Discovery of Cell Aggregate-Inducing Peptides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Peptide Synthesis
2.3. Cells and Culture
2.4. Cell Cytotoxicity Test
2.5. Cell Aggregate-Inducing Experiment
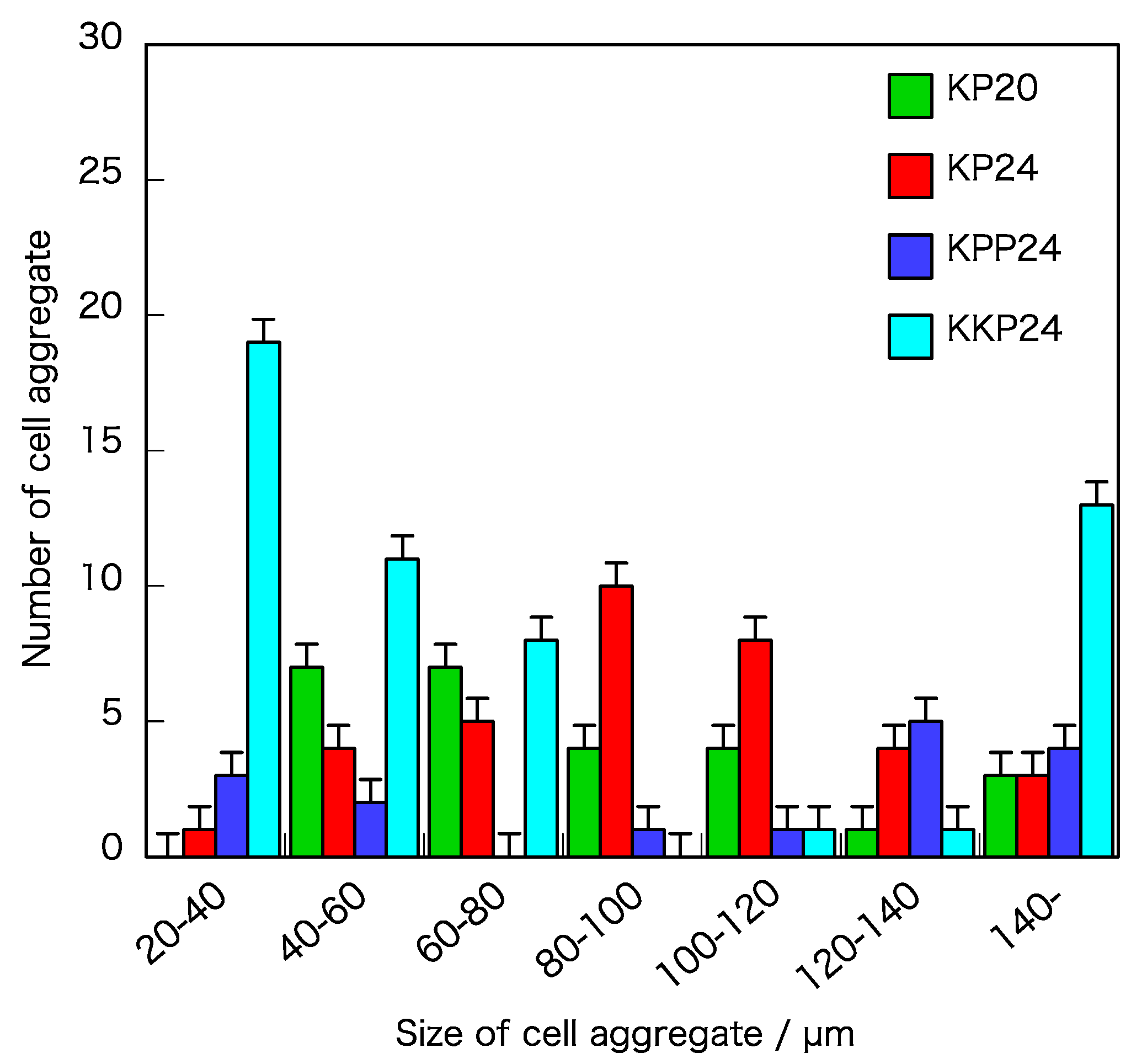
2.6. Measuring the Size of Cell Aggregates and Sphericity
3. Results and Discussion
3.1. Peptide Synthesis
3.2. Cell Cytotoxicity Test
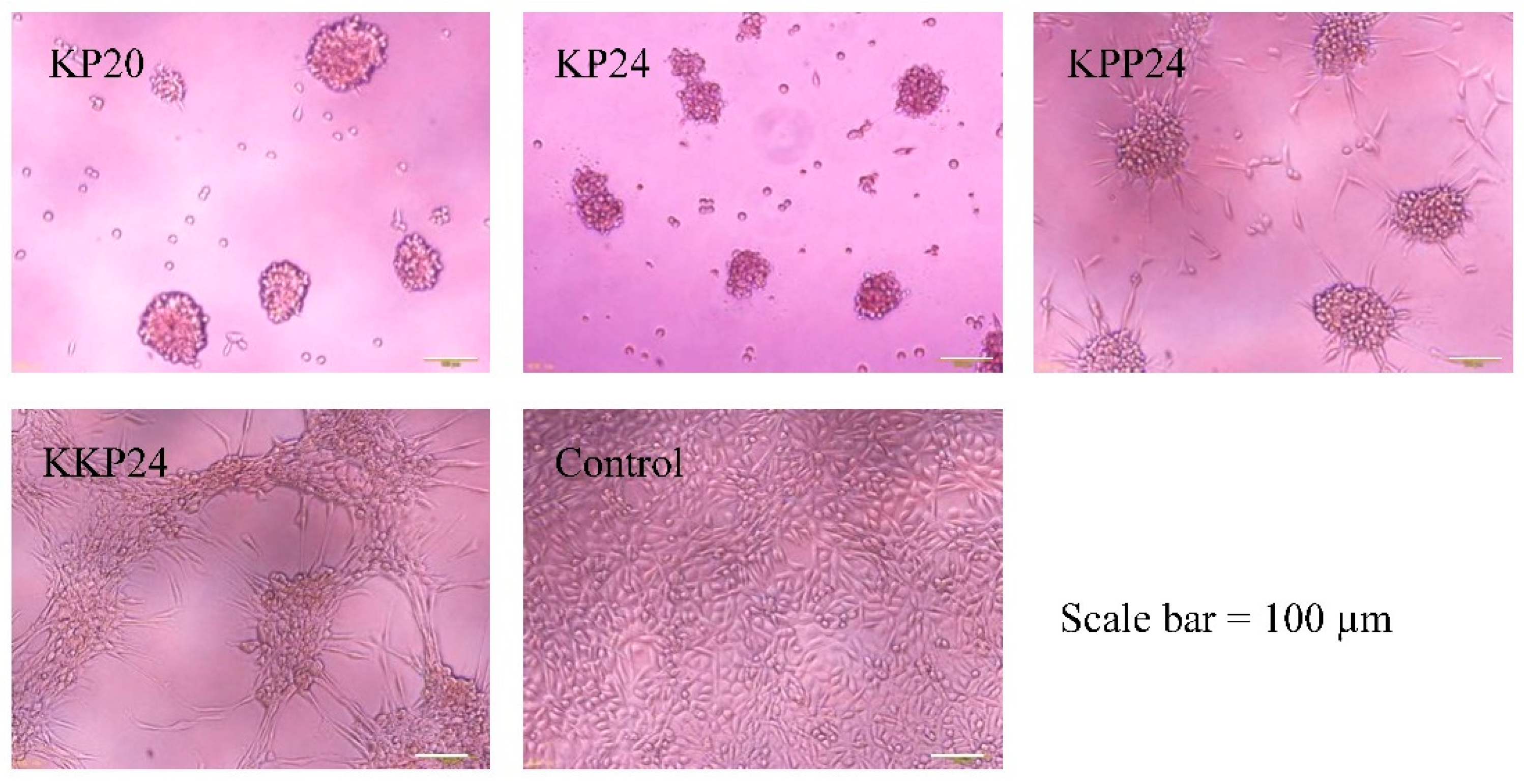
3.3. Cell Aggregate-Inducing Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef]
- Abbott, A. Cell culture: Biology’s new dimension. Nature 2003, 424, 870–872. [Google Scholar] [CrossRef]
- Kim, S.; Kim, E.-M.; Yamamoto, M.; Park, H.; Shin, H. Engineering Multi-Cellular Spheroids for Tissue Engineering and Regenerative Medicine. Adv. Healthc. Mater. 2020. [Google Scholar] [CrossRef]
- Lin, R.-Z.; Chang, H.-Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 2008, 3, 1172–1184. [Google Scholar] [CrossRef]
- Kozyra, M.; Johansson, I.; Nordling, A.; Ullah, S.; Lauschke, V.M.; Ingelman-Sundberg, M. Human hepatic 3D spheroids as a model for steatosis and insulin resistance. Sci. Rep. 2018, 8, 14297. [Google Scholar] [CrossRef]
- Bauer, S.; Huldt, C.W.; Kanebratt, K.P.; Durieux, I.; Gunne, D.; Andersson, S.; Ewart, L.; Haynes, W.G.; Maschmeyer, I.; Winter, A.; et al. Functional coupling of human pancreatic islets and liver spheroids on-a-chip: Towards a novel human ex vivo type 2 diabetes model. Sci. Rep. 2017, 7, 14620. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, T.; Xu, A.; Zhang, L. 3D spheroid culture enhances survival and therapeutic capacities of MSCs injected into ischemic kidney. J. Cell. Mol. Med. 2016, 20, 1203–1213. [Google Scholar] [CrossRef]
- Alhaque, S.; Themis, M.; Rashidi, H. Three-dimensional cell culture: From evolution to revolution. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170216. [Google Scholar] [CrossRef]
- Fennema, E.; Rivron, N.; Rouwkema, J.; van Blitterswijk, C.; de Boer, J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013, 31, 108–115. [Google Scholar] [CrossRef]
- Cesarz, Z.; Tamama, K. Spheroid Culture of Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 9176357. [Google Scholar] [CrossRef]
- Park, I.-S.; Chung, P.-S.; Ahn, J.C. Enhancement of Ischemic Wound Healing by Spheroid Grafting of Human Adipose-Derived Stem Cells Treated with Low-Level Light Irradiation. PLoS ONE 2015, 10, e0122776. [Google Scholar]
- Guo, L.; Ge, J.; Zhou, Y.; Wang, S.; Zhao, R.C.H.; Wu, Y. Three-dimensional spheroid-cultured mesenchymal stem cells devoid of embolism attenuate brain stroke injury after intra-arterial injection. Stem Cells Dev. 2014, 23, 978–989. [Google Scholar] [CrossRef]
- Hsu, S.H.; Hsieh, P.S. Self-assembled adult adipose-derived stem cell spheroids combined with biomaterials promote wound healing in a rat skin repair model. Wound Repair Regen. 2015, 23, 57–64. [Google Scholar] [CrossRef]
- Laschke, M.W.; Menger, M.D. Life is 3D: Boosting Spheroid Function for Tissue Engineering. Trends Biotechnol. 2017, 35, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, S.; Diaz-Romero, J.; Aigner, T.; Heini, P.; Mainil-Varlet, P.; Nesic, D. Micromass co-culture of human articular chondrocytes and human bone marrow mesenchymal stem cells to investigate stable neocartilage tissue formation in vitro. Eur. Cell Mater. 2010, 20, 245–259. [Google Scholar] [CrossRef]
- Li, Y.; Guo, G.; Li, L.; Chen, F.; Bao, J.; Shi, Y.; Bu, H. Three-dimensional spheroid culture of human umbilical cord mesenchymal stem cells promotes cell yield and stemness maintenance. Cell Tissue Res. 2015, 360, 297–307. [Google Scholar] [CrossRef]
- Kibschull, M. Differentiating Mouse Embryonic Stem Cells into Embryoid Bodies in AggreWell Plates. Cold Spring Harb. Protoc. 2017. [Google Scholar] [CrossRef]
- Fields, G.B.; Noble, R.L. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int. J. Pept. Protein Res. 1990, 35, 162–214. [Google Scholar] [CrossRef]
- Alberico, F.; Kneib-Cordonier, N.; Biancalana, S.; Gera, L.; Masada, R.I.; Hudson, D.; Barany, G. Preparation and application of the 5-(4-(9-fluorenylmethyloxycarbonyl)aminomethyl-3,5-dimethoxyphenoxy)-valeric acid (PAL) handle for the solid-phase synthesis of C-terminal peptide amides under mild conditions. J. Org. Chem. 1990, 55, 3730–3743. [Google Scholar] [CrossRef]
- Yajima, H.; Fujii, N.; Funakoshi, S.; Watanabe, T.; Murayama, E.; Otaka, A. New strategy for the chemical synthesis of proteins. Tetrahedron 1988, 44, 805–819. [Google Scholar] [CrossRef]
- Hioki, K.; Kobayashi, K.; Ohkihara, R.; Tani, S.; Kunishima, M. Preparation of weienreb amide using 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMT-MM). Chem. Pham. Bull. 2004, 52, 470–472. [Google Scholar] [CrossRef][Green Version]
- Cooper, T.M.; Stone, M.O. Investigation of Self-Assembly upon Formation of an Electrostatic Complex of Congo Red and a Helical Peptide. Langmuir 1998, 14, 6662–6668. [Google Scholar] [CrossRef]
- Nan, Y.H.; Lee, S.H.; Kim, H.J.; Shin, S.Y. Mammalian cell toxicity and candidacidal mechanism of Arg- or Lys-containing Trp-rich model antimicrobial peptides and their d-enantiomeric peptides. Peptides 2010, 31, 1826–1831. [Google Scholar] [CrossRef] [PubMed]
- Tamamura, H.; Arakaki, R.; Funakoshi, H.; Imai, M.; Otaka, A.; Ibuka, T.; Nakashima, H.; Murakami, T.; Waki, M.; Matsumoto, A.; et al. Effective lowly cytotoxic analogs of an HIV-cell fusion inhibitor, T22 ([Tyr5,12, Lys7]-polyphemusin II). Med. Chem. 1998, 6, 231–238. [Google Scholar] [CrossRef]
- Yang, S.T.; Shin, S.Y.; Lee, C.W.; Kim, Y.C.; Hahm, K.S.; Kim, J.I. Selective cytotoxicity following Arg-to-Lys substitution in tritrpticin adopting a unique amphipathic turn structure. FEBS Lett. 2003, 540, 229–233. [Google Scholar] [CrossRef]
- Turner, P.A.; Weeks, C.A.; McMurphy, A.J.; Janorkar, A.V. Spheroid organization kinetics of H35 rat hepatoma model cell system on elastin-like polypeptide-polyethyleneimine copolymer substrates. J. Biomed. Mater. Res. A 2014, 102, 852–861. [Google Scholar] [CrossRef]
- Weeks, C.A.; Aden, B.; Zhang, J.; Singh, A.; Hickey, R.D.; Kilbey, S.M.; Nyberg, S.L.; Janorkar, A.V. Effect of amine content and chemistry on long-term, three-dimensional hepatocyte spheroid culture atop aminated elastin-like polypeptide coatings. J. Biomed. Mater. Res. A 2017, 105, 377–388. [Google Scholar] [CrossRef]
- Weeks, C.A.; Newman, K.; Turner, P.A.; Rodysill, B.; Hickey, R.D.; Nyberg, S.L.; Janorkar, A.V. Suspension culture of hepatocyte-derived reporter cells in presence of albumin to form stable three-dimensional spheroids. Biotechnol. Bioeng. 2013, 110, 2548–2555. [Google Scholar] [CrossRef]
- Song, L.; Yuan, X.; Jones, Z.; Griffin, K.; Zhou, Y.; Ma, T.; Li, Y. Assembly of Human Stem Cell-Derived Cortical Spheroids and Vascular Spheroids to Model 3-D Brain-like Tissues. Sci. Rep. 2019, 9, 5977. [Google Scholar] [CrossRef] [PubMed]

| Type | Amino Acid Sequences (One Letter Amino Acid Code) | Code |
|---|---|---|
| KP type | KPKPKPKPKPKPKPKPKPKP | KP20 |
| KPKPKPKPKPKPKPKPKPKPKPKP | KP24 | |
| KPKPKPKPKPKPKPKPKPKPKPKPPKPKP | KP28 | |
| KPKPKPKPKPKPKPKPKPKPKPKPPKPKPPKPKP | KP32 | |
| KA type | KAKAKAKAKAKAKAKA | KA16 |
| KAKAKAKAKAKAKAKAKAKA | KA20 | |
| KAKAKAKAKAKAKAKAKAKAKAKA | KA24 | |
| KK type | KKKKKKKKKKKKKKKK | K16 |
| KKKKKKKKKKKKKKKKKKKK | K20 | |
| KKKKKKKKKKKKKKKKKKKKKKKK | K24 | |
| K and P type | KPPKPPKPPKPPKPPKPPKPPKPP | KPP24 |
| KKPKKPKKPKKPKKPKKPKKPKKP | KKP24 | |
| RP type | RPRPRPRPRPRPRPRPRPRPRPRP | RP24 |
| Code | MALDI-TOF-MS Data | Cell Toxicity (IC50: mg/mL) | Cell Aggregate-Inducing Activity | |||
|---|---|---|---|---|---|---|
| Fw (Cal.) | [M]+, [M+1]+ | [M+Na]+ | [M+K]+ | |||
| KP20 | 2271.01 | 2272.7 | - | 2311.4 | - | ○ |
| KP24 | 2721.61 | 2721.9 | 2743.4 | 2760.6 | - | ○ |
| KP28 | 3172.21 | 3170.1 | 3192.1 | - | 0.1 < 1.0 *1 | - |
| KP32 | 3622.81 | 3619.3 | 3643.2 | 3657.5 | 0.01 < 0.1 *2 | - |
| KA16 | 1612.09 | 1613.2 | - | - | - | - |
| KA20 | 2010.61 | 2011.7 | 2033.6 | - | - | - |
| KA24 | 2409.13 | 2410.9 | - | - | - | - |
| K16 | 2068.79 | - | 2091.4 | - | 0.1 < 1.0 *3 | - |
| K20 | 2581.45 | 2580.8 | 2603.3 | - | 0.1 < 1.0 *4 | - |
| K24 | 3094.15 | 3093.7 | 3115.7 | - | 0.1 < 1.0 *5 | - |
| KPP24 | 2597.37 | 2598.8 | 2620.1 | 2637.2 | - | ○ |
| KKP24 | 2845.85 | 2847.3 | - | - | - | ○ |
| RP24 | 3057.63 | 3057.3 | - | - | 0.01 < 0.1 *6 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Futaki, Y.; Amimoto, I.; Tanaka, M.; Ito, T.; Hirano, Y. Discovery of Cell Aggregate-Inducing Peptides. Processes 2021, 9, 538. https://doi.org/10.3390/pr9030538
Futaki Y, Amimoto I, Tanaka M, Ito T, Hirano Y. Discovery of Cell Aggregate-Inducing Peptides. Processes. 2021; 9(3):538. https://doi.org/10.3390/pr9030538
Chicago/Turabian StyleFutaki, Yudai, Ikumi Amimoto, Megumi Tanaka, Tomoki Ito, and Yoshiaki Hirano. 2021. "Discovery of Cell Aggregate-Inducing Peptides" Processes 9, no. 3: 538. https://doi.org/10.3390/pr9030538
APA StyleFutaki, Y., Amimoto, I., Tanaka, M., Ito, T., & Hirano, Y. (2021). Discovery of Cell Aggregate-Inducing Peptides. Processes, 9(3), 538. https://doi.org/10.3390/pr9030538






