Abstract
Graph theory is a well-established mathematical concept that is widely used in numerous applications such as in biology, chemistry and network analysis. The advancement in the theory of graph has led to the development of a concept called autocatalytic set. In this paper, a mathematical modeling technique namely graph-based dynamic modeling of palm oil refining process is introduced. The system parameters are identified in detail in the beginning of the paper. The parameters involved are the chemical compounds used or produced during the refining process. These identified parameters are then modeled as the vertices and edges of the graph. The dynamicity of the system is then simulated and analyzed. The system is simulated using MATLAB software programing. The two final products produced by the refining process agreed with results obtained from other published methods. Hence, the effectiveness and simplicity of the model are established.
1. Introduction
The refining of palm oil improves the production of palm oil and its quality. It is a process to extract oil from crude palm oil (CPO) into an edible form. Before the refining process is conducted, CPO needs to be extracted from palm oil fruits. Figure 1 illustrates the production stages for CPO.
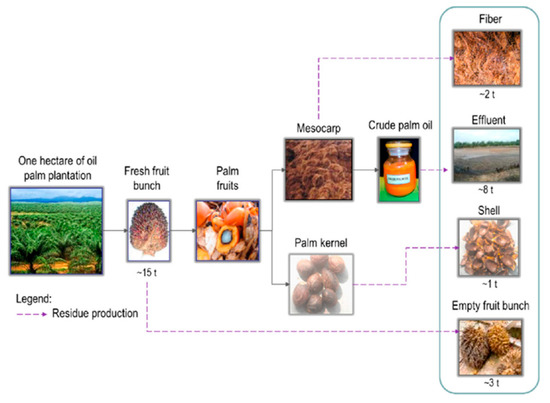
Figure 1.
Flow of crude palm oil production [1].
There are two types of refining processes, namely, chemical and physical [2]. Chemical compounds are used in former. On the other hand, less harmful elements and procedures are utilized for later. However, as time passed, the use of chemicals has been reduced due to its cost, time needed for processing, and its ill-effect when consumed by humans. As a result, physical-based refining was introduced at the end of the 1970s as an alternative to chemical-based refining [3]. Physical-based refining requires natural materials to refine palm oil. They are bleaching earth (earth clay) and phosphoric acid. Steam (water) injection is used at the end of the process to remove free fatty acids (FFA) from the palm oil [4].
There are three processes in physical-based refining: degumming, bleaching, and deodorization. In 2006, Morad et al. [2] has presented an overview of the design for degumming and bleaching process of palm oil. Further improvement for esterification reactions involving high free fatty acid content of crude palm oil and glycerol using zinc glycerolate and zinc soap catalysts was presented in the Sriwijaya International Seminar on Energy Science and Technology in September 2011 [5]. However, in this paper, a new mathematical method, namely, autocatalytic set, is described to model the palm oil refining process. The used technique demonstrates the process which occurs during the refining process in great detail. There are no studies that have been reported in literatures that utilize such method for refining process of palm oil. The rest of the paper is written in the following sections. Section 2 describes the refining process of palm oil in general and Section 3 focuses mainly on elements with respect to physical refining process. The methodology used in this study is introduced in Section 4 and followed by Section 5. The following two sections are on the implementation of the method to the refining process of palm oil. Section 8 is on the analysis of the model and Section 9 is the conclusion of the study.
2. Refining Process
The purpose of refining is to process crude palm oil (CPO) into edible oil. Impurities and other compounds are removed during the process. Figure 2 illustrates the stages involved in palm oil manufacturing, from the plantation to the refining. Firstly, the CPO is extracted from palm fruit [6]. It consists of desirable triglycerides and unsaponifiables compounds with a small amount of impurities [7]. These impurities are then removed during the refining process because they affect the quality of palm oil, in particular, color, flavor, odor, stability and produce foams. Morad et al. [2] used neural network to model degumming and bleaching procedures of palm oil for the refining process.
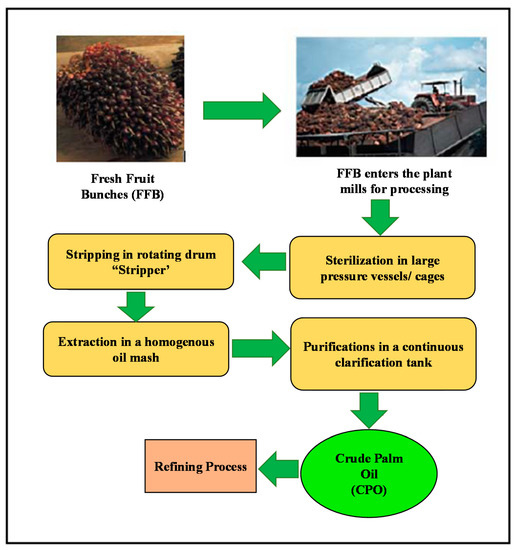
Figure 2.
Palm oil refining process.
Compositions of the crude palm oil (CPO) [8] can be categorized in several groups in general and listed in Table 1.

Table 1.
Crude palm oil (CPO) compounds.
In particular, Table 2 tabulated quantitavely the amount of CPO compounds of Malaysian crude palm oil [2].

Table 2.
The main compounds in Malaysian crude palm oil (CPO).
The acceptable amount with respect to CPO in Malaysia is maximum 5% only for fatty acid and 2.5% for moisture and impurities [9]. However, the standard specification of compounds for refined palm oil gazette by Palm Oil Refiners Association of Malaysia (PORAM) are summarized in Table 3.

Table 3.
PORAM (Palm Oil Refiners Association of Malaysia) standard specifications for refined palm oils.
There are two types of palm oil refining in Malaysia: i.e., chemical and physical. Figure 3 illustrates the stages for chemical and physical refining processes of palm oil. The chemical refining process requires more stages than physical refining. Hence, the total operating costs for physical refining is expected to be smaller than chemical refining. This is due to the use of high effluent treatment in chemical refining purpose. Yusoff and Thiagarajan [10] claimed that physical refining costs less operationally and capital inputs, fewer oil losses, less influent, and greater efficiency than chemical refining. As a result, chemical refining is less adopted than physical refining in Malaysia.
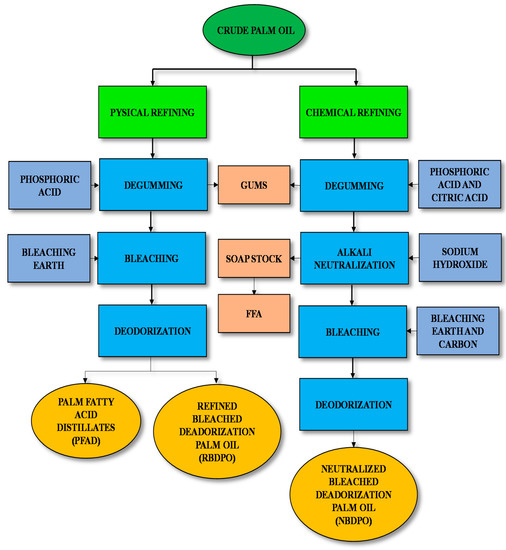
Figure 3.
Chemical and physical refining process of palm oil.
In 1985, Er [11] published a guideline contents for CPO, degummed bleached palm oil (DBPO) and refined bleached deodorized palm oil (RBDPO) (see Table 4). The DBPO is the resultant from the degumming and bleaching process, and RBDPO is the final product of the refining process [11].

Table 4.
Guideline contents for CPO, DBPO (degummed bleached) and RBDPO (refined bleached deodorized palm oil).
Basically, both refining processes are complex multidimensional systems. However, only physical refining process is considered in this study.
3. Physical Refining
The physical refining of palm oil consists of two stages, namely, pretreatment and deodorization. Pretreatment involves degumming and bleaching processes. The aim of the process is to remove undesirable impurities. This aim is achieved when chemical compounds, phosphoric acid and bleaching earth are added in the process. These compounds are used to adsorb the unwanted impurities whereby the color, flavor, and shelf-life stability of the finished product are monitored and controlled [12]. Furthermore, high temperatures are needed to remove free fatty acids (FFA) from CPO during steam distillation in the deodorization process [4]. The schematic diagram of the process is illustrated in Figure 4. The degumming, bleaching, and deodorization processes are described in detail in the following subsections.
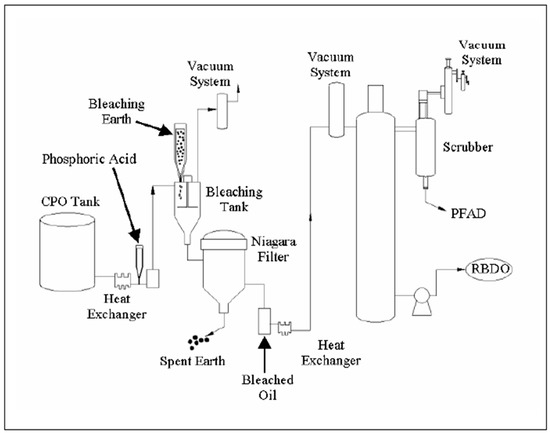
Figure 4.
Physical refining process [2].
3.1. Degumming
The purpose of the degumming is to remove unwanted gum from CPO using phosphoric acid. The amount of phosphoric acid is controlled during the degumming process. This is due to the fact that excess phosphoric acid is difficult to remove, which leads to further refining problems. The unwanted phosphatide is removed during the degumming. It is essential to remove phosphatide since it affects color, flavor, and lifespan of the oil. Phosphatide is the main culprit that affects the instability of oxidation in CPO. The degumming process starts by heating the CPO to up to . It is then treated by phosphoric acid. The amount of phosphoric acid is to of palm oil weight with concentrations of to . The decomposed phosphatide is then removed from the CPO.
3.2. Bleaching
The CPO resultant from the degumming process is then heated to and treated by bleaching earth. Bleaching earth is a solution that is added in ranges of to of the weight of palm oil. This mixture is then continuously blended for min. The purpose of this process is to remove the other unwanted compounds and to accelerate the reaction between compounds of the mixture. Then, the CPO is filtered before being transferred to the deodorization process. Phosphoric acid, pigments, trace metals, phosphatides, and oxidation compounds are removed during deodorization.
3.3. Deodorization
The palm oil is then heated up to –, under vacuum (2–4 mmHg) by direct steam injection with respect to % to of the weight of palm oil. This process is for deacidification and deodorization of the palm oil [12]. The Free Fatty Acids (FFA) are removed as palm fatty acid distillate (PFAD). The resultant products due to oxidation as well as carotenoid pigment are removed since they may affect the flavor of the oil. The final product of the deodorization is refined bleached deodorized palm oil (RBDPO). The RBDPO is then cooled and filtered before finally filled into the storage tanks.
The quality of palm oil depends on the amount of free fatty acids, moisture, heavy metals, oxidized products, and minor constituents. The details of these compounds are as follows:
- Free fatty acid (FFA)
- Free fatty acids (FFA) are formed when triglyceride, diglyceride, and monoglyceride are decomposed by chemical or enzymatic hydrolysis. Enzymes are used when there is a large amount of moisture in the palm oil [13]. Sometimes more than 2% FFA are produced. High FFA content must be avoided due to large refining losses and possible problems in the upcoming bleaching process [13].
- Moisture
- Palm oil consists of moisture. Under certain conditions, the triglyceride of palm oil is hydrolyzed into free fatty acids and glycerol [13]. However, the hydrolytic compound resultant from triglyceride molecules under 0.1% of moisture is negligible.C3H5(OOCR)3 + 3HOH → C3H5(OH) + 3HOOCRTherefore, in order to control the quantity of FFA, CPO must be stored with moisture content below 0.1% in order to eliminate further oxidation [9].
- Heavy (trace) metals
- Heavy metals or trace metals such as iron (Fe), copper (Cu), cadmium (Cd), lead (Pb), and manganese (Mn) are surrounded by phospholipids, proteins, lipids, and non-lipid compounds. The existence of trace metals due to corrosion reactions in the palm oil mill, storage and ship tanks, tankers, pipelines and refinery processes. Heavy metals are pro-oxidants that affect the quality of the palm oil. They act as catalyzers for hydro-peroxide decompositions. Iron (Fe) increases the rate of peroxide and copper (Cu) accelerates the decomposition process of hydro-peroxides [14].
- Oxidized products
- Oxidized products in palm oil are peroxides, aldehydes, ketones, and furfurals (from sugars) [8]. These compounds affect palm oil quality at the end of the refining process. They are very difficult to remove during refining. Therefore, palm oil must not be exposed to light, high temperatures, and pro-oxidant compounds.
- Minor constituents
- Phospholipids are complex esters that consist of nitrogen bases, phosphorus, sugars, and long-chain fatty acids. The existence of phospholipids in the mesocarp of palm oil are about 200 to 1000 ppm and in CPO are about 20 to 80 ppm [14]. These compounds affect the nutrition, stability, and bleaching process of palm oil. There are two types of phospholipids, namely, hydratable and non-hydratable phospholipids. Hydratable phospholipids are easy to remove by using water. These mixtures form liquid crystals. The non-hydratable phospholipids become hydratable phospholipids that are mixed with phosphoric acid or citric acid. Phospholipids are antioxidant-synergistic, and affect pro-oxidants to turn into inactive compounds. Phospholipids are used to remove metal compounds and hydrophilic salt. They decrease the oxidation process in the CPO, too. Phospholipids are removed using phosphoric acid during the degumming process.
- Phosphoric acid
- There are two types of acidic compounds: phosphoric acid (H3PO4) and nitric acid (HNO3). The (H3PO4) is widely used in palm oil refineries in Malaysia since it is easier to administer and cheaper than (HNO3). The (H3PO4) with concentration of 85% is mixed with CPO during the degumming process. Phosphoric acids are odorless and colorless.
- Bleaching earth
- Bleaching earth can be found in special strata with natural compounds. The role of bleaching earth in the bleaching process is to remove undesired pigment-type compounds in CPO. It is pro-oxidative compound and able to increase the quality of palm oil [15]. Acidic bleaching earth is formed when montmorillonite clay is treated by acidic compounds [16]. Acidic bleaching earth is an excellent metal adsorbent. Its roles are as follows [17]:
- (a)
- Decrease the existence of chlorophyll and palm oil color.
- (b)
- Remove phospholipids and soap compounds.
- (c)
- Decrease free fatty acid compounds in the bleaching process.
Most of these compounds are removed during the refining process. Nurul and Khairiyah [17] have applied Genetic Algorithms (GAs) optimization technique for palm oil refining process. The GA optimizes the dosage of bleaching earth and citric acid in order to improve the quality of the refined oil as well as to increase profit. In 2009, Vintila [18] presented a mathematical model for physical refining whereby a differential vaporization model is applied to simulate the deodorization process in physical refining. However, in this paper, a special type of mathematical modeling, namely graph-based autocatalytic set, is developed for physical palm oil refining process and will be elaborated in the following sections.
4. Graph
Graphs are defined as networks of points or nodes that are connected by links [19]. They are described as a set of lines that connect to a set of points [20].
Definition 1.
[19] A directed graph G = G (V, E) is defined by a set of V “vertices” also known as “nodes” and a set E of “edges” or “links” where each edge is an ordered pair of vertices.
All the graphs in Figure 5 are directed graphs. A set of vertices or nodes can be represented as and where the vertices and edges are also known as nodes and links, respectively. The adjacency matrix of a graph is presented as . An adjacency matrix is given in the following definition.
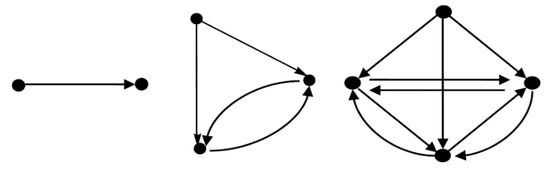
Figure 5.
Examples of graphs.
Definition 2.
[21] Adjacency matrix of graph G = G (V, E) with n vertices is a matrix, denoted by, where if E contains a directed link (j,i) (arrow pointing from vertices j to vertices i) and otherwise.
The adjacency matrix of graph G can be described as follows:
and the entries for matrix represent the connection between the and vertices. Figure 6 shows an example of graph G with four vertices and its adjacency matrix.
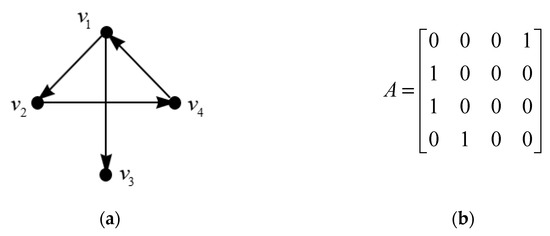
Figure 6.
(a) A directed graph; (b) Its adjacency matrix.
The definition of a graph given by Jain and Krishna is slightly different than Definition 1 [22]. The if and only if there is a link from node i to node j. Basically, the resultant adjacency matrix with respect to their definition of a graph is the transpose of the adjacency matrix described by Definition 2.
5. Autocatalytic Set and Dynamicity
A compound used to speed up a chemical reaction is called a catalyst. A catalyst is used to accelerate a slow chemical reaction with the addition of a foreign substance that is not consumed by the reaction itself [23]. In general, an autocatalytic set is defined as a set of entities or a collection of entities where the word entities can be anything such as people, molecules, or objects [24,25,26]. Jain and Krishna [22] formalized the definition of an autocatalytic set in the form of a graph.
Definition 3.
[22] An autocatalytic set is a subgraph, each of whose nodes has at least one incoming link from a node belonging to the same subgraph.
The concept of an autocatalytic set for a graph is defined as vertex j catalyst vertex i [27]. The simplest of ACS graph is a vertex with 1-cycle. Figure 7 illustrates some samples of autocatalytic sets.
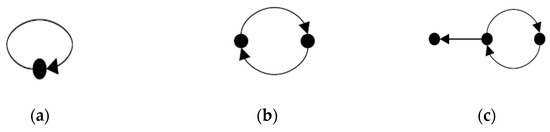
Figure 7.
(a) A 1-cycle and ACS; (b) A 2-cycle and ACS; (c) An ACS but not irreducible.
A dynamic system is governed by a set of coupled differential equations, that is given as follows [28,29,30,31]:
The dynamicity preserves the normalization of x as a state:
Equation (3) plays an important role in system dynamics. It has been used by several researchers such as Eigen et al. [24], who modeled the dynamic of relative populations of a self-replicating string and Jain and Krishna [30,31] applied for catalytically-reacting molecules.
In short, the variable is the relative population and designated as x . The set x of variables is exactly the set of vertices of the constructed graph such that . The time evolution of x depends on its interaction coefficients. Each variable has as its concentration. Hence, the relative concentration for each variable is given as follows:
and the sum of for all is as follows:
and
such that is a function at time t. It is a simple idealization of reaction rates in a well-stirred chemical reactor [32].
Sabariah [27] used ACS to model a Clinical Waste Incineration Process. The developed method by the researcher is outlined in the following steps.
Step 1: Construct matrix C with variables evolve through time t using equation (3) as follow:
and is large enough to get close to its attractor X, it is donated as X
Step 2: Determine the lowest value of of Perron-Frobenius Eigenvalue (PFE) from the set of vertices .
The lowest value of is removed from the system along with its edges. Thus, a graph C contains n-1 vertices.
Step 3: Matrix C is reduced to the dimension of . The remaining vertices and edges of graph C are fixed as they were before. All are updated so that . The step is repeated until matrix is produced.
In Step 1, Equation (3) is introduced to evaluate the dynamical system followed by Step 2 and 3 which describe the procedure to update the graph of the system.
6. Graph of Refining Process of Palm Oil
The graph-based model of physical refining process is elaborated in these subsections.
6.1. Chemical Compounds as Vertices
Firstly, a graphical representation of an ordinary physical refining process is classified as a crisp graph whereby V is denoted for the set of vertices and E is the set of edges of the graph that are needed to be determined systematically and properly.
As for refining process of palm oil, the CPO quality depends on the amount of free fatty acids, moisture, heavy metals, oxidized products, and minor constituents in the oil. The unwanted compounds are removed partially or completely during the refining process. The following variables are the vertices for the proposed graph.
6.1.1. Oil
Palm oil consists of free fatty acids (FFA), esterified, and glycerol. Free fatty acids are formed by triglyceride, diglyceride, and monoglyceride. Chemical compounds or enzymatic hydrolysis are used to decompose them.
6.1.2. Moisture
The standard amount of moisture in palm oil varies from 0.15% to 3.0%. It needs to be controlled to prevent FFA from increasing.
6.1.3. Heavy Metals
Heavy metals or trace metals are selected as a vertex in . This particular variable or vertex represents the metal compounds in the refining process. It comprises of iron (Fe), copper (Cu), cadmium (Cd), lead (Pb), and manganese (Mn).
6.1.4. Peroxide
Some oxidized compounds exist in palm oil. They produce odors and unpleasant flavors.
6.1.5. Phospholipids
Phospholipids are complex esters. These compounds consist of nitrogen bases, phosphorus, sugars, and long-chain fatty acids that amount up to 20 to 80 ppm.
6.1.6. Phosphoric Acid
Phosphoric acid (H3PO4) is added during the refining process. It is used to remove phosphatide during the degumming process.
6.1.7. Bleaching Earth
Bleaching earth consists of bentonite, attapulgite, and montmorillonite clay. A substantial amount of clay contains iron, magnesium, calcium, silica, and aluminum. Bleaching earth is added during the bleaching process. It is used to remove undesired pigment-type compounds in CPO.
The set of edges, V, of graph is described in the following subsection.
6.2. Chemical Reactions as Edges
The refining process of palm oil consists of seven variables as vertices as described in Section 6 earlier. Figure 8 illustrates the graphical representation of the refining process of palm oil . The edges are defined as connection such that a vertex catalyzes another vertex.
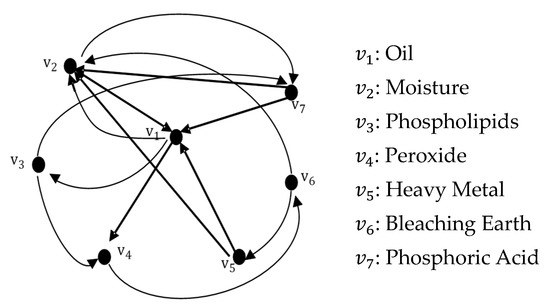
Figure 8.
Graph .
For example, represents edge catalyzes . The other fourteen edges are chemical reactions described and listed in Table 5.

Table 5.
The edges for graph .
7. Dynamicity of
The dynamicity of graph is determined using the procedure described in Section 5. The lowest value for PFE of is discarded; i.e., the chemical compound is depleted. The two surviving chemical compounds are the final products or the outputs of the refining process for palm oil. The quantitative (PFE) and descriptive resultant from the dynamicity of the graph are summarized in Table 6.

Table 6.
The dynamic of graph .
Figure 9 illustrates the updated stages of graph . Initially, it consists of seven vertices, which are reduced to two vertices in the end.
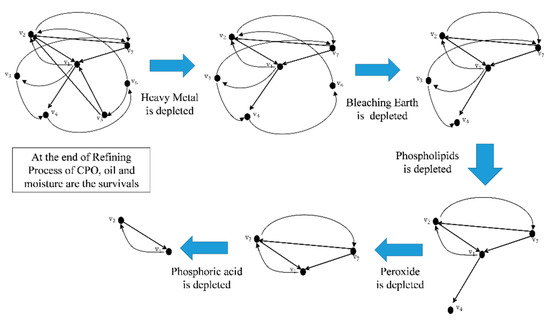
Figure 9.
The dynamic of physical refining process of palm oil .
8. Analysis of Gp
The dynamicity of graph is summarized in Table 6 of Section 7 whereby heavy metals are the first variable depleted in the refining process. The sequence of depleted compounds begins with bleaching earth, phospholipids, peroxide, and finally phosphoric acid. The remaining compounds at a given time, t, are summarized as follow (see Table 7):

Table 7.
Summary of remaining chemical compounds.
Five stages of depleted sequences in the refining process of palm oil are listed above. Further analysis on our obtained rate of change and dynamic of graph are elaborated in Table 8. The results obtained from the proposed method are significantly comparable to results documented in [2,8]. The comparisons are described in the third column of Table 8 (description).

Table 8.
Analysis of graph .
Furthermore, the resultant concentrations and dynamicity of graph in this study also concur to results of Er [11], Morad et al. [2], Khairil et al. [5] and Nanda et al. [35]. Moreover, the negativity concentration for some variables from the proposed method are perfectly justified by their role during the refining process.
9. Conclusions
In this paper, the graph-based model for the physical refining process of palm oil is presented. The multidimensional system, namely, the physical refining process for palm oil is defined as graph that consisted of seven vertices and fourteen edges. The concentration rate and dynamicity of graph are evaluated and the results show that oil and moisture are the surviving compounds. The results obtained from the model agreed to the one presented by Er [11] (see Table 8); i.e., these two compounds are the final products produced by the refining process. The FFA from oil compounds is formed as a palm fatty acid distillate (PFAD). The glycerol and moisture formed refined bleached deodorized palm oil (RBDPO). The PFAD is used as a soap product while RBDPO is utilized as cooking oil. On the other hand, undesired compounds such as phospholipid, peroxide, and heavy metals are removed during the refining process as explained in Table 8.
Author Contributions
Conceptualization, A.A. and T.A.; formal analysis, A.A.; writing—original draft preparation, A.A. and T.A.; writing—review and editing, S.R.A. and N.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by Ministry of Higher Education Malaysia (MOHE) Fundamental Research Grants FRGS/1/2020/STG06/UTM/01/1 and FRGS/1/2020/STG06/UTM/02/9.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Authors acknowledge the support of Universiti Teknologi Malaysia (UTM), and Ministry of Higher Education Malaysia (MOHE).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Harahap, F.; Leduc, S.; Mesfun, S.; Khatiwada, D.; Kraxner, F.; Silveira, S. Opportunities to optimize the palm oil supply chain in Sumatra, Indonesia. Energies 2019, 12, 420. [Google Scholar] [CrossRef]
- Morad, N.A. The Physical Properties of Palm Oil Mixtures for Design of Process Equipment. Ph.D. Thesis, University of Leeds, Leeds, UK, 2006. [Google Scholar]
- Ahmad, I.; Mohammad, J.A. Selected Readings on Palm Oil and its Uses; Palm Oil Familiarization Programme 1993; Palm Oil Research Institute of Malaysia: Kuala Lumpur, Malaysia, 1993; pp. 1–9.
- Ceriani, R.; Meirelles, A.J. Simulation of continuous physical refiners for edible oil deacidification. J. Food Eng. 2006, 76, 261–271. [Google Scholar] [CrossRef]
- Khairil, A.; Imam, P.; Agus, K.; Adiarso; Tatang, H.S. Esterification Reaction of High Free Fatty Acid Content of Crude Palm Oil and Glycerol Using Zinc Glycerolate and Zinc Soap Catalysts. In Proceedings of the Sriwijaya International Seminar on Energy Science and Technology, Palembang, Indonesia, 10–11 September 2011. [Google Scholar]
- Arrifin, D.; Fairus, H. Introduction to Malaysian Palm Oil Industry; Palm Oil Familiarization Programme 2002; Palm Oil Research Institute of Malaysia: Kuala Lumpur, Malaysia, 2002; pp. 1–15.
- Higuchi, M. Quality Control in Oil Refineries. In Proceedings of the Workshop of Quality in The Palm Oil Industry, Kuala Lumpur, Malaysia, 2–3 August 1983; pp. 240–245. [Google Scholar]
- Ariffin, A.A. The Effect of CPO Quality Parameters (FFA, M&I, IV, PV, AV, DOBI and Colour) on the Refinery Production Efficiency. In Proceedings of the 2000 National Seminar on Palm Oil Milling, Refining Technology, Quality and Environment, Genting highlands, Malaysia, 3–4 July 2001; pp. 79–88. [Google Scholar]
- Goh, E.M. Palm oil composition and quality. In Proceedings of the PORIM International Palm Oil Conference, Progress, Prospects Challenges Towards the 21st Century. Chemistry and Technology, Kuala Lumpur, Malaysia, 9–14 September 1993; pp. 268–278. [Google Scholar]
- Yusoff, M.S.A.; Thiagarajan, T. Refining and Downstreaming Processing of Palm Oil and Palm Kernel Oil. In Selected Readings on Palm Oil and Its Uses; Palm Oil Research Institute of Malaysia: Kuala Lumpur, Malaysia, 1993; pp. 150–174. [Google Scholar]
- Er, K.L. Quality control laboratories in refineries. In Proceedings of the Workshop on Quality in The Palm Oil Industry, Kuala Lumpur, Malaysia, 2–3 August 1985; pp. 203–208. [Google Scholar]
- Leong, W.L. The Refining and Fractionation of Palm Oil. In Palm Oil Mill Engineers-Executives Training Course 14th Semester 1; Palm Oil Research Institute of Malaysia: Kuala Lumpur, Malaysia, 1992; Volume 1, pp. 1–6. [Google Scholar]
- Formo, M.W.; Jungermann, E.; Norris, F.A.; Sonntag, N.O. Bailey’s Industrial Oil and Fat Products; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1979. [Google Scholar]
- Sambanthamurthi, R.; Sundram, K.; Tan, Y.A. Chemistry and biochemistry of palm oil. Prog. Lipid Res. 2000, 39, 507–558. [Google Scholar] [CrossRef]
- Bockisch, M. Fats and Oils Handbook; AOCS Press: Urbana, IL, USA, 1998. [Google Scholar]
- Howes, P.D.; Soon, T.C.; Lim, S.H.; Shaw, D.B.; Stemp, P.K. Bleaching Earths, Trends and developments in Bleaching. In Proceedings of the 1991 PORIM International Palm Oil Conference (Chemistry and Technology), Selangor, Malaysia, 9–14 September 1991; pp. 55–75. [Google Scholar]
- Rossi, M.; Gianazza, M.; Alamprese, C.; Stanga, F. The Role of Bleaching Clays and Synthetic Silica in Palm Oil Physical Refining. Food Chem. 2003, 82, 291–296. [Google Scholar] [CrossRef]
- Vintila, I. The Mathematical Model of Edible Oils Physical Refining. Sci. Study Res. 2009, 3, 260–264. [Google Scholar]
- Balakrishnan, R.; Ranganathan, K. A Textbook of Graph Theory, 2nd ed.; Springer Science and Business Media: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Epp, S. Discrete Mathematics with Applications; PWS Publishing Company: Boston, MA, USA, 1993. [Google Scholar]
- Harary, F. Graph Theory; Addison Wesley Publishing Company: Berkeley, CA, USA, 1969. [Google Scholar]
- Jain, S.; Krishna, S. Autocatalytic Sets and the Growth of Complexity in an Evolutionary Model. Phys. Rev. Lett. 1998, 81, 5684–5687. [Google Scholar] [CrossRef]
- Ostwald, W. Definition der Katalyse. Z. Phys. Chem. 1894, 15, 705–706. [Google Scholar]
- Eigen, M.; McCaskill, J.; Schuster, P. The Molecular Quasi-species. Adv. Chem. Phys. 1989, 75, 149–263. [Google Scholar]
- Kauffman, S.A. Autocatalytic Sets of Proteins. J. Theor. Biol. 1983, 119, 1–24. [Google Scholar] [CrossRef]
- Rossler, O.E. A System Theoretic Model of Biogenesis. Z. Nat. 1971, 26, 741–746. [Google Scholar] [CrossRef]
- Sabariah, B. Modelling of Clininal Waste Incinerator Process Using Novel Fuzzy Autocatalytic Set: Manifestation of Mathematical Thinking. Ph.D. Thesis, Universiti Teknologi Malaysia, Skudai, Malaysia, 2005. [Google Scholar]
- Jain, S.; Krishna, S. A model for the Emergence of Cooperation, Interdependence and Structure in Evolving Networks. In Proceedings of the National Academy of Sciences USA, Santa Fe Institute, Santa Fe, NM, USA, 16 January 2001; pp. 543–547. [Google Scholar] [CrossRef]
- Jain, S.; Krishna, S. Large Extinctions in an Evolutionary Model: The Role of Innovation and Keystone Species. In Proceedings of the National Academy of Sciences USA, University of Michigan, Ann Arbor, MI, USA, 19 February 2002; pp. 2055–2060. [Google Scholar] [CrossRef]
- Jain, S.; Krishna, S. 16 Graph Theory and the Evolution of Autocatalytic Networks. In Handbook of Graphs and Networks, 1st ed.; Bornholdt, S., Schuster, H.G., Eds.; John Wiley and VCH Publishers: Berlin, Germany, 2003; pp. 355–395. [Google Scholar]
- Jain, S.; Krishna, S. Formation and Destruction of Autocatalytic Sets in an Evolving Network Model. Ph.D. Thesis, Indian Institute of Science, Bangalore, India, 2003. [Google Scholar]
- Ashmore, P.G. Catalysis and Inhibition of Chemical Reactions; Butterworth: London, UK, 1963. [Google Scholar]
- Maheshwari, N. Clinical Biochemistry; Jaypee Brothers Publishers: New Delhi, India, 2008. [Google Scholar]
- Tamao, K.; Ishida, N.; Tanaka, T.; Kumada, M. Silafunctional compounds in organic synthesis. Part 20. Hydrogen peroxide oxidation of the silicon-carbon bond in organoalkoxysilanes. Organometallics 1983, 2, 1694–1696. [Google Scholar] [CrossRef]
- Nanda, M.R.; Yuan, Z.; Qin, W.; Poirier, M.A.; Chunbao, X. Purification of crude glycerol using acidification: Effects of acid types and product characterization. Austin J. Chem. Eng. 2014, 1, 1–7. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).