - | [102] | |
| TEMPO-CNFs | UF for Ca2+ and SO42− rejection. | 2.0 L·m−2·h−1 | - | 34% removal | [102] | |
| CNCs | Oil/water separation | ~750 L·m−2·h−1 | 31.6° | >99.9% removal | [133] | |
| CNFs | UF for virus removal | - | - | LRV ≥ 6.3 | [134] | |
| Filter Paper + AC + TEMPO-CNFs + CNFs | NF for E.coli removal | 425 L·m−2·h−1 | - | >96% removal | [135] | |
| Cellulose Microfiber + CNCs | UF for Ag+, Cu2+ and Fe2+/Fe3+ rejection | 900–4000 L·m−2·h−1 | - | >99.9% removal | [50] | |
| Cellulose Filter Paper + EPTMAC-CNFs | UF for NO3− removal | 30 L·m−2·h−1 | - | ~13 mg·g−1 | [100] | |
| Cellulose Filter Paper + Phosphorylated -CNFs | UF for Cu2+ removal | 2 L·m−2·h−1 | - | 19.6 mg·g−1 | [111] | |
| Electrospinning | PAN + PET + CNFs | MF for E.coli, MS2, Cr6+ and Pb2+ removal. | ~1300 L·m−2·h−1 | - | LRV of 6 (E. coli) LRV of 4 (MS2) 100 mg·g−1 (Cr6+) 260 mg·g−1 (Pb2+) | [67] |
| PAN + PET + CNCs | MF for E.coli, B. diminuta, MS2 and CV dye. | 1 138.7 L·m−2·h−1 | - | LRV of 6 (E. coli) LRV of 6 (B. diminuta) LRV of 2 (MS2) 4.3 mg·g−1 (CV dye) | [48] | |
| PVDF-HFP + CNCs | MD for salts rejections | 10.2–11.5 L·m−2·h−1 | 123° | 99% removal | [54] | |
| CA + CNCs | UF for 0.5–2.0 μm solid particles | >2500 L·m−2·h−1 | 0°–50.3° | 90–99% dye removal 20–56% particle removal | [136] | |
| Interfacial polymerization | PES + PIP + CNCs | NF for Na2SO4 and MgSO4 rejection | 16.8 L·m−2·h−1 | 37.5° | 98 % removal (Na2SO4) 97.5 % removal (MgSO4) | [63] |
| PES + PIP + CNCs | NF for dyes rejection | 16.8 L·m−2·h−1 | 37.5° | 99.75 % removal (Crystal violet) 98.98 % removal (Methylene blue) | [63] | |
| PSF + PA + CNCs | RO for salt rejection | 63 L·m−2·h−1 | 52.7° | 98.5% salt removal | [137] | |
| PET + PAN + CNFs | RO for NaCl rejection | 28.6 L·m−2·h−1 | - | 96.5% removal | [138] | |
| PET + PAN + PA + CNFs | NF for salt rejection | >28.6 L·m−2·h−1 | 55.8° | 91% removal | [139] | |
| PET + PAN + PEG + CNFs | UF for PEG 4600 rejection | 40 L·m−2·h−1 | 14° | >90% removal | [140] | |
| Freeze-drying | Chitosan + CNCs | UF for dyes removal | 64 L·m−2·h−1 | - | 98% removal (Victoria Blue 2B) 90% removal (Methyl Violet 2B) 78 % removal (Rhodamine 6G) | [126] |
| Alumina filter paper + CNFs | NF for rejection of 10 nm particles | 2.43–5.49 L·m−2·h−1 | - | >80% removal | [127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbakop, S.; Nthunya, L.N.; Onyango, M.S. Recent Advances in the Synthesis of Nanocellulose Functionalized–Hybrid Membranes and Application in Water Quality Improvement. Processes 2021, 9, 611. https://doi.org/10.3390/pr9040611
Mbakop S, Nthunya LN, Onyango MS. Recent Advances in the Synthesis of Nanocellulose Functionalized–Hybrid Membranes and Application in Water Quality Improvement. Processes. 2021; 9(4):611. https://doi.org/10.3390/pr9040611
Chicago/Turabian StyleMbakop, Sandrine, Lebea N. Nthunya, and Maurice S. Onyango. 2021. "Recent Advances in the Synthesis of Nanocellulose Functionalized–Hybrid Membranes and Application in Water Quality Improvement" Processes 9, no. 4: 611. https://doi.org/10.3390/pr9040611
APA StyleMbakop, S., Nthunya, L. N., & Onyango, M. S. (2021). Recent Advances in the Synthesis of Nanocellulose Functionalized–Hybrid Membranes and Application in Water Quality Improvement. Processes, 9(4), 611. https://doi.org/10.3390/pr9040611






