Exploration of Active Site-Directed Plasmin Inhibitors: Beyond Tranexamic Acid
Abstract
1. Introduction
2. Active Site (AS) and LBS-Directed Inhibitors
3. Design and Synthesis of Substrate-Based Inhibitors
4. Substitution of P2, P1 and P1′ Residues
4.1. Incorporation of TXA
4.2. Switch from Lys to Phe/Tyr
4.3. Discovery of H-TXA-Tyr(OPic)-NH-Octyl (36, YO-2)
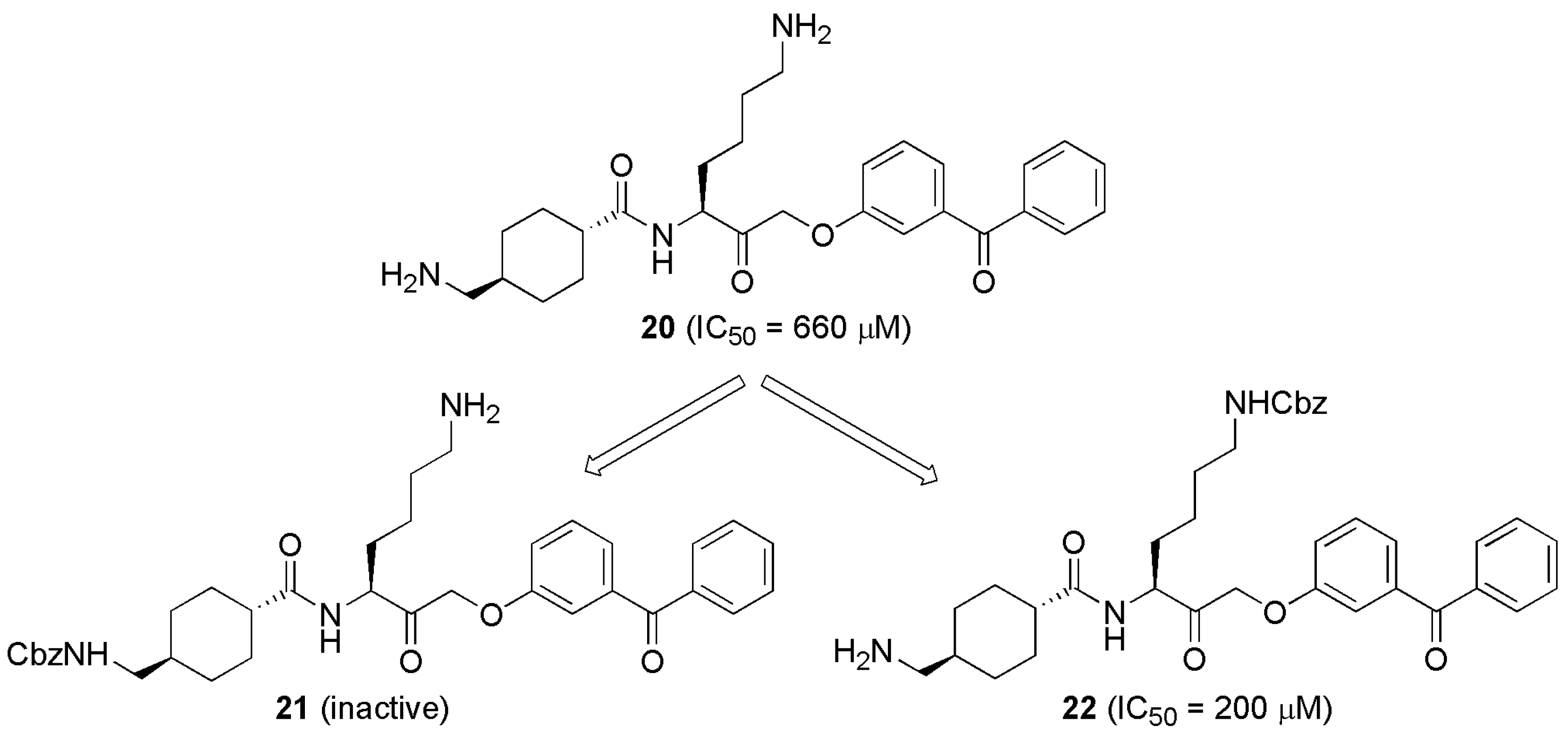
5. Improvement of Specificity (Plm/uPA)
6. X-ray Crystal Structures of 35 and 39 in the Complex with μPlm
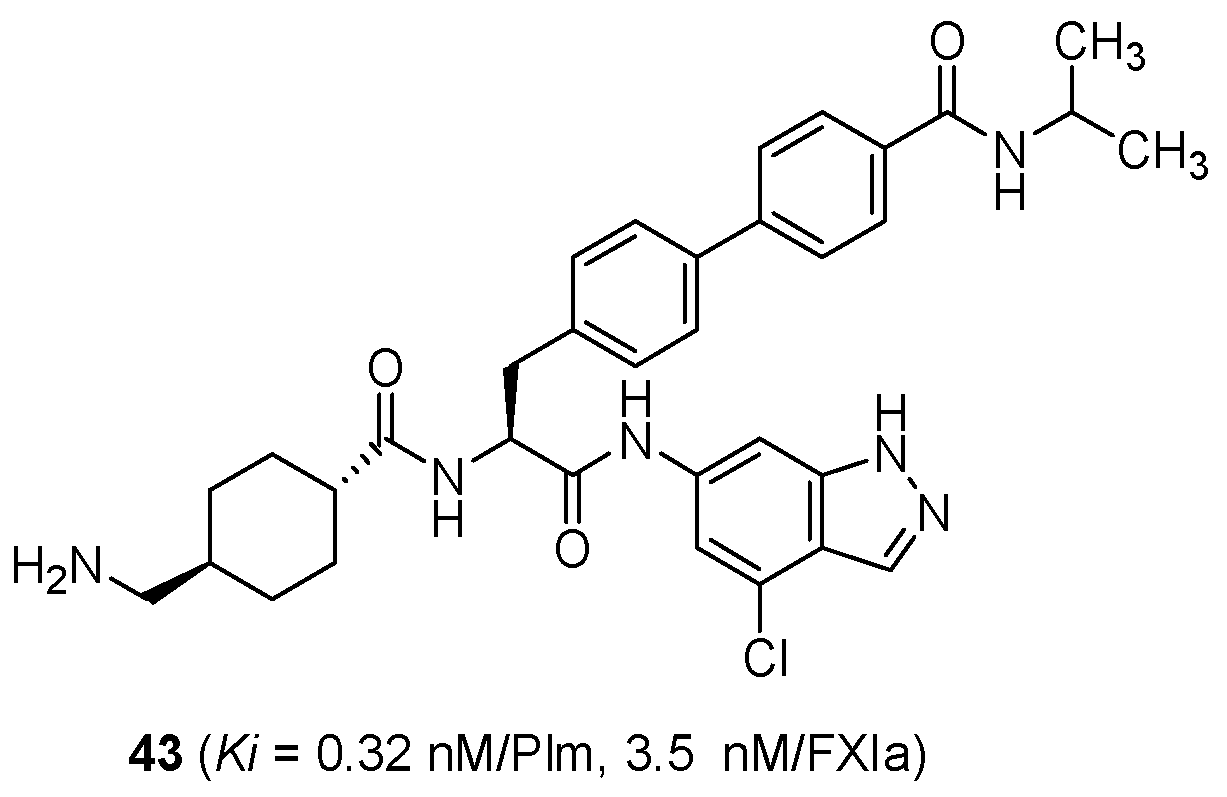
7. Challenge for Application beyond TXA
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bachmann, F. The Plasminogen-Plasmin Enzyme System (Chapter 84). Hemostasis and Thrombosis: Basic Principles and Clinical Practice, 3rd ed.; Colman, R.W., Hirsh, J., Marder, V.J., Zalzman, E.W., Eds.; Lippincott Company: Philadelphia, PA, USA, 1994; pp. 1592–1600. [Google Scholar]
- Angles-Cano, E. Overview on fibrinolysis: Plasminogen activation pathway on fibrin and cell surface. Chem. Phys. Lipids 1994, 68, 353–362. [Google Scholar] [CrossRef]
- Hoylaerts, M.; Rijken, D.C.; Lijinen, H.R.; Collen, D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J. Biol. Chem. 1982, 257, 2912–2919. [Google Scholar] [CrossRef]
- Lerch, P.G.; Rickli, E.E.; Lergier, W.; Gillessen, D. Localization of individual lysine-binding regions in human plasminogen and investigation on their complex-forming properties. Eur. J. Biochem. 1980, 107, 7–13. [Google Scholar] [CrossRef]
- Lijnen, H.R.; Van Hoef, B.; Collen, D. On the molecular interactions between fibrin, tissue-plasminogen activator and plasminogen. Thromb. Res. Suppl. 1990, 10, 45–54. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Cell surface remodeling by plasmin: A new function for an old enzyme. J. Biomed. Biotechnol. 2012, 2012, 564259. [Google Scholar] [CrossRef]
- Schaller, J.; Gerber, S.S. The plasmin-antiplasmin system, Structural and functional aspects. Cell Mol. Life Sci. 2011, 68, 785–801. [Google Scholar] [CrossRef] [PubMed]
- Lijnen, H.R. Plasmin and matrix metalloproteases in vascular remodeling. Thromb. Haemost. 2001, 86, 324–333. [Google Scholar]
- Tang, L.; Han, X. The urokinase plasminogen activator system in breast cancer invasion and metastasis. Biomed. Pharmacother. 2013, 67, 179–182. [Google Scholar] [CrossRef]
- Li, X.; Syrovent, T.; Ganze, F.; Pitterle, K.; Oberhunber, A.; Orend, K.H.; Simmet, T. Plasmin triggers chemotaxis of monocyte-derived dendritic cells through an Akt2-dependent pathway and promotes a T-helper type-1 response. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Syrovets, T.; Jendrach, M.; Rohwedder, A.; Scheule, A.; Simmer, T. Plasmin-induced expression of cytokines and tissue factor in human monocytes involves AP-1 and IKKbeta-mediated NF-kappaB activation. Blood 2001, 97, 3941–3950. [Google Scholar] [CrossRef]
- Li, Q.; Laumonnier, Y.; Simmet, T. Plasmin triggers cytokine induction in human monocyte-derived macrophages. Arterioscler Thromb. Vasc. Biol. 2007, 27, 1383–1389. [Google Scholar] [CrossRef]
- Okada, Y.; Tsuda, Y.; Tada, M.; Wanaka, K.; Okamoto, U.; Hijikata-Okunomiya, A.; Okamoto, S. Development of potent and selective plasmin and plasma kallikrein inhibitors and studies on the structure-activity relationship. Chem. Pharm. Bull. 2000, 48, 1964–1972. [Google Scholar] [CrossRef] [PubMed]
- Szende, B.; Okada, Y.; Tsuda, Y.; Horvath, A.; Bökönyi, G.; Okamoto, S.; Wanaka, K.; Kéri, G. A novel plasmin-inhibitor inhibits the growth of human tumor xenografts and decreases metastasis number. In Vivo 2002, 16, 281–286. [Google Scholar] [PubMed]
- Dietrich, W.; Barankay, A.; Dilthey, G.; Henze, R.; Niekau, E.; Sebening, F.; Richter, J.A. Reduction of homologous blood requirement in cardiac surgery by intraoperative aprotinin application--clinical experience in 152 cardiac surgical patients. Thorac. Cardiovasc. Surg. 1989, 37, 92–98. [Google Scholar] [CrossRef]
- Okamoto, S.; Nakajima, T.; Okamoto, U.; Watanabe, H.; Iguchi, Y.; Igawa, T.; Chien, C.; Hayashi, T. A suppressing effect of epsilon-amino-N-caproic acid on the bleeding of dogs, produced with the activation of plasmin in the circulatory blood. Keio J. Med. 1959, 8, 247–266. [Google Scholar] [CrossRef]
- Okamoto, S.; Okamoto, U. Amino-methyl-cyclohexane-carboxylic Acid: AMCHA. Keio J. Med. 1962, 11, 105–115. [Google Scholar] [CrossRef]
- Roberts, I.; Perel, P.; Prieto-Merino, D.; Shakur, H.; Coate, T.; Hunt, B.J.; Lecky, F.; Brohi, K.; Willett, K. Effect of tranexamic acid on mortality in patients with traumatic bleeding: Prespecified analysis of data from randomised controlled trial. Brit. Med. J. 2012, 345, 5839–5847. [Google Scholar] [CrossRef]
- Wanaka, K.; Okamoto, S.; Horie, N.; Hijikata-Okunomiya, A.; Okamoto, U.; Naito, T.; Ohno, N.; Bohgaki, M.; Tsuda, Y.; Okada, Y. Use of an active center-directed plasmin inhibitor elucidates the multiplicity of plasmin actions. Thromb. Res. 1996, 82, 79–86. [Google Scholar] [CrossRef]
- Sander, T.C.; Seto, C.T. 4-Heterocyclohexanone-based inhibitors of the serine protease plasmin. J. Med. Chem. 1999, 42, 2969–2976. [Google Scholar] [CrossRef] [PubMed]
- Abato, P.; Yuen, C.M.; Cubanski, J.K.; Seto, C.T. Inhibitors of plasmin that extend into both the S and S’ binding sites: Cooperative interactions between S1 and S2. J. Org. Chem. 2002, 67, 1184–1191. [Google Scholar] [CrossRef]
- Dietrich, W.; Nicklish, S.; Koster, A.; Spannabl, M.; Giersiefen, H.; Van de Locht, A. CU-2010-a novel small molecule protease inhibitor with antifibrinolytic and anticoagulant properties. Anesthesiology 2009, 110, 123–130. [Google Scholar] [CrossRef]
- Saupe, S.M.; Leubner, S.; Betz, M.; Klebe, G.; Steinmetzer, T. Development of new cyclic plasmin inhibitors with excellent potency and selectivity. J. Med. Chem. 2013, 56, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Hinkes, S.; Wuttke, A.; Saupe, S.M.; Ivanova, T.; Wagner, S.; Knörlein, A.; Heine, A.; Klebe, G.; Steinmetzer, T. Optimization of cyclic plasmin inhibitors: From benzamidines to benzylamines. J. Med. Chem. 2016, 59, 6370–6386. [Google Scholar] [CrossRef] [PubMed]
- Teno, N.; Gohda, K.; Wanaka, K.; Sueda, T.; Tsuda, Y. Identification of novel plasmin inhibitors possessing nitrile moiety as warhead. Bioorg. Med. Chem. Lett. 2011, 21, 6305–6309. [Google Scholar] [CrossRef]
- Teno, N.; Gohda, K.; Yamashita, Y.; Otsubo, T.; Yamaguchi, M.; Wanaka, K.; Tsuda, Y. Plasmin inhibitors with hydrophobic amino acid-based linker between hydantoin moiety and benzimidazole scaffold enhance inhibitory activity. Bioorg. Med. Chem. Lett. 2016, 26, 2259–2261. [Google Scholar] [CrossRef]
- Swedberg, J.E.; Harris, J.M. Plasmin substrate binding site cooperativity guides the design of potent peptide aldehyde inhibitors. Biochemistry 2011, 50, 8454–8462. [Google Scholar] [CrossRef]
- Okada, Y.; Tsuda, Y.; Teno, N.; Wanaka, K.; Bohgaki, M.; Hijikata-Okunomiya, A.; Naito, T.; Okamoto, S. Synthesis of active center-directed peptide inhibitors of plasmin. Chem. Pharm. Bull. 1988, 36, 1289–1297. [Google Scholar] [CrossRef]
- Teno, N.; Wanaka, K.; Okada, Y.; Tsuda, Y.; Okamoto, U.; Hijikata-Okunomiya, A.; Naito, T.; Okamoto, S. Development of active center-directed inhibitors against plasmin. Chem. Pharm. Bull. 1991, 39, 2340–2346. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Teno, N.; Wanaka, K.; Okada, Y.; Taguchi, H.; Okamoto, U.; Hijikata-Okunomiya, A.; Okamoto, S. Development of active center-directed plasmin and plasma kallikrein inhibitors and studies on the structure-inhibitory activity relationship. Chem. Pharm. Bull. 1993, 41, 1079–1090. [Google Scholar] [CrossRef][Green Version]
- Okada, Y.; Matsumoto, Y.; Tsuda, Y.; Tada, M.; Wanaka, K.; Hijikata-Okunomiya, A.; Okamoto, S. Development of plasmin-selective inhibitors and studies of their structure-activity relationship. Chem. Pharm. Bull. 2000, 48, 184–193. [Google Scholar] [CrossRef][Green Version]
- Gohda, K.; Teno, N.; Wanaka, K.; Tsuda, Y. Predicting subsite interactions of plasmin with substrates and inhibitors through computational docking analysis. J. Enzym. Inhib. Med. Chem. 2012, 27, 571–577. [Google Scholar] [CrossRef]
- Hidaka, K.; Gohda, K.; Teno, N.; Wanaka, K.; Tsuda, Y. Active site-directed plasmin inhibitors: Extension on the P2 residue. Bioorg. Med. Chem. 2016, 24, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Law, R.H.P.; Wu, G.; Leung, E.W.W.; Hidaka, K.; Quek, A.J.; Caradoc-Davies, T.T.; Jeevarajah, D.; Conroy, P.J.; Kirby, N.M.; Norton, R.S.; et al. X-ray crystal structure of plasmin with tranexamic acid-derived active site inhibitors. Blood Adv. 2017, 1, 766–771. [Google Scholar] [CrossRef]
- Munakata, S.; Tashiro, Y.; Nishida, C.; Sato, A.; Komiyama, H.; Shimazu, H.; Dhahri, D.; Salama, Y.; Eiamboonsert, S.; Takeda, K.; et al. Inhibition of plasmin protects against colitis in mice by suppressing matrix metalloproteinase 9-mediated cytokine release from myeloid cells. Gastroenterology 2015, 148, 565–578.e4. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, H.; Munakata, S.; Tashiro, Y.; Salama, Y.; Dhahri, D.; Eiamboonsert, S.; Ota, Y.; Onoda, H.; Tsuda, Y.; Okada, Y.; et al. Pharmacological targeting of plasmin prevents lethality in a murine model of macrophage activation syndrome. Blood 2017, 130, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Hangeland, J.J.; Friend, T.J.; Rossi, K.A.; Smallheer, J.M.; Wang, C.; Sun, Z.; Corte, J.R.; Fang, T.; Wong, P.C.; Rendina, A.R.; et al. Phenylimidazoles as potent and selective inhibitors of coagulation factor XIa with in vivo antithrombotic activity. J. Med. Chem. 2014, 57, 9915–9932. [Google Scholar] [CrossRef]
- Corte, J.R.; Fang, T.; Hangeland, J.J.; Friend, T.J.; Rendina, A.R.; Rossi, K.A.; Wei, A.; Ramamurthy, V.; Morin, P.E.; Seiffert, D.A.; et al. Pyridine and pyridinone-based factor XIa inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 925–930. [Google Scholar] [CrossRef]
- Clark, C.G.; Rossi, K.A.; Corte, J.R.; Fang, J.M.; Smallheer, J.M.; De Lucca, I.; Nirschl, D.S.; Orwat, M.J.; Pinto, D.J.P.; Hu, Z.; et al. Structure based design of macrocyclic factor XIa inhibitors: Discovery of cyclic P1 linker moieties with improved oral bioavailability. Bioorg. Med. Chem. Lett. 2019, 29, 126604. [Google Scholar] [CrossRef]
- Corte, J.R.; Pinto, D.J.P.; Fang, T.; Osuna, H.; Yang, W.; Wang, Y.; Lai, A.; Clark, C.G.; Sun, J.H.; Rampulla, R.; et al. Potent, orally bioavailable, and efficacious macrocyclic inhibitors of factor XIa. Discovery of pyridine-based macrocycles possessing phenylazole carboxamide P1 groups. J. Med. Chem. 2020, 63, 784–803. [Google Scholar] [CrossRef]
- Roehn, U.; Ellermann, M.; Strassburger, J.; Wendt, A.; Roehrig, S.; Webster, R.A.; Schmidt, M.V.; Tersteegen, A.; Bayer, K.; Schaefer, M.; et al. Preparation of substituted phenylalanine derivatives as FXIa modulators for treating thrombotic and thromboembolic diseases. WO2015044163A1, 2015. [Google Scholar]
- Steinmetzer, T.; Pilgram, O.; Wenzel, B.M.; Wiedemeyer, S.J.A. Fibrinolysis inhibitors: Potential drugs for the treatment and prevention of bleeding. J. Med. Chem. 2020, 63, 1445–1472. [Google Scholar] [CrossRef]

| Comp. | IC50 (μM) | ||
|---|---|---|---|
| S-2251 | Fibrin | ||
| 1 |  | (Km = 20 μM) | 180 |
| 2 | 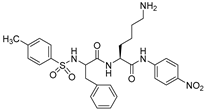 | (Km = 200 μM) | 250 |
| 3 | 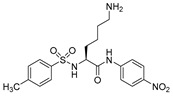 | 700 | 780 |
| Comp. | IC50 (μM) | ||
|---|---|---|---|
| S-2251 | Fibrin | ||
| 4 | 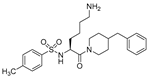 | 300 | 150 |
| 5 | 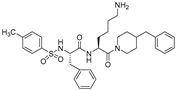 | 210 | 100 |
| 6 |  | 3,000 | 2,100 |
| 7 |  | 140 | 150 |
| 8 | 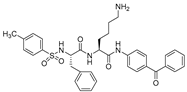 | >100 | >25 |
| 9 | 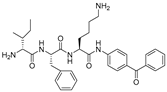 | 69 | 180 |
| 10 |  | 210 | 250 |
| Comp. | R: | IC50 (μM) | ||
|---|---|---|---|---|
| S-2251 | Fibrin | |||
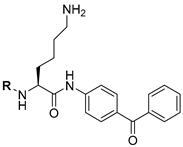 | 11 | 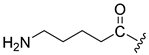 | 16 | 17 |
| 12 | 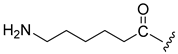 | 12 | >10 (18%) | |
| 13 |  | 15 | 6.1 | |
| 14 | 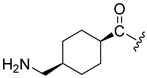 | 400 | 260 | |
| Comp. | R: | IC50 (μM) | |||
|---|---|---|---|---|---|
| S-2251 | Fibrin | Fibrinogen | |||
 | TXA |  | 75,000 | 60 | 9,500 |
| 13 | 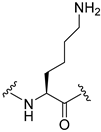 | 15 | 6.1 | 13 | |
| 15 |  | >200 (0%) | >200 (0%) | ND | |
| 16 | 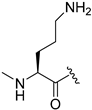 | >300 (33%) | >300 (43%) | ND | |
| Comp. | R: | IC50 (μM) | ||
|---|---|---|---|---|
| S-2251 | Fibrin | |||
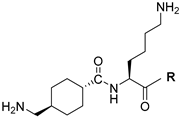 | 13 | 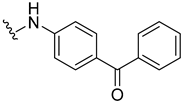 | 15 | 6.1 |
| 17 | 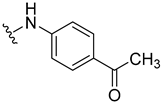 | 39 | 9.3 | |
| 18 |  | 24 | 170 | |
| 19 |  | >500 (7%) | >500 (16%) | |
| Comp. | R: | IC50 (μM) | |||||
|---|---|---|---|---|---|---|---|
| Plm | PK | uPA | Thr | ||||
| S-2251 | Fibrin | S-2302 | S-2444 | S-2238 | |||
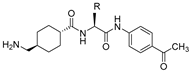 | 23 | 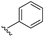 | 36 | 21 | 0.85 | 58 | >1000 |
| 24 | 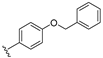 | 1.8 | 0.40 | 0.63 | 31 | >200 (10%) | |
| 25 |  | 0.64 | 0.29 | 0.58 | 45 | >100 (11%) | |
| 26 | 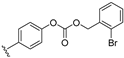 | 0.23 | 0.051 | 0.37 | 43 | 63 | |
| Comp. | R: | IC50 (μM) | |||||
|---|---|---|---|---|---|---|---|
| Plm | PK | uPA | Thr | ||||
| S-2251 | Fibrin | S-2302 | S-2444 | S-2238 | |||
 | 27 | 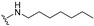 | 1.1 | 0.43 | 10 | >50 | >50 |
| 28 |  | 0.80 | 0.23 | 16 | 50 | >50 | |
| 29 | 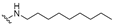 | 0.50 | 0.10 | 22 | >10 | 100 | |
| 30 |  | 0.46 | 0.056 | 2.1 | 260 | 70 | |
| 31 | 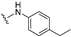 | 0.63 | 0.098 | 0.71 | >50 | 89 | |
| 32 | 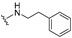 | 1.1 | 0.30 | 7.0 | >50 | >50 | |
| 33 | 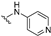 | 0.98 | 0.17 | 0.29 | 19 | 91 | |
| 34 |  | 5.3 | 1.40 | 14.0 | 72 | 240 | |
| Comp. | R: | IC50 (μM) | |||
|---|---|---|---|---|---|
| Plm | PK | uPA | |||
| S-2251 | S-2302 | S-2444 | |||
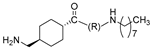 | 28 | 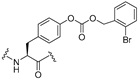 | 0.80 | 16 | >50 |
| 35 | 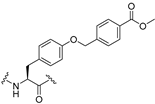 | 0.65 | 38 | >50 | |
| 36 | 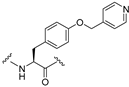 | 0.53 | 30 | 5.3 | |
| 37 | 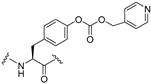 | 1.0 | 12 | 31 | |
| Comp. | R: | IC50 (μM) | |||
|---|---|---|---|---|---|
| Plm | uPA | trypsin | |||
| S-2251 | S-2444 | S-2238 | |||
 | 38 |  | 0.34 | >100 | 93 |
| 39 | 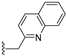 | 0.22 | 77 | 1.4 | |
| 40 | 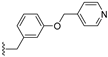 | 0.97 | >50 | 1.3 | |
| 41 | 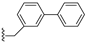 | 0.75 | >100 | 6.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuda, Y.; Hidaka, K.; Hojo, K.; Okada, Y. Exploration of Active Site-Directed Plasmin Inhibitors: Beyond Tranexamic Acid. Processes 2021, 9, 329. https://doi.org/10.3390/pr9020329
Tsuda Y, Hidaka K, Hojo K, Okada Y. Exploration of Active Site-Directed Plasmin Inhibitors: Beyond Tranexamic Acid. Processes. 2021; 9(2):329. https://doi.org/10.3390/pr9020329
Chicago/Turabian StyleTsuda, Yuko, Koushi Hidaka, Keiko Hojo, and Yoshio Okada. 2021. "Exploration of Active Site-Directed Plasmin Inhibitors: Beyond Tranexamic Acid" Processes 9, no. 2: 329. https://doi.org/10.3390/pr9020329
APA StyleTsuda, Y., Hidaka, K., Hojo, K., & Okada, Y. (2021). Exploration of Active Site-Directed Plasmin Inhibitors: Beyond Tranexamic Acid. Processes, 9(2), 329. https://doi.org/10.3390/pr9020329






