Enzymatic Saccharification with Sequential-Substrate Feeding and Sequential-Enzymes Loading to Enhance Fermentable Sugar Production from Sago Hampas
Abstract
1. Introduction
2. Materials and Methods
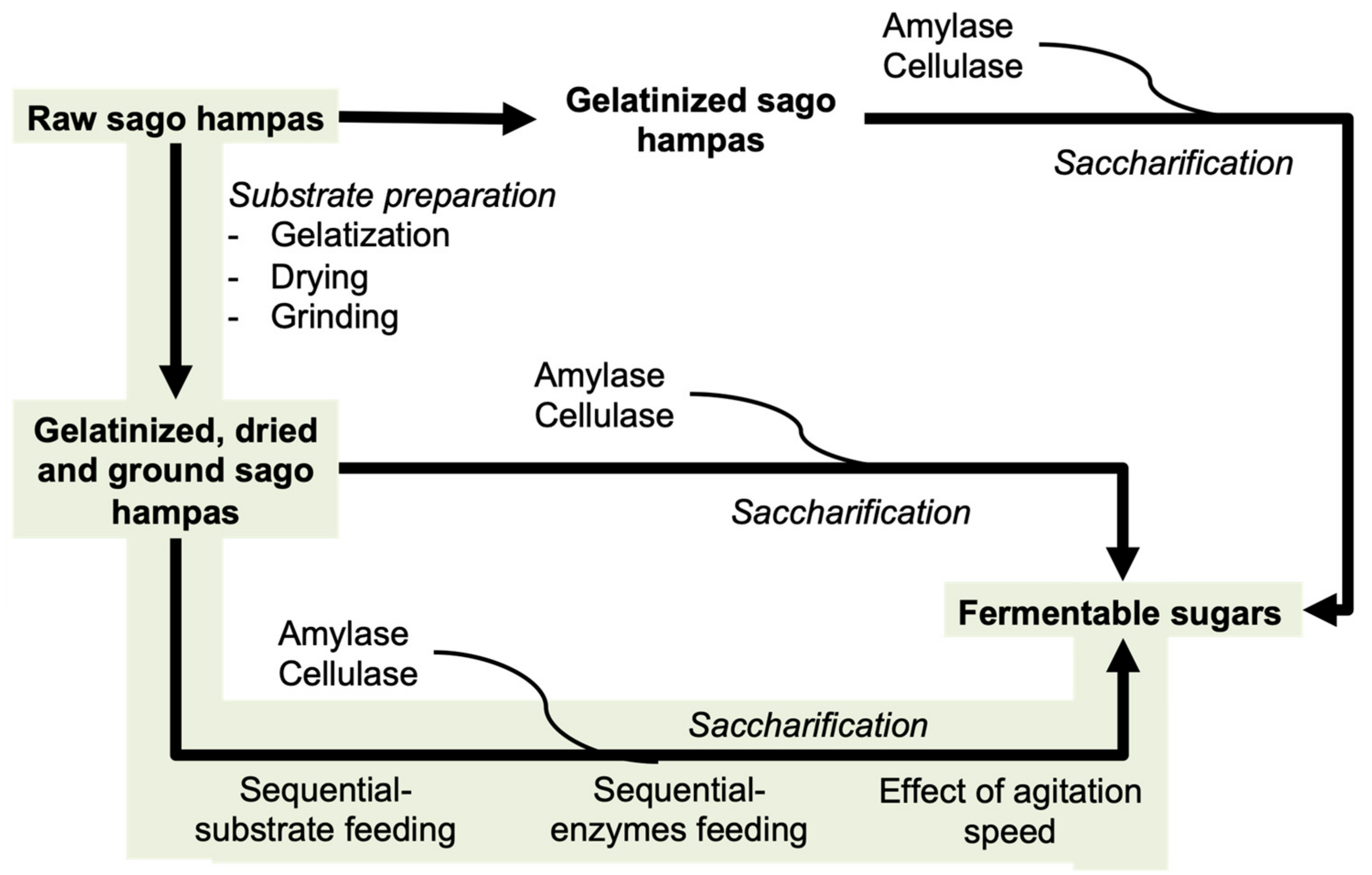
2.1. Substrate Preparation
2.2. Batch Saccharification
2.3. Sacchatification with Sequential-Substrate Feeding and Sequential-Enzyme Loading
2.3.1. Feeding Interval
2.3.2. Enzymes Loading
2.3.3. Agitation Speed
2.4. Analytical Procedures
3. Results and Discussion
3.1. Characteristics of Sago Hampas
3.2. Saccharification of Sago Hampas
3.2.1. Effect of Wet and Dried Sago Hampas
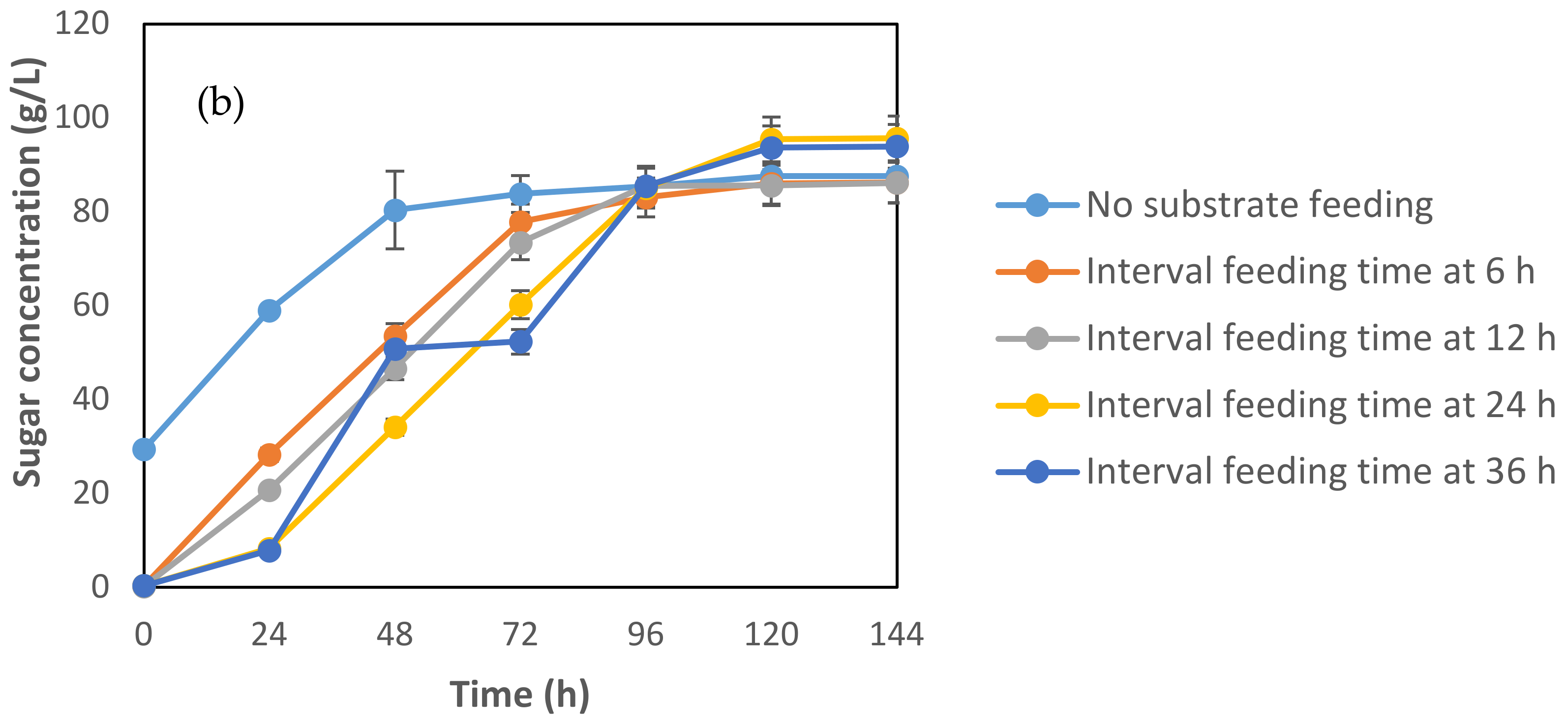
3.2.2. Effect of Feeding Interval
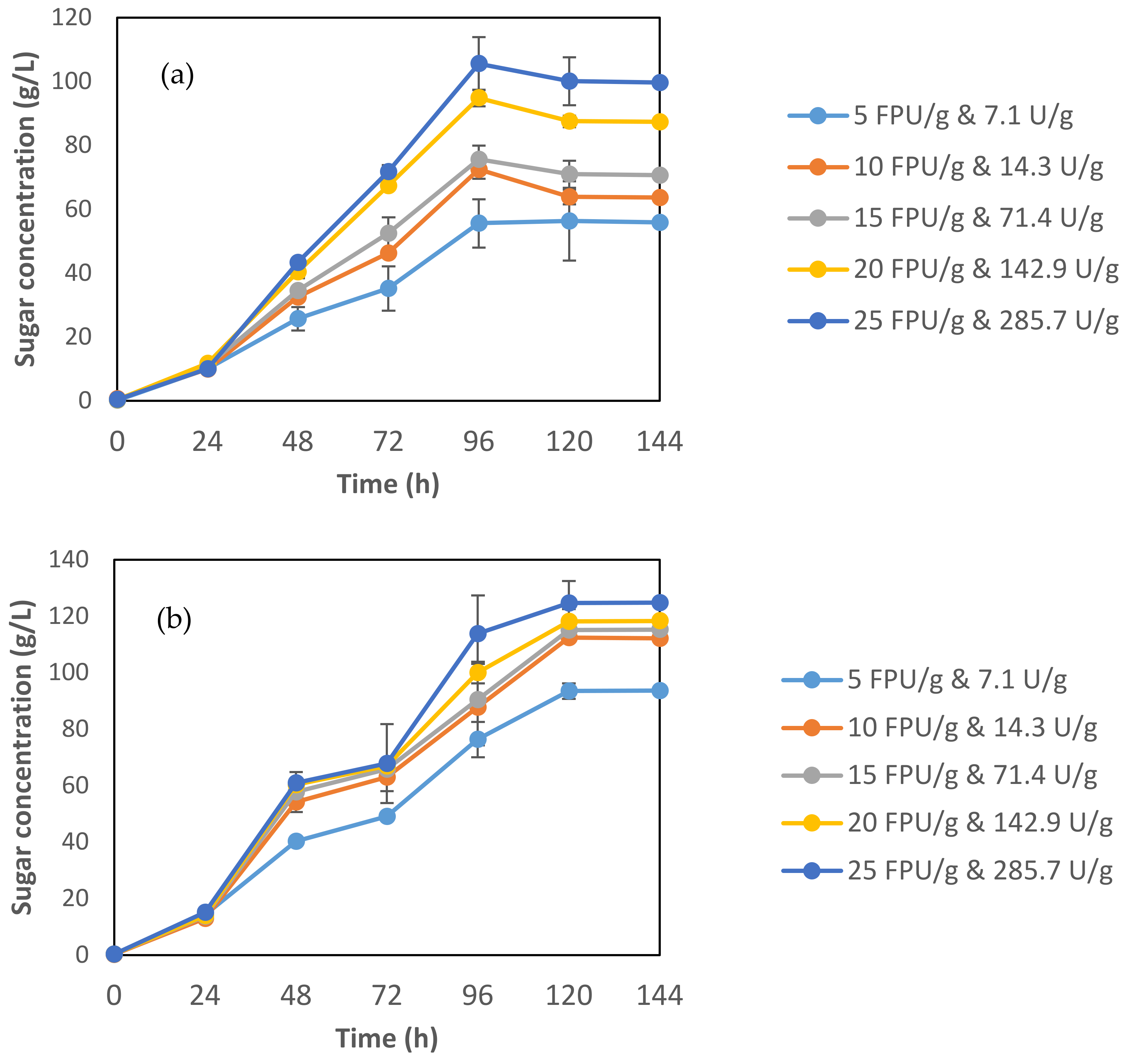
3.2.3. Effect of Enzymes Loading
3.2.4. Effect of Agitation Speed
3.3. Comparison Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bujang, K. Potential of sago for commercial production of sugars. In Proceedings of the the 10th International Sago Symposium, Bogor, Indonesia, 29–31 October 2011; pp. 1–7. [Google Scholar]
- Pei-Lang, A.T.; Mohamed, A.M.D.; Karim, A.A. Sago starch and composition of associated components in palms of different growth stages. Carbohydr. Polym. 2006, 63, 283–286. [Google Scholar] [CrossRef]
- Ishizaki, A. Production, purification, and health benefits of sago sugar. In Proceedings of the Concluding Remarks for the 6th International Sago Symposium; Sago Comm: Riau, Indonesia, 1997; pp. 22–24. [Google Scholar]
- Karim, A.A.; Tie, A.P.L.; Manan, D.M.A.; Zaidul, I.S.M. Starch from the sago (Metroxylon sagu) palm tree-Properties, prospects, and challenges as a new industrial source for food and other uses. Compr. Rev. Food Sci. Food Saf. 2008, 7, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Awg-Adeni, D.S.; Abd-Aziz, S.; Bujang, K.B.; Hassan, M.A. Bioconversion of Sago Residue into Value Added Products. Afr. J. Biotechnol. 2010, 9, 2016–2021. [Google Scholar]
- Ngaini, Z.; Wahi, R.; Halimatulzahara, D.; Mohd Yusoff, N.A.-N. Chemically modified sago waste for oil absorption. Pertanika J. Sci. Technol. 2014, 22, 153–161. [Google Scholar]
- Husin, H.; Ibrahim, M.F.; Kamal Bahrin, E.; Abd-Aziz, S. Simultaneous saccharification and fermentation of sago hampas into biobutanol by Clostridium acetobutylicum ATCC 824. Energy Sci. Eng. 2018, 7, 66–75. [Google Scholar] [CrossRef]
- Jenol, M.A.; Ibrahim, M.F.; Phang, L.Y.; Salleh, M.M. Sago biomass as a sustainable source for biohydrogen production by Clostridium butyricum A1. BioResources 2014, 9, 1007–1026. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Verma, P. An overview of key pretreatment processes employed for bioconversion of lignocellulosic biomass into biofuels and value added products. 3 Biotech 2013, 3, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Canilha, L.; Chandel, A.K.; Dos Santos Milessi, S.T.; Antunes, F.A.F.; Da Costa Freitas, L.W.; Das Graças Almeida Felipe, M.; Da Silva, S.S. Bioconversion of sugarcane biomass into ethanol: An overview about composition, pretreatment methods, detoxification of hydrolysates, enzymatic saccharification, and ethanol fermentation. J. Biomed. Biotechnol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Van Der Maarel, M.J.E.C.; Van Der Veen, B.; Uitdehaag, J.C.M.; Leemhuis, H.; Dijkhuizen, L. Properties and applications of starch-converting enzymes of the α-amylase family. J. Biotechnol. 2002, 94, 137–155. [Google Scholar] [CrossRef]
- Alcázar-Alay, S.C.; Meireles, M.A.A. Physicochemical properties, modifications and applications of starches from different botanical sources. Food Sci. Technol. 2015, 35, 215–236. [Google Scholar] [CrossRef]
- Roy, I.; Gupta, M.N. Hydrolysis of starch by a mixture of glucoamylase and pullulanase entrapped individually in calcium alginate beads. Enzyme Microb. Technol. 2004, 34, 26–32. [Google Scholar] [CrossRef]
- Goyal, N.; Gupta, J.K.; Soni, S.K. A novel raw starch digesting thermostable α-amylase from Bacillus sp. I-3 and its use in the direct hydrolysis of raw potato starch. Enzyme Microb. Technol. 2005, 37, 723–734. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K.; Kumar, R. Cellulose Solvent-Based Pretreatment for Enhanced Second-Generation Biofuel Production: A Review; Royal Society of Chemistry: London, UK, 2019; Volume 3, ISBN 8415683111. [Google Scholar]
- Gao, Y.; Xu, J.; Zhang, Y.; Liu, Y.; Liang, C. Optimization of fed-batch enzymatic hydrolysis from alkali-pretreated sugarcane bagasse for high-concentration sugar production. Bioresour. Technol. 2014, 167, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.M.; Li, Y.-H.; Liu, C.-G.; Zhao, X.-Q.; Bai, F.-W. Fed-batch Saccharification and ethanol fermentation of Jerusalem Artichoke Stalks by an Inulinase Producing Saccharomyces cerevisiae MK01. R. Soc. Chem. 2015, 5, 107112–107118. [Google Scholar] [CrossRef]
- Nakamura, L.K. Lactobacillus amylovorus, a new starch-hydrolyzing species from cattle waste-corn fermentations. Int. J. Syst. Bacteriol. 1981, 31, 56–63. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples Laboratory Analytical Procedure (LAP); Issue Date: 12/08/2006; Midwest Research Institute: Golden, CO, USA, 2008. [Google Scholar]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass: Laboratory Analytical Procedure (LAP); Issue Date 7/17/2005; Midwest Research Institute: Golden, CO, USA, 2008. [Google Scholar]
- Awg-Adeni, D.S.; Bujang, K.B.; Hassan, M.A.; Abd-Aziz, S. Recovery of glucose from residual starch of sago hampas for bioethanol production. Biomed Res. Int. 2013, 2013, 935852. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Y.; Sun, J.; Li, D.; Huang, Y.; Feng, Y. Effect of extractives on digestibility of cellulose in corn stover with liquid hot water pretreatment. BioResources 2016, 11, 54–70. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, L.; Wang, C.; Yu, J.; Yang, Z. Investigating the influence of extractives on the oil yield and alkane production obtained from three kinds of biomass via deoxy-liquefaction. Bioresour. Technol. 2011, 102, 7190–7195. [Google Scholar] [CrossRef]
- Vincent, M.; Jabang, E.; Nur, N.M.; Esut, E.; Unting, L.B.; Awg-Adeni, D.S. Simultaneous co-Saccharification and Fermentation of Sago Hampas for Bioethanol Production. Agric. Eng. Int. CIGR J. 2015, 17, 160–167. [Google Scholar]
- Jenol, M.A.; Ibrahim, M.F.; Bahrin, E.K.; Kim, S.W.; Abd-Aziz, S. Direct bioelectricity generation from sago hampas by clostridium beijerinckii sr1 using microbial fuel cell. Molecules 2019, 24, 2397. [Google Scholar] [CrossRef]
- Mustafa Kamal, M.; Baini, R.; Mohamaddan, S.; Selaman, O.S.; Ahmad Zauzi, N.; Rahman, M.R.; Abdul Rahman, N.; Chong, K.H.; Atan, M.F.; Abdul Samat, N.A.S.; et al. Effect of temperature to the properties of sago starch. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206. [Google Scholar] [CrossRef]
- Yan, S.; Yao, J.; Yao, L.; Zhi, Z.; Chen, X.; Wu, J. Fed batch enzymatic saccharification of food waste improves the sugar concentration in the hydrolysates and eventually the ethanol fermentation by saccharomyces cerevisiae H058. Braz. Arch. Biol. Technol. 2012, 55, 183–192. [Google Scholar] [CrossRef]
- Kumar, L.; Arantes, V.; Chandra, R.; Saddler, J. The lignin present in steam pretreated softwood binds enzymes and limits cellulose accessibility. Bioresour. Technol. 2012, 103, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Bommarius, A.S.; Katona, A.; Cheben, S.E.; Patel, A.S.; Ragauskas, A.J.; Knudson, K.; Pu, Y. Cellulase kinetics as a function of cellulose pretreatment. Metab. Eng. 2008, 10, 370–381. [Google Scholar] [CrossRef]
- Lu, C.; Dong, J.; Yang, S.-T. Butanol production from wood pulping hydrolysate in an integrated fermentation-gas stripping process. Bioresour. Technol. 2013, 143, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Van Dyk, J.S.; Pletschke, B.I. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes-Factors affecting enzymes, conversion and synergy. Biotechnol. Adv. 2012, 30, 1458–1480. [Google Scholar] [CrossRef] [PubMed]
- Leu, S.Y.; Zhu, J.Y. Substrate-Related Factors Affecting Enzymatic Saccharification of Lignocelluloses: Our Recent Understanding. Bioenergy Res. 2013, 6, 405–415. [Google Scholar] [CrossRef]
- Mathew, G.M.; Sukumaran, R.K.; Singhania, R.R.; Pandey, A. Progress in research on fungal cellulases for lignocellulose degradation. J. Sci. Ind. Res. 2008, 67, 898–907. [Google Scholar]
- Kim, D. Physico-chemical conversion of lignocellulose: Inhibitor effects and detoxification strategies: A mini review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef]
- Yoon, J.-J.; Kim, K.-Y.; Cha, C.-J. Purification and characterization of thermostable β-glucosidase from the brown-rot basidiomycete Fomitopsis palustris grown on microcrystalline cellulose. J. Microbiol. 2008, 46, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, K.; Kafarov, V. Exergy analysis of enzymatic hydrolysis reactors for transformation of lignocellulosic biomass to bioethanol. Chem. Eng. J. 2009, 154, 390–395. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Fernandes, M.; Milagres, A.M.F.; Roberto, I.C. The effect of agitation speed, enzyme loading and substrate concentration on enzymatic hydrolysis of cellulose from brewer’s spent grain. Cellulose 2008, 15, 711. [Google Scholar] [CrossRef]
- Hu, J.; Arantes, V.; Saddler, J.N. The enhancement of enzymatic hydrolysis of lignocellulosic substrates by the addition of accessory enzymes such as xylanase: Is it an additive or synergistic effect? Biotechnol. Biofuels 2011, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hawker, J.; Jenner, C. High temperature affects the activity of enzymes in the committed pathway of starch synthesis in developing wheat endosperm. Aust. J. Plant Physiol. 1993, 20, 197–209. [Google Scholar] [CrossRef]
- Amit, K.; Nakachew, M.; Yilkal, B.; Mukesh, Y. A review of factors affecting enzymatic hydrolysis of pretreated lignocellulosic Biomass. Res. J. Chem. Environ. 2018, 22, 62–67. [Google Scholar]
- Sorimachi, K.; Le Gal-Coëffet, M.F.; Williamson, G.; Archer, D.B.; Williamson, M.P. Solution structure of the granular starch binding domain of Aspergillus niger glucoamylase bound to β-cyclodextrin. Structure 1997, 5, 647–661. [Google Scholar] [CrossRef]
- Xu, Q.S.; Yan, Y.S.; Feng, J.X. Efficient hydrolysis of raw starch and ethanol fermentation: A novel raw starch-digesting glucoamylase from Penicillium oxalicum. Biotechnol. Biofuels 2016, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Eveleigh, D.; Mandels, M.; Andreotti, R.; Roche, C. Measurement of saccharifying cellulase. Biotechnol. Biofuels 2009, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.K. Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 1–41. [Google Scholar] [CrossRef]
- Liu, Z.; Smith, S.R. Enzyme Recovery from Biological Wastewater Treatment. Waste Biomass Valorization 2020. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, J.; Xia, L. Enzymatic hydrolysis of maize straw polysaccharides for the production of reducing sugars. Carbohydr. Polym. 2008, 71, 411–415. [Google Scholar] [CrossRef]
- Ibrahim, M.F.; Linggang, S.; Jenol, M.A.; Yee, P.L.; Abd-Aziz, S. Effect of Buffering System on Acetone-Butanol-Ethanol Fermentation by Clostridium acetobutylicum ATCC 824 using Pretreated Oil Palm Empty Fruit Bunch. BioResources 2015, 10, 3890–3907. [Google Scholar] [CrossRef]
- Salleh, M.S.M.; Ibrahim, M.F.; Roslan, A.M.; Abd-Aziz, S. Improved Biobutanol Production in 2-L Simultaneous Saccharification and Fermentation with Delayed Yeast Extract Feeding and in-situ Recovery. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]



| Time Interval for Substrate Feeding (h) | Initial Substrate (%) | Sequential-Substrate Feeding (%) | Total Amount of Substrate Feeding (%) |
|---|---|---|---|
| 0 (control) | 20 | No substrate added | 20 |
| 6 | 2 | 1.5 | |
| 12 | 2 | 3 | |
| 24 | 2 | 6 | |
| 36 | 2 | 9 |
| Time Interval for Substrate Feeding and Enzymes Loading (h) | Sequential-Substrate Feeding (%) | Total Substrate Loading (%) | Sequential-Enzymes Loading | |
|---|---|---|---|---|
| Amylase (U/g) | Cellulase (FPU/g) | |||
| 24 | 6 | 20 | 7.1 | 5 |
| 14.3 | 10 | |||
| 71.4 | 15 | |||
| 142.9 | 20 | |||
| 285.7 | 25 | |||
| Sago Hampas Collection | Composition (%) | References | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Starch | Cellulose | Hemicellulose | Lignin | Moisture | Extractives | Others | |||
| Water | Solvent | ||||||||
| Pusa, Sarawak | 49.5 | 26.0 | 14.5 | 7.5 | n.d | n.d | n.d | 2.5 | [8] |
| Pusa, Sarawak | 58.0 | 23.5 | 8.2 | 6.3 | n.d | n.d | n.d | 2.3 | [24] |
| Mukah, Sarawak | 58.0 | 21.0 | 13.4 | 5.4 | 4.7 | n.d | n.d | 3.13 | [25] |
| Mukah, Sarawak | 56.0 | 20.7 | 11.2 | 3.1 | 6.35 | 2.33 | 0.67 | 6.1 | This study |
| Study | Initial Substrate Concentration (g/L) | Total Substrate Loading (g/L) | Feeding Time Interval (h) | Enzyme Loading (per g of the Substrate) | * Sugar Concentration (g/L) | |
|---|---|---|---|---|---|---|
| Amylase (U/g) | Cellulase (U/g) | |||||
| Batch saccharification (wet substrate) | 7 | 7 | Null | 71.4 | 20 | 43.29 ± 2.54 |
| Batch saccharification (dried substrate) | 7 | 7 | Null | 71.4 | 20 | 46.23 ± 0.76 |
| Batch saccharification (control) | 20 | 20 | Null | 71.4 | 20 | 80.33 ± 0.02 |
| Saccharification with sequential-substrate feeding | 2 | 20 | Every 24 h | 71.4 | 20 | 94.88 ± 2.59 |
| Saccharification with sequential-substrate feeding and sequential-enzymes loading | 2 | 20 | Every 24 h | 14.3 | 10 | 119.90 ± 0.32 |
| Saccharification Operations | Substrate Concentration (g/L) | Enzyme Used per g of Substrate | Sugar Concentration (g/L) | References |
|---|---|---|---|---|
| Batch saccharification | 5 g/L oil palm empty fruit bunch | 0.1 g/mL crude cellulase cocktail | 12 g/L glucose | [47] |
| Batch saccharification | 5 g/L oil palm empty fruit bunch | 15 FPU/mL celluclast | 31 g/L reducing sugars | [48] |
| Batch saccharification | 9 g/L sago hampas | 71.4 U/g Dextrozyme amylase + 20 FPU/g Acremonium cellulase | 66.9 g/L reducing sugars | [7] |
| Fed-batch saccharification | 20 g/L Jerusalem artichoke stalks | 20 FPU/g cellulase | 83.7 g/L glucose | [17] |
| Saccharification with sequential-substrate feeding and sequential-enzymes loading | 20 g/L sago hampas | 14.3 U/g Dextrozyme amylase + 10 FPU/g Acremonium cellulase | 119.90 g/L glucose ± 0.32 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alias, N.H.; Abd-Aziz, S.; Yee Phang, L.; Ibrahim, M.F. Enzymatic Saccharification with Sequential-Substrate Feeding and Sequential-Enzymes Loading to Enhance Fermentable Sugar Production from Sago Hampas. Processes 2021, 9, 535. https://doi.org/10.3390/pr9030535
Alias NH, Abd-Aziz S, Yee Phang L, Ibrahim MF. Enzymatic Saccharification with Sequential-Substrate Feeding and Sequential-Enzymes Loading to Enhance Fermentable Sugar Production from Sago Hampas. Processes. 2021; 9(3):535. https://doi.org/10.3390/pr9030535
Chicago/Turabian StyleAlias, Nurul Haziqah, Suraini Abd-Aziz, Lai Yee Phang, and Mohamad Faizal Ibrahim. 2021. "Enzymatic Saccharification with Sequential-Substrate Feeding and Sequential-Enzymes Loading to Enhance Fermentable Sugar Production from Sago Hampas" Processes 9, no. 3: 535. https://doi.org/10.3390/pr9030535
APA StyleAlias, N. H., Abd-Aziz, S., Yee Phang, L., & Ibrahim, M. F. (2021). Enzymatic Saccharification with Sequential-Substrate Feeding and Sequential-Enzymes Loading to Enhance Fermentable Sugar Production from Sago Hampas. Processes, 9(3), 535. https://doi.org/10.3390/pr9030535









